Evaluation of Ceftriaxone Residue in Cow Milk and its Toxicity on Danio rerio
Article Information
Jayanta Chowdhury1, Riya Mukherjee2, Debanjan Dutta2, Tapan Kumar Mandal3, Tarakdas Basu2, Sandhimita Mondal1*
1Department of Microbiology, Techno India University, West Bengal, EM 4, Sector V, Salt Lake City, Kolkata-700 091, West Bengal, India.
2Department of Biochemistry and Biophysics, University of Kalyani, Kalyani - 741 235, West Bengal, India.
3Department of Pharmacology and Toxicology, W.B. University of Animal and Fishery Sciences, 37-K.B.Sarani, Kolkata-37, India
*Corresponding Author: Sandhimita Mondal, Department of Microbiology, Techno India University, EM-4 Sector-V, Salt Lake City, Kolkata 700091, West Bengal, India;
Received: 06 August 2022; Accepted: 17 August 2022; Published: 18 November 2022
Citation: Jayanta Chowdhury, Riya Mukherjee, Debanjan Dutta, Tapan Kumar Mandal, Tarakdas Basu, Sandhimita Mondal. Evaluation of Ceftriaxone Residue in cow milk and its Toxicity on Danio rerio. Journal of Food Science and Nutrition Research 5 (2022): 716-723.
Share at FacebookAbstract
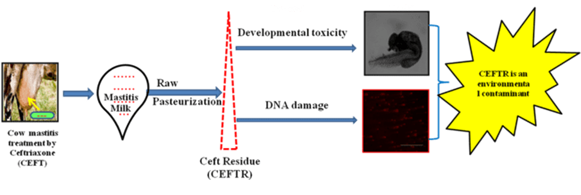
Ceftriaxone (CEFT), a widely used broad-spectrum beta -lactam cephalosporin antibiotic, is used to treat bovine mastitis, caused by a variety of bacteria. If used injudiciously, this antibiotic leaves a residue that persists after pasteurization. Antibiotic residue contamination occurs when antibiotic residue exceeds its Maximum Residue Limits (MRLs). This has negative impacts on both public health and the environment. The aim of a recent study was to determine the concentration of ceftriaxone residue (CEFTR) in raw and pasteurized mastitis cow milk, and its role in developmental toxicity and genotoxicity in the zebra fish model. The CEFTR concentrations in raw and pasteurized milk were several times higher than CEFT's MRL. CEFTR showed a decrease in body length and yolk sac region of zebra fish larvae 7-amino cephalosporanic acid (7-ACA), C3 and C7 are the cephalosporin components produced by the degradation of CEFT that may present in CEFTR, and have an impact on the zebra fish embryo in this stage of development. Comet Assay or Single Cell Gel Electrophoresis (SCGE) also exhibited highest percentage of tail DNA, and tail moment (DNA migration) that is the ultimate indicator of DNA damage by breaking DNA strands and incorporating guanine residue into the genome that ultimately damages the DNA. As a result, the CEFTR is extremely concerning for public health and the environment. The toxic effects of the CEFTR in zebra fish model have not yet been studied. This may be the first comprehensive study.
Keywords
Ceftriaxone-residue, Zebra fish, Developmental-toxicity, Genotoxicity, HPLC
Ceftriaxone-residue articles; Zebra fish articles; Developmental-toxicity articles; Genotoxicity articles; HPLC articles
Ceftriaxone-residue articles Ceftriaxone-residue Research articles Ceftriaxone-residue review articles Ceftriaxone-residue PubMed articles Ceftriaxone-residue PubMed Central articles Ceftriaxone-residue 2023 articles Ceftriaxone-residue 2024 articles Ceftriaxone-residue Scopus articles Ceftriaxone-residue impact factor journals Ceftriaxone-residue Scopus journals Ceftriaxone-residue PubMed journals Ceftriaxone-residue medical journals Ceftriaxone-residue free journals Ceftriaxone-residue best journals Ceftriaxone-residue top journals Ceftriaxone-residue free medical journals Ceftriaxone-residue famous journals Ceftriaxone-residue Google Scholar indexed journals Zebra fish articles Zebra fish Research articles Zebra fish review articles Zebra fish PubMed articles Zebra fish PubMed Central articles Zebra fish 2023 articles Zebra fish 2024 articles Zebra fish Scopus articles Zebra fish impact factor journals Zebra fish Scopus journals Zebra fish PubMed journals Zebra fish medical journals Zebra fish free journals Zebra fish best journals Zebra fish top journals Zebra fish free medical journals Zebra fish famous journals Zebra fish Google Scholar indexed journals Developmental-toxicity articles Developmental-toxicity Research articles Developmental-toxicity review articles Developmental-toxicity PubMed articles Developmental-toxicity PubMed Central articles Developmental-toxicity 2023 articles Developmental-toxicity 2024 articles Developmental-toxicity Scopus articles Developmental-toxicity impact factor journals Developmental-toxicity Scopus journals Developmental-toxicity PubMed journals Developmental-toxicity medical journals Developmental-toxicity free journals Developmental-toxicity best journals Developmental-toxicity top journals Developmental-toxicity free medical journals Developmental-toxicity famous journals Developmental-toxicity Google Scholar indexed journals Genotoxicity articles Genotoxicity Research articles Genotoxicity review articles Genotoxicity PubMed articles Genotoxicity PubMed Central articles Genotoxicity 2023 articles Genotoxicity 2024 articles Genotoxicity Scopus articles Genotoxicity impact factor journals Genotoxicity Scopus journals Genotoxicity PubMed journals Genotoxicity medical journals Genotoxicity free journals Genotoxicity best journals Genotoxicity top journals Genotoxicity free medical journals Genotoxicity famous journals Genotoxicity Google Scholar indexed journals HPLC articles HPLC Research articles HPLC review articles HPLC PubMed articles HPLC PubMed Central articles HPLC 2023 articles HPLC 2024 articles HPLC Scopus articles HPLC impact factor journals HPLC Scopus journals HPLC PubMed journals HPLC medical journals HPLC free journals HPLC best journals HPLC top journals HPLC free medical journals HPLC famous journals HPLC Google Scholar indexed journals DNA tail articles DNA tail Research articles DNA tail review articles DNA tail PubMed articles DNA tail PubMed Central articles DNA tail 2023 articles DNA tail 2024 articles DNA tail Scopus articles DNA tail impact factor journals DNA tail Scopus journals DNA tail PubMed journals DNA tail medical journals DNA tail free journals DNA tail best journals DNA tail top journals DNA tail free medical journals DNA tail famous journals DNA tail Google Scholar indexed journals DNA damage articles DNA damage Research articles DNA damage review articles DNA damage PubMed articles DNA damage PubMed Central articles DNA damage 2023 articles DNA damage 2024 articles DNA damage Scopus articles DNA damage impact factor journals DNA damage Scopus journals DNA damage PubMed journals DNA damage medical journals DNA damage free journals DNA damage best journals DNA damage top journals DNA damage free medical journals DNA damage famous journals DNA damage Google Scholar indexed journals live stock articles live stock Research articles live stock review articles live stock PubMed articles live stock PubMed Central articles live stock 2023 articles live stock 2024 articles live stock Scopus articles live stock impact factor journals live stock Scopus journals live stock PubMed journals live stock medical journals live stock free journals live stock best journals live stock top journals live stock free medical journals live stock famous journals live stock Google Scholar indexed journals toxic effect articles toxic effect Research articles toxic effect review articles toxic effect PubMed articles toxic effect PubMed Central articles toxic effect 2023 articles toxic effect 2024 articles toxic effect Scopus articles toxic effect impact factor journals toxic effect Scopus journals toxic effect PubMed journals toxic effect medical journals toxic effect free journals toxic effect best journals toxic effect top journals toxic effect free medical journals toxic effect famous journals toxic effect Google Scholar indexed journals non-biodegradable articles non-biodegradable Research articles non-biodegradable review articles non-biodegradable PubMed articles non-biodegradable PubMed Central articles non-biodegradable 2023 articles non-biodegradable 2024 articles non-biodegradable Scopus articles non-biodegradable impact factor journals non-biodegradable Scopus journals non-biodegradable PubMed journals non-biodegradable medical journals non-biodegradable free journals non-biodegradable best journals non-biodegradable top journals non-biodegradable free medical journals non-biodegradable famous journals non-biodegradable Google Scholar indexed journals
Article Details
Graphical Abstract
Article Highlights
- Concentration of ceftriaxone residue (CEFTR) was determined in raw and pasteurized mastitis cow milk.
- Developmental toxicity of CEFTR was evaluated on zebra fish (Danio rerio) embryos.
- Percentage of Tail DNA and DNA tail moment were measured as indicator of DNA damage
Introduction
Mastitis is a chronic, economically important inflammatory disease that affecting the dairy industry around the world. Bovine mastitis costs India a significant amount of money every year. Mastitis is caused by a variety of bacteria that are extremely susceptible to ceftriaxone [1]. Ceftriaxone (CEFT) is a broad-spectrum, third-generation cephalosporin antibiotic with a beta -lactam structure. Because of its low toxicity, it is commonly used in bacterial infections such as meningitis, pneumonia, diarrhoea, joint infections, sepsis, and nosocomial infections [2]. Antibiotics including cephalosporin have Active Pharmaceutical Ingredients (API) that have a harmful impact on the environment. It acts as an emerging contaminant [3]. Antibiotics are manufactured in approximately 248,000 tonnes each year and according to the findings, 48 percent of them being used to prevent and treat bacterial infections in humans and animals, according to the findings. 52 percent are used as growth promoter in agriculture allied sector. These are therapeutically administered to livestock to treat bacterial infections. These are also used as at subtherapeutic doses for disease prevention, growth promotion, and mortality reduction by lowering immune system function in live stock. These strategies help to minimize waste and toxin production [5-8]. Various antibiotics have stable structures and have a harmful toxic effect and due to which non-biodegradable parts of such antibiotics are directly discharged into a variety of environmental compartments leading to human health hazards. The usage of antibiotics are huge, despite the lack of official survey data on the detailed use of various types of antibiotics [9]. Excessive use of these antibiotics lead to the presence of their residual forms in milk. Antibiotics are contained in various organ tissues, secreted through milk, foods, and eggs, and are also excreted in feces and urine [10]. Among them, the duration and severity of exposure to antibiotic like, ceftriaxone have a significant impact on the implication of blaCTX-M resistance gene in the gut flora and leads to antibiotic resistance, which is the major cause of significant health care hazards [11,12]. Cephalosporrin antibiotic contamination has a strong negative impact on human health and ecosystem due to the presence of toxic substances in the waste water. This pollution affects marine ecosystems and it also impede ecosystem roles and affect organisms which are exposed during their life cycle [13]. Antibiotic residues in river water affect water quality and vary seasonally as a result of antibiotic runoff. Antibiotics are widely used in the treatment of animal diseases and as, growth promoter. Since 2006, European Union (EU) legislation has banned the use of antibiotics as a growth promoter [14]. Antibiotic residues from human and animal sources are discharged into many water bodies and posing a health risk to human beings [15]. Drug metabolites have carcinogenic and mutagenic properties, as well as have a negative impact on the various processes of the human body [8]. Antibiotic residues have now become a global problem [16]. Antibiotics have slowed the growth of starter cultures used to make fermented milk products [17]. Humans are the final consumers of antibiotic residue-containing products [8]. The pasteurization process removes the bacterial load from milk, but is unable to reduce drug residues [18]. As a result, maintaining the quality of milk necessitates the monitoring of antibiotic residue levels in milk. In this regard, regulatory agencies have set the higher limit known as the Maximum Residue Limits (MRLs) for a variety of anti-infective agents [19]. The main component of Cephalosporin containing wastewater is intermediate residues, which produce the fewest non-recycled materials, most of which are heterocyclic macromolecular compounds. Water which contains a variety of toxic organic compounds can pose a threat to organisms living in the environment [3]. It is estimated that 50% of all antimicrobials exist worldwide are for veterinary use. Bacteria that inevitably develop antibiotic resistance in animals comprise of food-borne pathogens, opportunistic pathogens and commensal bacteria. The same antibiotic resistance genes and gene transfer mechanisms can be found in the microflora of animals and humans. Direct contact, with such resistant bacteria, which reside in food and water contaminates animal and human habitats. It is reported that, resistant bacteria are accumulated by the use of antibiotics in agriculture and veterinary practices which spread via agriculture or veterinary tools and by direct contamination. The zebrafish embryo is a common model for mechanism-based research [20]. Antibiotics affect a variety of biomarkers in zebra fish [21]. Tetracycline causes oxidative stress in zebrafish embryos, which slows development [22]. Some pesticides are genotoxic, and also affect fishes [23]. The objectives of this study are (i) to quantify the CEFTR in raw and pasteurized milk from mastitis suffered cow, (ii) to investigate developmental toxicity of CEFTR in zebra fish, and (iii) to study its role in DNA damage.
Materials and Methods
Chemicals and reagents
SRL laboratories provided the analytical grade solvents used in the HPLC (India). Milli Q Elix (USA) filtered water was used. The analytical grade Ceftriaxone (CEFT) was also purchased from Sigma Aldrich (USA).
Collection of milk samples
40 milk samples (100ml / sample) were collected from cows from about 10 herds in the dairy farming villages around Kalyani, Nadia, West Bengal, India, after assessing the udder health of cows with a history of clinical and subclinical mastitis (i.e. 20 from clinical mastitis case and 20 from subclinical mastitis case). The mastitis milk samples were validated by the California Mastitis Test (CMT) and the White Side Test (WST) [24]. The herds were labeled on the day of the first screening, and 30 days later, anamnesis of the treatment regime was obtained from the owners of those identified herds, and samples were collected and stored in sterile containers at 4° C for future use. This research did not include any animal testing.As a result, in this study no animals were killed or anaesthetized.
Ceftriaxone residue (CEFTR) screening and quantification in milk samples
To detect CEFTR in raw milk samples, all samples were run through a High Performance Liquid Chromatography (HPLC) device (Schimadzu Lc-20 AT system attached to Thermo ODS Hypersil C18 column (250 mm,4.6 mm-ID; 5 µm particle size, USA) according to the standard protocol [25]. For the study of CEFT residues in milk samples, the simple and responsive HPLC method was optimized and validated [26]. Pasteurization was carried out on all of them. For the residue, pasteurized milk samples were also subjected to HPLC following the same process. The light at a wavelength of 254 nm was detected using a UV Vis SPD 20 detector. The mobile phase in this method consisted of acetonitrile and milipore purified water after filtration and sonication. The result derived from this experiment by comparing the curves obtained from experiments to the normal curve to examine the results. HPLC was used to separate drug residue from 20 µl of processed milk samples abiding a standard protocol by utilizing “LC Real Time Analysis” software. The CEFTR quantification was done by comparing the peak area of the sample with that of the standard CEFT having the corresponding chromatogram [27].
Outcome of CEFTR on Zebrafish (Danio rerio)
Maximum Residual Limit (MRL) determination of samples for the in vivo study
To standardize the process, drug residue aliquots were obtained in acetonitrile, and the multiples of the Maximum Residual Limit (MRL) in each sample were determined. Observed multiples of MRL of Ceftriaxone Residue in 1 ml sample container, which is calculated in this manner, i.e., 141 X MRL in 1 ml i.e., 1000 µl. So, 141 X MRL=1000 µl i.e., 1XMRL= 7 µl (approx.) of Ceftriaxone residue. So, 7 µl contain 0.1ppm (As, MRL of CEFT is 0.1ppm). Therefore, we inoculate the drug residue fraction collected from HPLC in 1 ml of E3 medium containing 10 nos. of just hatched 24hpf zebra fish larvae at 25 well plate to check their livability under 1xMRL inoculums for 24 h in case of ceftriaxone Residue (CEFTR). We did same for acetonitrile also as drug residue dissolved in the acetonitrile. In this study, we got tolerance to acetonitrile upto 20 µl. Livability of Ceftriaxone Residue treated just hatched zebrafish larvae was checked under 1X MRL to 3 X MRL, i.e., the amount of that subjected drug residue is 7 µl to 21 µl. It was observed that from point of 3X MRL of the drug residue maximum larvae was died. So, tolerance observed for Ceftriaxone residue is upto 2 x MRL, that means 14 µl. Therefore, just hatched and survived zebra fish larvae in 1ml E3 medium was charged with 14 µl of aliquot which contains Ceftriaxone residue i.e 0.2 ppm of concentration. Concentration of same pure drug was also charged in E3 medium containing the same quantity of zebra fish larva and 20 µl of acetonitrile was also charged in E3 medium containing the same quantity of larvae. Untreated larvae at E3 medium was considered as control. Zebra fish eggs were maintained and hatched by following the modified method reported by Oliviera et al. [28].
Role of CEFTR on morphological traits of Zebra fish embryo
For this experiment, 48 hpf zebrafish embryos were used. The effects of CEFT, CEFTR, and ACTN on the developmental process of 48hpf embryos for 24 hours were investigated. The characteristics that were measured were body length, yolk sack length, and yolk sac height. Using an EVOS FL Auto fluorescence microscope(Invitrogen), these traits were measured for at 72 hpf of each zebrafish. The temperature was held at 28°C. Image J 2.0 software was used to assess the stated parameters of the larvae.
Role of CEFTR on DNA damage of Zebra fish embryo
The Comet Assay, also known as Single Cell Gel Electrophoresis (SCGE), is a sensitive and fast technique used in assessing DNA damage, repair, genotoxicity testing, and genotoxicity evaluation [29,30]. Cell samples, including controls, were prepared by centrifuging trypsin-treated cells from 24 hpf zebra fish embryo for this test. For 24 hours, zebra fish embryos were exposed to 2X MRL Ceftriaxone drug residue (CEFTR), i.e. 14 µl of aliquot, 20 µl of Acetonitrile (ACTN), and 14 µl of pure Ceftriaxone (CEFT). The cells were then processed and dyed according to the standard protocol [31-33]. Comet score 2.0.0.0 software was used to analyze the comet formation of each and every cell one by one in sample under consideration. For the detection of DNA damage, the percentage of tail DNA and DNA migration (tail moment) were calculated [35]. The sample size was sufficient for statistical data analysis to estimate DNA damage caused by the stated treatment using the experimental model chosen.
Results
Detection and quantification of CEFTR by HPLC
CEFTR was detected in raw and pasteurized milk samples (Figure1), which was verified by comparing the peak of the CEFT standard drugs obtained by the HPLC. CEFTR concentrations were 1.75±0.44 and 0.56±0.03 ppm, respectively, in raw and pasteurized milk samples (Figure 1). CEFTR levels in raw and pasteurized mastitis milk were found to be approximately 17.5 and 5.6 times higher than MRL in this recent report, which is very significant and alarming.
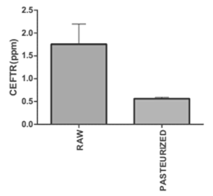
Figure 1: Concentration of Ceftriaxone residue (CEFTR) in raw and pasteurized cow milk having the report of mastitis, determined by using High-Performance Liquid Chromatography (HPLC). These experiments were performed for three times with three sets. The data are shown as average values ± SD. P<0.05 defines significant difference of concentration between raw milk and pasteurized milk.
Morphological traits
CEFTR showed detrimental effects on the growth and development of zebrafish after the treatment. These treatments reduced the body length (Figure 2b, 2c, 2d and 3a) while compared with the untreated group (Figure 2a, 3a). The CEFTR treated zebrafish shows shortest yolk sac length 430.2±28.94 µm and yolk sack height 72.33±3.84, and shorter body length 1489±38.44µm (Figure 2c,3b, 3c, 3a). The growth of the body length of the embryos was measured at 72hpf and the results are shown in Figure 2a,2b,2c,2d and 3a and the yolk sack length and height are shown in Figure 3b and 3c. CEFT and acetonitrile do not affect significantly on body length compared to the controls. But CEFTR has the detrimental effect on the growth of the embryo of zebra fish.
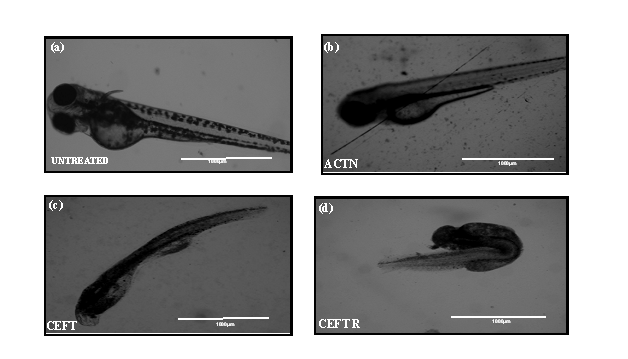
Figure 2: (a) Image of untreated D. rerio embryos at 72 hpf. (b) Image of D. rerio embryos malformations after ACTN treatment at 72 hpf (c) Image of D. rerio embryos malformations after CEFT treatment at 72 hpf (d) Image of D. rerio embryos malformations after CEFTR treatment at 72 hpf.
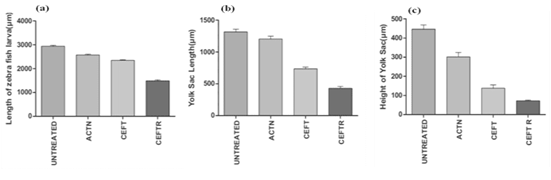
Figure 3: (a) Body length of D. rerio embryos without treatment (untreated) and with treatment of ACTN, CEFT and CEFTR at 72 hpf (b) Length of yolk sac of D. rerio embryos without treatment (untreated) and with treatment of ACTN, CEFT and CEFTR at 72 hpf.(c) Height of yolk sac of D. rerio embryos without treatment (untreated) and with treatment of ACTN, CEFT and CEFTR at 72 hpf. The data was presented as average values ± SD, with a significance level P<0.05 indicating a significant difference between untreated and treated embryos. This procedure was carried out three times.
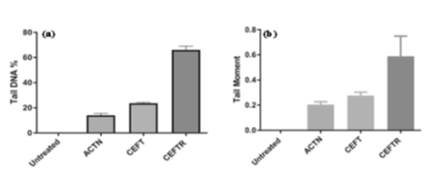
Figure 4: (a) Tail DNA (%) in cells of D. rerio without treatment (Untreated) and treatment with ACTN, CEFT and CEFTR at 48hpf (b) Tail DNA moment in cells of D. rerio without treatment (Untreated) and treatment with ACTN, CEFT and CEFTR at 48hpf. The data was presented as average values ± SD, with a significance level P<0.05 indicating a significant difference between untreated and treated embryo. This procedure was carried out three times.
DNA damage
CEFTR was demonstrated for developmental toxicity in this study. This residue was also utilized to examine the DNA damage in cells of zebrafish embryos (24hpf). This was measured by % tail DNA and DNA migration (tail moment). CEFT treated cell shows 23.45±0.59 % tail DNA. No damage in the untreated group (Figure 4a). ACTN treatment exhibits 13.93±0.64 % tail DNA while CEFTR treated showed highest 65.95±0.78% tail DNA (Fig.4a) after only 24h treatment. Tail moment is significant i.e.0.58 in CEFTR respect to ACTN treated and CEFT treated samples, where tail moment were 0.20 and 0.27 respectively (Figure 4b).With its high sensitivity, the Comet assay detects early DNA damage.
Discussion
In veterinary medicine, antibiotics are often used to treat various infectious diseases among which mastitis is most considerable one. Ceftriaxone (CEFT) residue was found in thirty out of forty samples. As a result, CEFTR was our main priority regarding drug residue. According to previous report, Tetracycline residue was not fully removed from milk at 700 C, depending on milk product matrix and its properties. Ceftriaxone has a Maximum Residue Limit (MRL) of 0.1ppm in milk, according to European Commission regulation 2377/90/EC. Antibiotic residues in milk and milk products that exceed the MRL cause significant health issues for consumers [37]. Antibiotic residues in food have a negative impact on health and lead to the emergence of antibiotic resistance genes [36]. After initial degradation, ceftriaxone produces some intermediate products. This intermediate was then broken down further into small molecules [4]. In our current study, CEFTR has the detrimental effect on the growth of the embryo of zebra fish. It is reported that the body length is a key indicator of embryo development, and nutrient losses can result in a shorter body length. Since the yolk sac is an embryo's only source of nutrition, therefore it plays an important role during the early stages of development, and its physical size decreases as the embryo develops. Cephalosporin C (CPC) is a fungus-derived (fungus of the genus Acremonium sp) antibiotic of the cephalosporin class that is converted to 7-amino cephalosporanic acid (7-ACA) [3]. This noxious cephalosporin derived 7-amino cephalosporanic acid (7-ACA) affects Zebra fish embryos during organogenesis in aqueous medium. Finally, it produces developmental toxicity, resulting in morphological malformations. C3 and C7 are cephalosporin substituents that are much more poisonous than 7-ACA. The toxicity of C3 and C7 may be exerted individually or in a synergistic manner. It is also reported that toxicity is also caused by the N-methylthiotetrazole (MTT) ring, which is a C3 substituent of cephalosporin and induces haemorrhage and hypoprothrombinemia by causing vitamin K deficiency and inhibiting aldehyde dehydrogenase. Platelet dysfunction is caused by the –COO group, which is present at C7 location. The role of genes, proteins, and biomarkers in the toxicity process is unknown at this time [3]. In our present study, 7-ACA, C3 and C7 may present in CEFTR that slowed the absorption of the yolk sac and causes zebrafish embryos to mature more slowly. This constituents may responsible for CEFTR-induced developmental toxicity in zebrafish embryos. The findings of our current research are close to those previously published literature. After a brief exposure period, erythromycin, lincomycin, and ibuprofen cause DNA damage [34]. CEFTR raises the tail moment substantially, according to a new analysis (Figure 4b). After a short time exposure, atorvastatin and gemfibrozil confirm a clear DNA damage, according to the some study [34]. Some antibiotics have been confirmed to cause a significant increase in DNA migration (tail moment). As a result, these drugs are a pollutant to the environment. Cephalosporin is said to damage DNA by breaking DNA strands. The oxidized guanine residues are incorporated into the genome [39]. CEFT is the cephalosporin drug. In our study, it is confirmed that CEFTR causes DNA damage. From the previous report, it is explained that intermediated product which may present in CEFTR damage DNA by breaking DNA strands and incorporating oxidized guanine residue in the genome [37]. However, since there is availability of scanty report on effect of antibiotic residues on the host system in the past, so, our recent study may be the first to show that antibiotic residues cause DNA damage. This may reports first time that DNA damage occurred by antibiotic residue like CEFTR. In our study, CEFTR affect on body length and yolk sack area of zebrafish embryo. 7-ACA, C3 and C7 are the constituents of CEFTR, have an impact on the zebra fish embryo in the stage of development. CEFTR also surge the percentage of tail DNA and the tail moment by breaking DNA strands and incorporating oxidized guanine residue in the genome that ultimately damages DNA. Our findings showed that the antibiotic could have a successful therapeutic application with a low risk of drug residue in milk, which could aid in the rational selection of antibiotics for the treatment of mastitis without causing residue in the food chain. Long-term prospective investigations with several antibiotics and more cow herds are needed for this research.
Conclusion
The CEFTR levels in raw and pasteurized mastitis milk were found to be approximately 17.5 and 5.6 times higher than MRL. It is concerning because pasteurization is a common practice among people before consuming milk. However, there is ample evidence that the drug residue is harmful to the host system. Our findings showed that the antibiotic could have a successful therapeutic application with a low risk of drug residue in milk, which could aid in the rational selection of antibiotics for the treatment of mastitis without causing residue in the food chain. While the pasteurization process removes bacteria from milk, an experiment found that conventional pasteurization is insufficient to remove drug residue from the food chain. CEFTR has an impact on the zebra fish embryo in the stages of development. CEFTR has an effect on body length and yolk sack area, according to a recent report. It also escalates the percentage of tail DNA and the tail moment as a consequence of CEFTR aided DNA damage. Long-term prospective investigations with several antibiotics and more cow herds in other districts of West Bengal, India in the first phase, and then across all Indian provinces in the second phase, are needed for this further research
Ethics
The Institutional Animal Ethics Committee of WBUAFS, Kolkata [Ref. No. IAEC/67 / xiv(B)] authorized the collection of milk samples and experimental procedures described in this manuscript on August 19, 2019, following the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India.
Author’s Contribution
JC: Data Curation, Methodology, Validation, Writing Original draft, & Editing; RM and DD: Methodology; TKM: Resources, Project Supervision; TB*: Methodology, Resources, Project Supervision; SM*: Conceptualization, Methodology, Investigation, Data Curation, Project Supervision, Writing - Original Draft, Review & Editing
Funding
This research does not have any funding sources
Availability of data and materials
On reasonable request, the corresponding author will provide the datasets analyzed during the current work.
Acknowledgments
The authors express their gratitude to the Chancellor of Techno India University, West Bengal for providing the requisite laboratory and infrastructural support
Conflict of interest
There are no conflicting interests.
References
- Bhat AM, Soodan JS, Singh R, et al. Incidence of bovine clinical mastitis in Jammu region and antibiogram of isolated pathogens. Vet. World 10 (2017): 984-989.
- Sonda TB, Horumpende PG, Kumburu HH, et al. Ceftriaxone use in a tertiary care hospital in Kilimanjaro, Tanzania: A need for a hospital antibiotic stewardship programme. Plos one 14 (2019): e0220261.
- Das N, Madhavan J, Selvi A. An overview of cephalosporin antibiotics as emerging contaminants: a serious environmental concern. Biotech 9 (2019): 231.
- Zhao Y, Liang X, Wang Y. et al. Degradation and removal of Ceftriaxone sodium in aquatic environment with Bi2WO6/g-C3N4 J. Colloid Interface Sci 523 (2018): 7-17.
- Ray P, Knowlton KF, Shang C. Development and Validation of a UPLC-MS/MS Method to Monitor Cephapirin Excretion in Dairy Cows following Intramammary Infusion. Plos one 9 (2014): e112343.
- Elizalde-Velázquez A, Gómez-Oliván LM, Galar-Martínez M, et al. Amoxicillin in the Aquatic Environment, Its Fate and Environmental Risk, in: Larramendy M, Soloneski S. (Eds.), Environmental Health Risk - Hazardous Factors to Living Species. InTech (2016).
- Graham JP, Boland JJ, Silbergeld E. Growth Promoting Antibiotics in Food Animal Production: An Economic Analysis. Public Health Rep 122 (2007): 79-87.
- Nisha A. Antibiotic Residues - A Global Health Hazard. Vet. World 2 (2008): 375.
- Feng Li, Cheng Y, Zhang Y, et al. Distribution and human health risk assessment of antibiotic residues in large-scale drinking water sources in Chongqing area of the Yangtze River. Environ. Res 185 (2020): 109386.
- Sachi S, Ferdous J, Sikder M, et al. Antibiotic residues in milk: Past, present, and future. J. Adv. Vet. Anim. Res 6 (2019): 315.
- Sileshi A, Tenna A, Feyissa M, et al. Evaluation of ceftriaxone utilization in medical and emergency wards of Tikur Anbessa specialized hospital: a prospective cross-sectional study. BMC Pharmacol Toxicol 17 (2016): 7.
- Meletiadis J, Turlej-Rogacka A, Lerner A, et al. Amplification of Antimicrobial Resistance in Gut Flora of Patients Treated with Ceftriaxone. Antimicrob. Agents Chemother 61 (2017): 473-477.
- Kraemer SA, Ramachandran A, Perron GG. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 7 (2019): 180.
- Cháfer-Pericás C, Maquieira Á, Puchades R. Fast screening methods to detect antibiotic residues in food samples. TrAC Trends Anal. Chem. 29 (2010): 1038-1049.
- Zhao Y, Liang X, Wang Y. et al. Degradation and removal of Ceftriaxone sodium in aquatic environment with Bi2WO6/g-C3N4 J. Colloid Interface Sci 523 (2018): 7-17.
- Teuber M. Veterinary use and antibiotic resistance. Curr. Opin. Microbiol 4 (2001): 493-499.
- Seymour EH, Jones GM, McGilliard ML, et al. Persistence of residues in milk following antibiotic treatment of dairy cattle. J. Dairy Sci. 71 (1988): 2292-2296.
- Ruegg PL. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci 100 (2017): 10381-10397.
- Beyene T. Veterinary Drug Residues in Food-animal Products: Its Risk Factors and Potential Effects on Public Health. J. Vet. Sci. Technol 7 (2015)
- Nishimura Y, Inoue A, Sasagawa S, et al. Using zebrafish in systems toxicology for developmental toxicity testing: Zebrafish and developmental toxicity. Congenit. Anom 56 (2016): 18-27.
- Oliveira R, McDonough S, Ladewig JCL, et al. Effects of oxytetracycline and amoxicillin on development and biomarkers activities of zebrafish (Danio rerio). Environ. Toxicol. Pharmacol 36 (2013): 903-912.
- Zhang Q, Cheng J, Xin Q, et al. Effects of tetracycline on developmental toxicity and molecular responses in zebrafish (Danio rerio) embryos. Ecotoxicology 24 (2015): 707-719.
- D’Costa AH, Shyama SK, Praveen KMK, et al. Induction of DNA damage in the peripheral blood of zebrafish (Danio rerio) by an agricultural organophosphate pesticide, monocrotophos. Int. Aquat. Res 10 (2018): 243-251.
- Mpatswenumugabo JP, Bebora LC, Gitao GC, et al. Prevalence of Subclinical Mastitis and Distribution of Pathogens in Dairy Farms of Rubavu and Nyabihu Districts, Rwanda. J. Vet. Med 11 (2017): 1-8.
- Schenck FJ, Callery PS. Chromatographic methods of analysis of antibiotics in milk. J. Chromatogr. A 812 (1998): 99-109.
- Ibrahim FA, Nasr JJM. Direct determination of ampicillin and amoxicillin residues in food samples after aqueous SDS extraction by micellar liquid chromatography with UV detection. Anal. Methods 6 (2014): 1523.
- Kurjogi M, Issa MYH, Alghamdi S, et al. Detection and determination of stability of the antibiotic residues in cow’s milk. Plos one 14 (2019): e0223475.
- Chowdhury J, Mandal TK, Mondal S. Genotoxic impact of emerging contaminant amoxicillin residue on zebra fish (Danio rerio) embryos. Heliyon 6 (2020): e05379.
- Ostling O, Johanson KJ. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem. Biophys. Res. Commun. 123 (1984): 291-298.
- Singh NP, McCoy MT, Tice RR, et al. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res 175 (1988): 184-191.
- Frenzilli G, Lenzi P, Scarcelli V, et al. Effects of loud noise exposure on DNA integrity in rat adrenal gland. Environ. Health Perspect 112 (2004): 1671-1672.
- Olive PL, Durand RE. Heterogeneity in DNA damage using the comet assay. Cytom. Part J. Int. Soc. Anal. Cytol. 66 (2005): 1-8.
- Speit G, Rothfuss A. The comet assay: a sensitive genotoxicity test for the detection of DNA damage and repair. Methods Mol. Biol. Clifton NJ 920 (2012): 79-90.
- Rocco L, Peluso C, Stingo V. Micronucleus test and comet assay for the evaluation of zebrafish genomic damage induced by erythromycin and lincomycin. Environ. Toxicol 27 (2012): 598-604.
- Van den Meersche T, Pamel EV, Poucke CV, et al. Development, validation and application of an ultra high performance liquid chromatographic-tandem mass spectrometric method for the simultaneous detection and quantification of five different classes of veterinary antibiotics in swine manure. J. Chromatogr 1429 (2016): 248-257.
- Hassan MM. Antimicrobial Resistance Pattern against E. coli and Salmonella in Layer Poultry. Res. J. Vet. Pract 2 (2014): 30-35.
- Shapiro RS. Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens. Plos Pathog 11 (2015): e1004678.
