Estimation of Ivermectin Treatment Demand for the Management of Strongyloidiasis Cases Worldwide: A Systematic Review
Article Information
Heron Gezahegn Gebretsadik*
School of Global Health and Bioethics, Euclid University, Banjul, Gambia
*Corresponding Author: Heron Gezahegn Gebretsadik, School of Global Health and Bioethics, Euclid University, Banjul, Gambia.
Received: 29 March 2023; Accepted: 05 April 2023; Published: 01 May 2023
Citation:
Heron Gezahegn. Estimation of Ivermectin Treatment Demand for the Management of Strongyloidiasis Cases Worldwide: A Systematic Review. Journal of Orthopedics and Sports Medicine 5 (2023): 214-221.
Share at FacebookAbstract
Background: Strongyloides stercoralis is an intestinal helminth that infects humans through contact with larval soil. The infection is often asymptomatic and can only be detected by eosinophilia in peripheral blood. Therefore, serology is the most reliable method for diagnosing the infection. The disease is treated with 2 × 200 μg/kg ivermectin administered orally at two-week intervals. However, the need for this medication to treat strongyloidiasis cases worldwide is unknown. All available data were systematically reviewed and summarized to estimate the global need for ivermectin treatment.
Methods: A systematic electronic literature search was performed in the PubMed, WHOLIS, and ISI Web of Science databases for articles published between January 1990 and May 2017. Articles with quantitative data on prevalence, incidence, duration of infection, remission/cure, and mortality in humans were included. After obtaining the raw data from the systematic review, adjustments were made for diagnostic accuracy, reference population selection, and adjustments for age and reference year 2017 as a prerequisite for estimating the total number of strongyloidiasis cases in humans worldwide. The corresponding ivermectin treatment needs were then derived.
Results: One hundred and sixty-six articles were identified as relevant for data analysis. The analysis revealed that an estimated 159 542 655 people were infected with S. stercoralis worldwide in the best-case scenario and 260 710 055 in the worst-case scenario. Accordingly, the corresponding ivermectin treatment requirements were calculated to be 422,043,504 and 1,379,330,000 ivermectin 3-mg tablets for the best-case and worst-case scenarios. The calculation assumed a single tablet per day for 1- and 2-day treatment recommendations.
Conclusions: To begin disease control, approximately 422,043,504 to 1,379,330,000 ivermectin tablets would be required based on the results of this study, not including occasional ivermectin treatment by other programs. This estimate could be important for policymakers and other stakeholders to address the disease worldwide.
Keywords
Strongyloidiasis; Ivermectin; Estimate, Strongyloides stercoralis, Diagnosis
Strongyloidiasis articles Strongyloidiasis Research articles Strongyloidiasis review articles Strongyloidiasis PubMed articles Strongyloidiasis PubMed Central articles Strongyloidiasis 2023 articles Strongyloidiasis 2024 articles Strongyloidiasis Scopus articles Strongyloidiasis impact factor journals Strongyloidiasis Scopus journals Strongyloidiasis PubMed journals Strongyloidiasis medical journals Strongyloidiasis free journals Strongyloidiasis best journals Strongyloidiasis top journals Strongyloidiasis free medical journals Strongyloidiasis famous journals Strongyloidiasis Google Scholar indexed journals filariform larvae articles filariform larvae Research articles filariform larvae review articles filariform larvae PubMed articles filariform larvae PubMed Central articles filariform larvae 2023 articles filariform larvae 2024 articles filariform larvae Scopus articles filariform larvae impact factor journals filariform larvae Scopus journals filariform larvae PubMed journals filariform larvae medical journals filariform larvae free journals filariform larvae best journals filariform larvae top journals filariform larvae free medical journals filariform larvae famous journals filariform larvae Google Scholar indexed journals Gastrointestinal tract articles Gastrointestinal tract Research articles Gastrointestinal tract review articles Gastrointestinal tract PubMed articles Gastrointestinal tract PubMed Central articles Gastrointestinal tract 2023 articles Gastrointestinal tract 2024 articles Gastrointestinal tract Scopus articles Gastrointestinal tract impact factor journals Gastrointestinal tract Scopus journals Gastrointestinal tract PubMed journals Gastrointestinal tract medical journals Gastrointestinal tract free journals Gastrointestinal tract best journals Gastrointestinal tract top journals Gastrointestinal tract free medical journals Gastrointestinal tract famous journals Gastrointestinal tract Google Scholar indexed journals Rhabditiform articles Rhabditiform Research articles Rhabditiform review articles Rhabditiform PubMed articles Rhabditiform PubMed Central articles Rhabditiform 2023 articles Rhabditiform 2024 articles Rhabditiform Scopus articles Rhabditiform impact factor journals Rhabditiform Scopus journals Rhabditiform PubMed journals Rhabditiform medical journals Rhabditiform free journals Rhabditiform best journals Rhabditiform top journals Rhabditiform free medical journals Rhabditiform famous journals Rhabditiform Google Scholar indexed journals Nematodes articles Nematodes Research articles Nematodes review articles Nematodes PubMed articles Nematodes PubMed Central articles Nematodes 2023 articles Nematodes 2024 articles Nematodes Scopus articles Nematodes impact factor journals Nematodes Scopus journals Nematodes PubMed journals Nematodes medical journals Nematodes free journals Nematodes best journals Nematodes top journals Nematodes free medical journals Nematodes famous journals Nematodes Google Scholar indexed journals Autoinfections articles Autoinfections Research articles Autoinfections review articles Autoinfections PubMed articles Autoinfections PubMed Central articles Autoinfections 2023 articles Autoinfections 2024 articles Autoinfections Scopus articles Autoinfections impact factor journals Autoinfections Scopus journals Autoinfections PubMed journals Autoinfections medical journals Autoinfections free journals Autoinfections best journals Autoinfections top journals Autoinfections free medical journals Autoinfections famous journals Autoinfections Google Scholar indexed journals Thighs articles Thighs Research articles Thighs review articles Thighs PubMed articles Thighs PubMed Central articles Thighs 2023 articles Thighs 2024 articles Thighs Scopus articles Thighs impact factor journals Thighs Scopus journals Thighs PubMed journals Thighs medical journals Thighs free journals Thighs best journals Thighs top journals Thighs free medical journals Thighs famous journals Thighs Google Scholar indexed journals Hemoptysis articles Hemoptysis Research articles Hemoptysis review articles Hemoptysis PubMed articles Hemoptysis PubMed Central articles Hemoptysis 2023 articles Hemoptysis 2024 articles Hemoptysis Scopus articles Hemoptysis impact factor journals Hemoptysis Scopus journals Hemoptysis PubMed journals Hemoptysis medical journals Hemoptysis free journals Hemoptysis best journals Hemoptysis top journals Hemoptysis free medical journals Hemoptysis famous journals Hemoptysis Google Scholar indexed journals Dyspnea articles Dyspnea Research articles Dyspnea review articles Dyspnea PubMed articles Dyspnea PubMed Central articles Dyspnea 2023 articles Dyspnea 2024 articles Dyspnea Scopus articles Dyspnea impact factor journals Dyspnea Scopus journals Dyspnea PubMed journals Dyspnea medical journals Dyspnea free journals Dyspnea best journals Dyspnea top journals Dyspnea free medical journals Dyspnea famous journals Dyspnea Google Scholar indexed journals Throat irritation articles Throat irritation Research articles Throat irritation review articles Throat irritation PubMed articles Throat irritation PubMed Central articles Throat irritation 2023 articles Throat irritation 2024 articles Throat irritation Scopus articles Throat irritation impact factor journals Throat irritation Scopus journals Throat irritation PubMed journals Throat irritation medical journals Throat irritation free journals Throat irritation best journals Throat irritation top journals Throat irritation free medical journals Throat irritation famous journals Throat irritation Google Scholar indexed journals Hyperinfection syndrome articles Hyperinfection syndrome Research articles Hyperinfection syndrome review articles Hyperinfection syndrome PubMed articles Hyperinfection syndrome PubMed Central articles Hyperinfection syndrome 2023 articles Hyperinfection syndrome 2024 articles Hyperinfection syndrome Scopus articles Hyperinfection syndrome impact factor journals Hyperinfection syndrome Scopus journals Hyperinfection syndrome PubMed journals Hyperinfection syndrome medical journals Hyperinfection syndrome free journals Hyperinfection syndrome best journals Hyperinfection syndrome top journals Hyperinfection syndrome free medical journals Hyperinfection syndrome famous journals Hyperinfection syndrome Google Scholar indexed journals
Article Details
Abbreviations:
WHO: World Health; NTDs: Neglected Tropical Diseases; MeSH: Medical Subject Headings; PubMed: U.S. National Library of Medicine, National Institute of Health; WHOLIS: World Health Organization Library Information System; ISI: Institute for Scientific Information; URL: Uniform Resource Locator; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analyses
1. Introduction
The parasitic character and the free-living reproductive cycle make Strongyloides species special among nematodes [1-3]. The life cycle in Figure 1 begins with the excretion of first-stage rhabditiform larvae by the host [4]. Within a few days in the soil, the rhabditiform larvae can either develop directly into re-infectious filariform larvae or become adult males and females [5,6].
The adult males and females mate and produce eggs from which new rhabditiform larvae hatch [7]. The rhabditiform larvae may then develop into infective filariform larvae or a new generation of free-living adults. Upon direct contact, the infective filariform larvae can pierce human skin and migrate into the submucosa, then into the venous circulation and toward the right heart and lungs [8]. There they break through the capillaries and travel up the pulmonary tree and trachea. The cough reflex helps expel the larvae from the bronchial tree and trachea. However, once the larvae reach the larynx, they are swallowed and migrate to the stomach and small intestine. In the gastrointestinal tract, the larvae mature in about two weeks into tiny adult females that live in the mucosa and submucosa of the duodenum and jejunum [6,7]. The females reproduce asexually (by parthenogenesis) and release eggs from which the rhabditiform first-stage larvae hatch in the intestinal lumen and are excreted back into the environment [8].
Of note, some nematodes, including Strongyloides species, have the unique property of causing autoinfections [9]. This occurs when noninfectious rhabditiform larvae develop into infectious filariform larvae before leaving the body. The filariform larvae can penetrate the intestinal wall or perianal skin and reinfect the person, i.e., the parasites never completely leave the host but (re)enter the host through the intestinal circulation (endo-autoinfection) or the perianal skin of the buttocks and thighs (exo-autoinfection). In this way, a person can remain infected for years, even up to 35 years, according to some sources [10], without coming into contact again with contaminated sources of infection. Infectious filariform larvae can also be transmitted directly from the anus back to the host (fecal-oral route) via the hands (dirty fingers and fingernails) or food contaminated with feces [7]. However, the most common route of transmission is contact with contaminated soil and direct penetration of larvae into human skin [11-12].
It is also noteworthy that, as mentioned earlier, the parasitic parthenogenetic female worms can live in the small intestinal mucosa of various mammals, reptiles, amphibians, and birds [12]. Therefore, the soil may be contaminated by both human and animal feces [13]. Among domestic animals, dogs frequently harbor S. stercoralis and contribute greatly to human infections through their fecal contamination of the environment [4,11]. Cats can become infected, but the infection appears to be transient, as the parasites die off after several months [14].
As mentioned earlier, most humans infected with Strongyloides remain largely asymptomatic [14,15]. When symptoms do occur, they are a consequence of the parasite's migration through various organs of the host body and may be dermatologic (urticarial rash, itchy and red rash on the skin where the larvae have invaded, recurrent raised red rash, typically along the thighs buttocks, and around the anus), pulmonary and anatomic, and around the anus), pulmonary (cough, difficulty breathing, throat irritation, hemoptysis, dyspnea, wheezing, and tachypnea), and/or gastrointestinal (abdominal cramps and bloating, constipation, intermittent watery diarrhea, anorexia, nausea, and loss of appetite) [16-18]. In addition, Strongyloides larvae can cause alveolar capillary hemorrhage and severe eosinophilic inflammation during maturation, which can lead to eosinophilic pneumonitis. In addition, neurologic symptoms (focal seizures, meningitis, brain abscess, and headache) may occur with central nervous system involvement. Cachexia and fatigue, weight loss, and heartburn are other possible signs and symptoms of strongyloidiasis [19].
Strongyloidiasis is usually diagnosed by examination of host feces for eggs or larvae [17]. Feces may be collected from the floor or directly from the rectum in large domestic animals or from stool samples from patients in humans. Laboratory examination can be performed either by direct microscopic examination of fresh stool samples, which is known to be relatively insensitive, by concentration methods (e.g., formalin-ethyl acetate concentration), or by collection of larvae using the Baermann funnel technique or the Koga agar plate culture method [20]. Repeated stool examinations may be necessary because the sensitivity of a single stool examination may be low, especially in chronic infections with a low worm burden. Immunodiagnostic testing for strongyloidiasis is also indicated when infection is suspected despite a negative stool examination [19]. Given the relatively poor performance of stool examination, serology and polymerase chain reaction testing have been used as alternatives [20].
In individuals diagnosed with strongyloidiasis, whether symptomatic or asymptomatic, treatment is recommended to (i) avoid the risk of developing a hyperinfection syndrome and/or disseminated strongyloidiasis and (ii) reduce transmission of the disease. Ivermectin, thiabendazole, and albendazole are currently anthelmintics for the treatment of S. stercoralis infections [21]. The efficacy of treating strongyloidiasis with ivermectin has been demonstrated, and it is the current drug of choice [18-23]. However, a major challenge is the severely limited availability and access to ivermectin in many low- and middle-income countries where strongyloidiasis is most prevalent [18]. Nevertheless, this study was initiated to assess the global ivermectin treatment needs for the management of strongyloidiasis cases.
2. Methodology
2.1 Systematic review: search strategy
This systematic review primarily focused on estimating the ivermectin treatment demand for the management of strongyloidiasis cases worldwide. To do so quantitative data pertaining to strongyloidiasis-related prevalence, incidence, duration of infection, remission/cure, and mortality in humans in the currently existing literature were identified systematically. The review was carried out based on PRISMA guidelines and included published literature between January 1, 1990, and May 23, 2017 [24]. The search was performed on May 23, 2017, in the PubMed, WHOLIS, and ISI Web of Science databases. The keywords used were "Strongyloides" and "strongyloidiasis" combined with the Boolean operator "OR." For searches in the PubMed and WHOLIS databases, the terms were used as Medical Subject Headings (MeSH).
2.2 Systematic review: extraction of data
A simple Microsoft Excel data extraction template was used to record epidemiologic data relevant for analysis. The extracted information included the bibliographic details of the reference, the study location, the study population, the sample size, the period of data collection, the sex and age of the studied population, and key epidemiological parameters (i.e., prevalence, incidence, duration of infection, remission/cure, mortality, severity breakdown), and any additional relevant comments, e.g., potential bias, other potentially relevant metadata, or other potentially relevant references.
2.3 Data analysis
The raw relevant epidemiologic data were adjusted for diagnostic accuracy, reference population selection, population growth, and age structure. Then, the total global numbers of infections and the needed Ivermectin demand were calculated one after the other. Of note, prior to calculating the Ivermectin treatment demand, the total cases were divided into different age groups, particularly children and adults. This information played a vital role in estimating the Ivermectin treatment demand by age group.
2.4 Correction for age group data
If the identified strongyloidiasis prevalence estimates originated from a specific age group (e.g., schoolchildren, adults over the age of 20, etc.), the respective reference population was also age-adjusted. The population percentage in the specific age group was calculated from the United Nations Report of Global Population [25] to identify the corresponding reference population for the respective age group. In general, estimating the proportion of children and/or adults out of the respective entire population and adjustment of the estimated number to the reference year 2017 were crucial steps to estimate the Ivermectin treatment demand.
2.5 Estimating the Ivermectin treatment demand
The ivermectin treatment demand by a number of 3 mg tablets calculated based on the minimum and the maximum number of strongyloidiasis cases estimated for children age group (≤ 19 years old) and adults age groups (>19 years old) obtained from age-specific prevalence profile by the WHO, the average 15.5-kg weight of children and the 62-kg average weight of adult globally [26,27], the ivermectin treatment dosage guidelines for strongyloidiasis on body weight [28] and the conceptual formula below.
Number of Ivermectin 3mg tablets required = (number of infected adults (children) *ivermectin dose by body weight (200 µg/kg) *average adult (children) weight by the kg) / 3
No ethical clearance was needed for the proposed research.
2.7 Findings
2.7.1 Databases search results
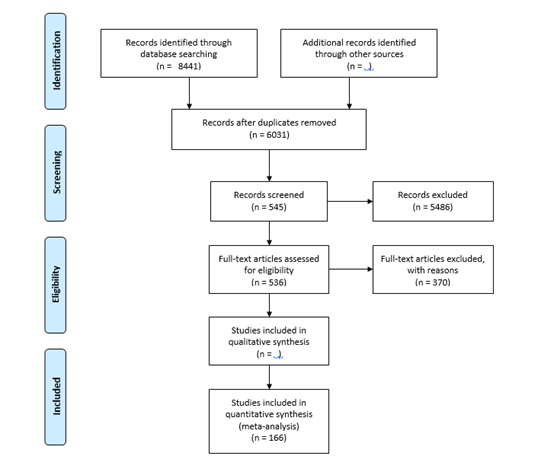
The initial electronic search yielded a total of 8441 hits. 2022 and 6419 articles were found in PubMed and ISI Web of Science using MeSH terms and keywords, respectively. No study was found in WHOLIS. A total of 2410 articles were identified as duplicates and excluded using the EndNote (1678) and Manual (732) search strategies. Of the 6031 articles without duplicates, 545 were classified as potentially relevant based on their title and abstract. In addition, nine articles were identified by searching the bibliographies of the included full texts, personal archives, and recommendations from colleagues and collaborators.
A total of 554 articles were checked for full-text accessibility using EndNote full-text search, URL search, Google search, the Swiss Tropical and Public Health Institute, and the University of Basel Library. Approximately 97% (n=536) of the total articles reviewed for full-text accessibility were found, retrieved, and finally evaluated against the inclusion criteria. After thoroughly reading all available full text, 166 articles containing relevant data for the analysis were finally included. The following flowchart illustrates the search strategy and methodology used in the literature search.
2.7.2 Total number of infected populations by age group
Data analysis yielded an estimated 159,542,655 and 260,710,055 infections in the best and worst scenarios, respectively. Of these, 82,962,181 and 135,569,229 were adults in the best and worst scenarios, respectively. In the best and worst scenarios, 76,580,474 and 125,140,826 children were estimated to be infected, respectively. Of note, the age category was based on the WHO age classification system (>19 years old = adults; ≤19 years old = children). To better estimate ivermectin requirements, obtaining age-specific prevalence data for Strongyloides stercoralis infection was essential, as mentioned previously.
2.7.3 Estimates of the ivermectin treatment demand due to strongyloidiasis
Depending on the available children and adults number of infected cases data from this systematic review, the ivermectin treatment dosage guidelines for strongyloidiasis on body weight [28] and the 15.5 kg and 62 kg global average weight of children and adults, respectively [27], the ivermectin treatment demand was roughly estimated (Table 1). Of note; depending on the respective treatment schedule, children and adults with an average body weight of 15.5 kg and 62 kg would need to take 3 mg and 12 mg of Ivermectin (4 Ivermectin 3 mg tablets) orally and per day, either for 1 or 2 days [28].
|
Human Strongyloidiasis cases |
Ivermectin treatment for 1-day recommendation |
Ivermectin treatment for 2 days recommendation |
|
|
Best case scenario, i.e., using a minimum number of infected estimates |
Best case scenario, i.e., using a minimum number of infected estimates |
Worst case scenario, i.e., using the maximum number of infected estimates |
|
|
Children |
79133156 |
158266313 |
258624374 |
|
Adults |
342910348 |
685820696 |
1120705626 |
|
Total |
422043504 |
844087009 |
1379330000 |
Table 1: Ivermectin treatment demand in terms of 3mg tablets required for treating strongyloidiasis cases globally in different scenarios.
3. Discussion
Oral administration of ivermectin has been shown to be effective in the treatment of patients with strongyloidiasis. The cure rate of a single and a double dose of ivermectin is 85.7% and 90.5%, respectively, with very mild and transient side effects [22]. In patients diagnosed with chronic Strongyloides infection, a double dose of ivermectin two weeks apart has been shown to be more effective than a single dose of ivermectin, thiabendazole, and a 7-day course of albendazole [18, 22].
Oral administration of ivermectin 200 µg/kg, once daily for 1 or 2 days, is used for uncomplicated infections and is generally well tolerated. Before treatment with ivermectin, clinicians should screen patients for coinfection with Loa loa, as ivermectin can cause severe reactions in loiasis patients. An alternative is the oral administration of albendazole 400 mg, twice daily for 7 days. Prolonged therapy or repeat treatment may be required in immunocompromised patients. In severely ill patients who cannot take oral medications, rectal ivermectin preparations or sometimes the subcutaneous animal ivermectin formulation have been used [21,23].
Preventive measures against strongyloidiasis include wearing sturdy footwear in endemic areas, washing vegetables and fruits thoroughly, prohibiting the use of feces as fertilizer, providing improved latrines and sanitation facilities, community information and mobilization campaigns to avoid contact with feces or sewage, identification and treatment of infected pets, patient screening, high-sensitivity diagnosis, and worldwide introduction and availability of ivermectin for treatment, chemoprophylaxis, and, especially in the case of Strongyloides hyperinfection syndrome, parenteral administration [23].
Ivermectin is the current drug of choice for the treatment of strongyloidiasis. Indeed, it is much more effective than other anthelmintics such as albendazole. The reduction of strongyloidiasis by mass administration of ivermectin has also been documented in various situations [18]. Ivermectin treatment requirements were calculated based on the available age-specific information in this review and drug treatment guidelines [28]. Accordingly, 422,043,504 and 689,665,000 ivermectin 3-mg tablets are needed to treat the estimated minimum and maximum number of infected persons worldwide once per day, respectively. The number of ivermectin-3-mg tablets would double with the recommended oral administration once daily for two days. Careful screening of patients is required prior to ivermectin administration. Loa loa infection, pregnant or nursing mothers, and hypersensitivity to benzimidazole compounds are considered contraindicated. In patients with disseminated strongyloidiasis, ivermectin treatment for two weeks is recommended until stool and/or sputum tests are negative. In such cases of hyperinfection syndrome, the need for ivermectin tablets is much higher than estimated in this review. In severe cases where oral and rectal treatment is not possible, subcutaneous ivermectin may be used [21,23].
4. Conclusion
Strongyloides is a parasitic infection that can cause hyperinfection syndrome and disseminated infection years after exposure. There are limited data on the best therapeutic option to treat the disseminated form of the disease and hyperinfection. On the other hand, thiabendazole, ivermectin, and albendazole are recommended for the treatment of uncomplicated Strongyloides infection. Nowadays, ivermectin is the first choice for treatment due to its better tolerability. However, it is reported that the distribution of the drug is disproportionate and inadequate. The results of this series of systematic reviews could serve as a basis for distributing the right amount of the medication to affected people worldwide.
Acknowledgement
I want to thank Dr. Thomas Fuest and Prof. Peter Odermatt for their excellent supervision throughout the research work. Without their sound scientific support, this work would not have been accomplished. My thanks also go to Dr. Dora Buonfrate for her contribution during the data extraction phase of the project.
Conflict of Interest
The author declares no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Cabada MM, Lopez M, Arque E, et al. Prevalence of soil-transmitted helminths after mass albendazole administration in an indigenous community of the Manu jungle in Peru. Pathog Glob Health 108 (2014): 200-205.
- Conlan JV, Khamlome B, Vongxay K, et al. Soil-transmitted helminthiasis in Laos: a community-wide cross-sectional study of humans and dogs in a mass drug administration environment. Am J Trop Med Hyg 86 (2012): 624-634.
- Fontes G, Oliveira KKL, Oliveira AKL, et al. Influence of specific treatment of intestinal parasites and schistosomiasis on prevalence in students in Barra de Santo Antonio, AL. Rev Soc Bras Med Trop 36 (2003): 625-628.
- Heukelbach J, Winter B, Wilcke T, et al. Selective mass treatment with Ivermectin to control intestinal helminthiases and parasitic skin diseases in a severely affected population. Bull World Health Organ 82 (2004): 563-571.
- WHO | Strongyloidiasis (2017).
- Adekeye TA, Thompson E, Awobode HO. Environmental contamination and public health risk of soil parasites in Ibadan South East Local Government Area, Nigeria Zool Ecol 26 (2016): 150-157.
- Prevention CDC. CDC - Strongyloides – Biology (2017).
- Mangali A, Sasabone P, Syafruddin AK, et al. Prevalence of intestinal helminthic infections in Kao District, North Halmahera, Indonesia. Southeast Asian J Trop Med Public Health 25 (1994): 737-744.
- Amor A, Rodriguez E, Saugar JM, et al. High prevalence of Strongyloides stercoralis in school-aged children in a rural highland of north-western Ethiopia: the role of intensive diagnostic work-up. Parasit Vectors 9 (2016).
- CDC C, Prevention. CDC Responds to Disease Outbreaks 24-7 | About | CDC (2014).
- Schär F, Giardina F, Khieu V, et al. Occurrence of and risk factors for Strongyloides stercoralis infection in South-East Asia. Acta Trop 159 (2016): 227-238.
- Khieu V, Schär F, Marti H, et al. Prevalence and risk factors of Strongyloides stercoralis in Takeo Province, Cambodia. Parasit Vectors 7 (2014): 221.
- Machado ER, Costa-Cruz JM. Strongyloides stercoralis and other enteroparasites in children at Uberlandia City, state of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz 93 (1998): 161-164.
- Steinmann P, Zhou XN, Du ZW, et al. Occurrence of Strongyloides stercoralis in Yunnan Province, China, and comparison of diagnostic methods. PLOS Negl Trop Dis 1 (2007): e75.
- Traversa D, Di Cesare A, Conboy G. Canine and feline cardiopulmonary parasitic nematodes in Europe: emerging and underestimated. Parasit Vectors 3 (2010): 62.
- Kassalik M, Mönkemüller K. Strongyloides stercoralis Hyperinfection Syndrome and Disseminated Disease. J Gastroenterol Hepatol 7 (2011): 766-768.
- Khieu V, Schar F, Marti H, et al. Diagnosis, treatment and risk factors of Strongyloides stercoralis in schoolchildren in Cambodia. PLOS Negl Trop Dis 7 (2013): e2035.
- Khieu V, Schär F, Marti H, et al. Diagnosis, Treatment and Risk Factors of Strongyloides stercoralis in Schoolchildren in Cambodia. PLOS Negl Trop Dis 7 (2013): e2035.
- de Almeida JA, Araujo MB, Rodrigues ML, et al. Prevalence of intestinal parasitosis in feces collected during necropsies. Rev Soc Bras Med Trop 24 (1991): 27-29.
- Krolewiecki AJ, Ramanathan R, Fink V, et al. Improved diagnosis of Strongyloides stercoralis using recombinant antigen-based serologies in a community-wide study in northern Argentina. Clin Vaccine Immunol 17 (2010): 1624-1630.
- Shikiya K, Kinjo N, Uehara T, et al. Efficacy of Ivermectin against Strongyloides stercoralis in humans. Intern Med 31 (1992): 310-312.
- Forrer A, Khieu V, Schindler C, et al. Ivermectin Treatment and Sanitation Effectively Reduce Strongyloides stercoralis Infection Risk in Rural Communities in Cambodia. PLOS Negl Trop Dis 10 (2016).
- Suputtamongkol Y, Premasathian N, Bhumimuang K, et al. Efficacy and Safety of Single and Double Doses of Ivermectin versus 7-Day High Dose Albendazole for Chronic Strongyloidiasis. PLOS Negl Trop Dis 5 (2011): e1044.
- Transparent reporting of systematic reviews and meta-analyses (2015).
- UNPD Total population (both sexes combined) by five-year age group, region, sub-region, and country, 1950-2100 (thousands) (2015).
- Definition of key terms (2013).
- Walpole SC, Prieto-Merino D, Edwards P, et al. The weight of nations: an estimation of adult human biomass. BMC Public Health 12 (2012): 439.
- Ivermectin Dosage (2018).