Elevated Maternal Testosterone Levels Alter PFOA Elimination and Tissue Distribution in Pregnant Rats
Article Information
Pankaj Yadav1, Jay S Mishra1 and Sathish Kumar1, 2, 3*
1Department of Comparative Biosciences, School of Veterinary Medicine, University of Wisconsin, Madison, Wisconsin, United States of America
2Department of Obstetrics and Gynecology, School of Medicine and Public Health, University of Wisconsin, Madison, Wisconsin, United States of America
3Endocrinology-Reproductive Physiology Program, University of Wisconsin, Madison, Wisconsin, United States of America
*Corresponding Author: Sathish Kumar. Department of Comparative Biosciences, School of Veterinary Medicine, University of Wisconsin, Madison, Wisconsin, United States of America
Received: 19 July 2023; Accepted: 26 July 2023; Published: 03 August 2023.
Citation:
Pankaj Yadav, Jay S Mishra and Sathish Kumar. Elevated Maternal Testosterone Levels Alter PFOA Elimination and Tissue Distribution in Pregnant Rats. Journal of Environmental Science and Public Health. 7 (2023): 131-139.
Share at FacebookAbstract
Perfluorooctanoic acid (PFOA) is an enduring synthetic chemical that harms human health. Recent studies indicate heightened bioaccumulation of PFOA, particularly in pregnant women experiencing preeclampsia. Since plasma testosterone levels are elevated in pregnant women with preeclampsia, we hypothesized that hyperandrogenic conditions during pregnancy may hinder PFOA elimination and contribute to their higher body burden. Pregnant Sprague-Dawley rats were s/c injected with vehicle or testosterone propionate from gestational day (GD) 15 to 20 to increase plasma testosterone levels by 2-fold, similar to levels in preeclampsia. On GD 16, [14C]-PFOA (9.4 pmol/kg) was given intravenously, and subsequently, 14C radioactivity was measured in maternal blood, urine, feces, and tissues. PFOA was primarily eliminated through urine; however, less PFOA was excreted in urine of pregnant rats with elevated testosterone levels than controls. Fecal excretion of PFOA was minimal and did not significantly differ between groups. The total elimination of PFOA (urine plus feces) was significantly reduced by 12% in pregnant rats with elevated testosterone levels. In controls, PFOA distribution was highest in placenta, followed by the kidneys, liver, brain, heart, lungs, and spleen. Pregnant rats with elevated testosterone levels displayed 12% higher concentrations of PFOA in these tissues than controls. Furthermore, the renal expression of Oat2 and Oat3 was significantly decreased, while Oatp1 and Oat-k expression was significantly increased in pregnant rats with elevated testosterone levels than controls. In conclusion, elevated maternal testosterone levels decrease urinary elimination of PFOA, possibly through altered expression of renal transporters leading to increased tissue concentrations of PFOA in pregnant rats.
Keywords
Perfluorooctanoic acid, Bioaccumulation,Testosterone, Pregnant rats, Kidney, Transporters.
Article Details
1. Introduction
Per- and poly-fluoroalkyl substances (PFAS) are a family of unique eight-carbon fluorinated synthetic compounds. Their remarkable chemical stability, resistance to heat, water and oil repellence, and surfactant properties make them versatile compounds used in various industrial processes and consumer goods, including water-resistant fabrics, food packaging, and cleaning products (1-4). Consequently, PFAS has become a globally pervasive pollutant. Human exposure to PFAS occurs through multiple routes, such as drinking water, consumption of PFAS-contaminated fish and food, and dermal contact with products containing PFAS (5). These compounds have been detected in humans (4), wildlife (6, 7), and livestock (8, 9), with a half-life of approximately 3.5 years in human serum (10).
Despite efforts to reduce the use of PFAS, our understanding of their detrimental health effects, particularly in vulnerable populations, is rapidly evolving (5). Numerous studies have focused on assessing PFAS levels in pregnant women. Higher concentrations of PFAS have been linked with adverse pregnancy complications such as gestational diabetes, gestational hypertension, and preeclampsia (11). PFAS are also transferred across the placenta to the fetus and can be detected in human umbilical cord serum (12-14), placenta (15, 16), and fetal organs (17). Moreover, they are known to be transferred through breast milk (18). Exposure to PFAS during pregnancy or through breastfeeding has been associated with fetal growth restriction (19) and various adverse health effects in childhood, including obesity, immunosuppression, endocrine disorders, neurodevelopmental issues, and cardiovascular risks (5, 20-24). Although higher PFAS concentrations are consistently detected and linked with adverse maternal and fetal outcomes, why such higher PFAS levels are observed in these pregnancy complications is unclear.
Studies have shown that following exposure to PFOA, the most prevalent PFAS, male rats tend to have higher concentrations of organic fluorine in their liver and serum than female rats (25). Furthermore, sex-related variations in the toxicological effects of PFOA have been observed in rats, with females displaying greater resistance to PFOA toxicity than males (25, 26). This sex-related disparity can be attributed, at least in part, to the faster elimination of PFOA in females compared to males (25). Additionally, it has been demonstrated that testosterone exerts inhibitory effects on the renal excretion of PFOA in male rats (27). These findings suggest that sex hormones and PFOA elimination mechanisms play a role in the sex-specific response to PFOA toxicity.
Pregnant women experience sudden and dramatic changes in endocrine hormone levels. Alterations in the hormonal milieu, particularly increased maternal testosterone levels, have been linked with pregnancy complications. For example, plasma testosterone levels are elevated approximately 1.4- to 3.4-fold in pathological pregnancies such as preeclampsia (28). Despite the well-documented impact of sex-related changes in PFOA excretion and the existing circumstantial evidence of elevated maternal testosterone levels in preeclampsia, the influence of testosterone on PFOA elimination during pregnancy has not been studied. This study investigated the effects of elevated maternal testosterone levels on the elimination and tissue distribution of PFOA in pregnant rats. We hypothesized that hyperandrogenic conditions during pregnancy may hinder PFOA elimination and contribute to their higher body burden.
2. Materials and Methods
All the experiments were conducted as per National Institutes of Health guidelines (NIH Publication No. 85–23, revised 1996) with approval by the Institutional Animal Care and Use Committee at the University of Wisconsin at Madison (IACUC protocol V005847). Timed-pregnant Sprague-Dawley rats, purchased from Envigo Laboratories (Indianapolis, IN) on day 12 of gestation, were maintained under controlled conditions with a 12L:12D photoperiod in a temperature-regulated room (23°C). These rats were provided with unlimited access to food and water. On day 14 of gestation, the rats were divided into two groups: the control group received subcutaneous administration of vehicle (sesame oil), while the treatment group received subcutaneous injections of testosterone propionate (1 mg/kg) from Day 15 to Day 20 of gestation. The dosage and duration of testosterone administration were chosen to simulate the increased testosterone levels observed in pregnant women with preeclampsia (29).
On day 16 of gestation, 14C-PFOA (PerkinElmer, 99% purity, specific activity 5.44 mCi/mmol) was administered via the tail vein at a dose rate of 9.4 pmol/kg, diluted in phosphate-buffered saline to both control and testosterone-treated rats (n=6 in each group). The rats were housed in metabolic cages that were designed for the separate collection of urine and feces. Feces and urine and were collected daily for 4 consecutive days after 14C-PFOA administration. Blood samples were collected daily through the tail vein for 4 days. On day 20 of gestation (four days after 14C-PFOA administration), the rats were euthanized using CO2 asphyxiation. Maternal blood, liver, lung, kidney, heart, spleen, brain placentas (from both male and female fetuses), and fetal liver (from both male and female fetuses) were removed, weighed, and preserved in trichloroacetic acid (TCA). One half of the kidney was snap-frozen in liquid nitrogen for RNA isolation, while the other half was stored in TCA. The TCA-stored organ samples were homogenized, followed by centrifugation at 3000 g for 5 minutes at 4°C. The resulting supernatant was then suspended in bioscint-biosol biodegradable liquid scintillation solution (National Diagnostics, Atlanta, Georgia, USA). The quantification of 14C-PFOA radioactivity in all samples was performed using a Packard liquid scintillation analyzer (Model 2000CA), with quench correction executed by the Packard DPM 1-2-3 software program.
Total RNA from kidneys was isolated using the RNeasy mini kit (QIAGEN, Valencia, CA, USA) per the manufacturer's instructions. The RNA concentration and integrity were estimated by measuring the UV absorption at 260 nm using a Nano photometer (Implen, Inc. CA, USA). One µg of total RNA was reverse transcribed (iScript cDNA synthesis, Bio-Rad, Hercules, CA, USA). cDNA was diluted five times, corresponding to 200 ng of RNA, and amplified by qRT-PCR using a CFX 96 real-time thermal cycler (Bio-Rad, Hercules, CA, USA). Primer sequences were selected from the previously published article of Kudo et al. for organo-anion transporter genes Oat1, Oat2, Oat3, Oatp1, Oatp2, Oat-K, Gapdh and purchased from Integrated DNA Technologies (Coralville, IA, USA) (26). The primer sequence is specified in Table 1. Results were analyzed (2–ΔΔCT method) and expressed as fold change in the testosterone-treated vs. control group. All reactions were done in duplicates, and Gapdh was used as an internal control.
Table 1: Primer sequences used for qRT-PCR
|
Transporter |
Accession number |
Primer |
Sequence |
|
Oat1 (Slc22a6) |
AB004559 |
Forward |
5′-ACAAGCAAGGACAACCCGAA-3′ |
|
Reverse |
5′-AGACATAGCCAATCAAGGTGCC-3' |
||
|
Oat2 (Slc22a7) |
L27651 |
Forward |
5′-GCAGCCTCCATCAACTACATCA-3′ |
|
Reverse |
5′-GCGCACAAGGAAGTAGACCATA-3′ |
||
|
Oat3 (Slc22a8) |
AB017446 |
Forward |
5′-TGGAGGACCTGTGATTGGAGAA-3′ |
|
Reverse |
5′-ATAGAACCAGCCAGCGTATGGA-3′ |
||
|
Oatp1 (Slc21a1) |
L19031 |
Forward |
5′-CATGAGTGTACTTCTCTCTTGG-3′ |
|
Reverse |
5′-ATTCTGCTGGGTCTTGCGTTGG-3′ |
||
|
Oatp2 (Slc21a5) |
U95011 |
Forward |
5′-GCCTAAGTATCTGGAACAGCAA-3′ |
|
Reverse |
5′-CAGCGAGTATATGAAACAGCCA-3′ |
||
|
Oat-k (Slc21a4) |
AB012662 |
Forward |
5′-TCGCATTCTGCCTATCCTTGTC-3′ |
|
Reverse |
5′-GCCTTTATTACACAGCCCCAGG-3′ |
||
|
Gapdh |
M17701 |
Forward |
5′-GACCCCTTCATTGACCTCAACTACA-3′ |
|
Reverse |
5′-TGATGGCATGGACTGTGGTCATGAG-3’ |
Statistical analysis
Data analyses were performed using Prism software (GraphPad Prism 9.1), and results were expressed as mean ± SEM. Comparisons between the control and testosterone groups were performed using unpaired Student t-tests. P<0.05 was considered significant compared to control.
3. Results
Testosterone-treated dams exhibited plasma testosterone levels of 2.31 ± 0.26 ng/mL, whereas vehicle-treated control dams had 1.1 ± 0.22 ng/mL levels. Elevated maternal testosterone levels did not significantly alter the duration of gestation or the average litter size. However, on GD 20, fetal weights (control: 2.71 ± 0.07 g; testosterone-treated: 2.05 ± 0.12 g) and placental weights were significantly reduced (control: 0.52 ± 0.09 g; testosterone-treated: 0.41 ± 0.22 g) in the testosterone-treated group compared to the control group, corroborating findings from our prior studies (30).
PFOA Elimination
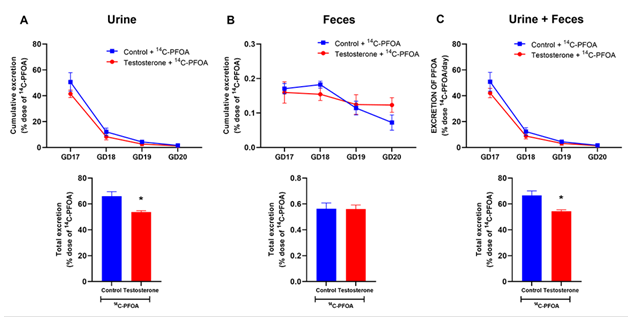
PFOA was primarily eliminated in the urine, with most eliminated within the first 24 hours, and only minimal amounts were detected after 4 days. In control pregnant rats, 65.98 ± 3.52% of the administered PFOA dose was excreted in the urine in 4 days. However, in the presence of elevated maternal testosterone levels, pregnant rats exhibited reduced cumulative urinary excretion of PFOA (53.69 ± 1.14%) compared to the controls (Figure 1A).
Fecal excretion of PFOA accounted for less than 0.6% of the total administered dose, and the cumulative percentage of PFOA eliminated through feces over 4 days remained similar in pregnant rats regardless of with and without elevated testosterone levels (Figure 1B).
The total elimination of PFOA from the body in a 4-day timeframe, considering both urine and feces, was significantly diminished by 12% of the administered dose in pregnant rats with elevated maternal testosterone levels (66.58 ± 3.55%) compared to controls (54.31 ± 1.19%) (Figure 1C).
Figure 1: Elimination of 14C-PFOA in urine and feces. Vehicle (blue color) and testosterone (red color) treated pregnant rats were administered with 14C-PFOA (9.4 µmol/kg, i.v.). Feces and urine were collected daily for 4 days. Daily elimination of 14C-PFOA from urine (A), feces (B), and urine + feces (C) are presented in the top panel. The cumulative percentage of 14C-PFOA eliminated through urine, feces, and urine + feces over 4 days is presented in the bottom panel. Data are presented in Mean ± SEM, n = 6 in each group. *P<0.05 compared to control.
Total accumulation of PFOA in the body
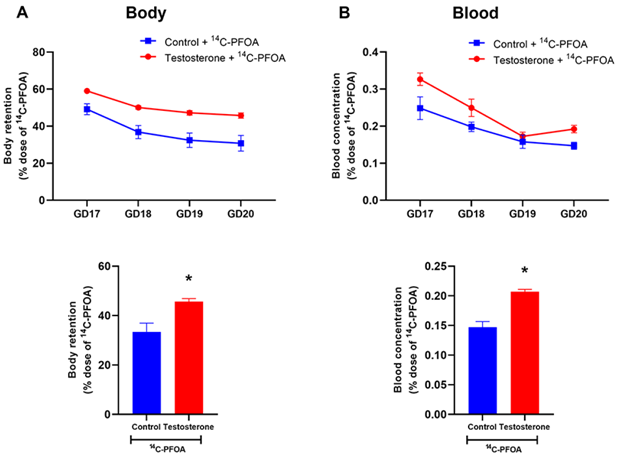
Figure 2A presents the total body burden (i.e., accumulation/retention within the body) of PFOA in pregnant rats, as determined by subtracting the cumulative percentage of the dose excreted in urine and feces from 100%. The total body burden through the 4 days remained higher in the pregnant rats with elevated testosterone than in controls. The total body burden of PFOA after the 4-day time period was 33.42 ± 3.55% in the controls, whereas, in the presence of elevated maternal testosterone levels, this burden significantly increased to 45.69 ± 1.19%.
As shown in Figure 2B, the pregnant rats with elevated testosterone levels displayed higher concentrations of PFOA in their blood and exhibited a slower elimination rate than controls.
Figure 2: Total body burden and blood concentration of 14C-PFOA. Vehicle (blue color) and testosterone (red color) treated pregnant rats were administered with 14C-PFOA, and blood was collected daily for 4 days. Daily total 14C-PFOA accumulation in rat body (A) and blood (B) are presented in the top panel. The bottom panel presents the body burden and blood concentration of 14C-PFOA after 4 days. Data are presented in Mean ± SEM, n = 6 in each group. *P<0.05 compared to control.
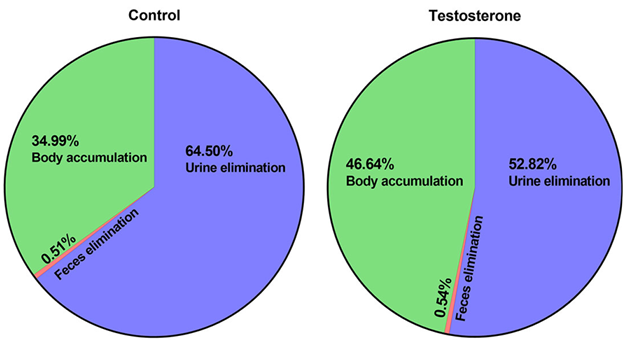
Collectively, the presence of elevated maternal testosterone levels decreased urinary elimination by 12% and increased body burden by 12% after 4 days of PFOA administration (Figure 3).
PFOA Tissue Concentrations
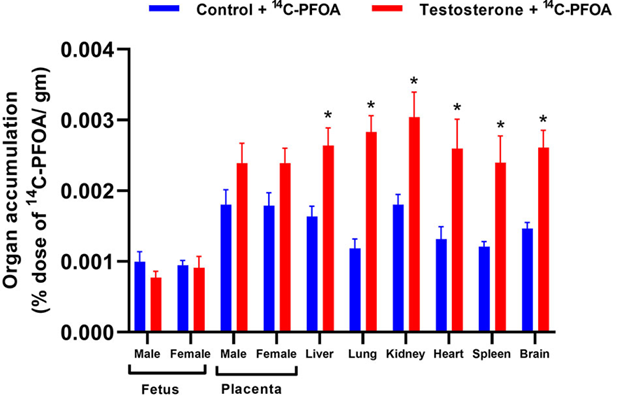
PFOA distribution in major tissues of pregnant rats was examined 4 days after PFOA administration. Among the controls, the placenta exhibited the highest concentration of PFOA, followed by the kidneys, liver, brain, heart, lungs, and spleen (Figure 4).
The pregnant rats with elevated testosterone levels displayed higher concentrations of PFOA in their kidneys, liver, brain, heart, lungs, and spleen compared to controls (Figure 4). There was a trend of increased PFOA concentration in male and female placentas of pregnant rats with elevated testosterone compared to their control counterparts. However, there were no significant differences in the PFOA concentrations in the male and female fetal livers between the control and testosterone groups.
Figure 4: Tissue distribution of 14C-PFOA in the organs. Vehicle (blue color) and testosterone (red color) treated pregnant rats were administered with 14C-PFOA, and organs were collected after 4 days. The total accumulation of 14C-PFOA in the organs is presented in Mean ± SEM, n = 6 in each group. *P<0.05 compared to respective controls.
Expression of organic anion transporters in the kidney
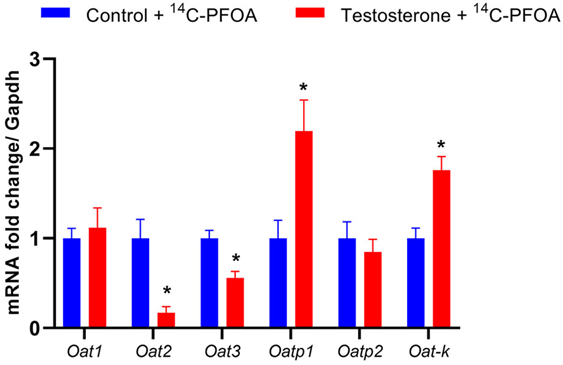
Since kidneys were the major route of PFOA excretion, we examined if elevated testosterone altered the mRNA expression of organic anion transmembrane transporter Oat1, Oat2, Oat3, and organic anion-transporting polypeptide Oatp1, Oatp2, Oat-k. The pregnant female rats with elevated testosterone levels showed significantly decreased Oat2 and Oat3 expression, while Oatp1 and Oat-K expression were significantly increased compared to controls (Figure 5). The levels of Oat1 and Oatp2 were not significantly different between the control and testosterone groups.
Figure 5: Changes in mRNA levels of organo-anion transporters in the kidney of control and testosterone-treated pregnant rats. Real-time PCR was used to assess renal organo-anion transporter mRNA expression. Quantitation of mRNA expression was normalized relative to Gapdh. n=5 in each group. *P<0.05 vs. compared to controls.
4. Discussion
The major findings of this study are: 1) The kidneys play a prominent role as the primary excretory pathway for PFOA in pregnant rats; 2) Elevated maternal testosterone levels, akin to those observed in preeclamptic pregnancies, decrease urinary elimination of PFOA, resulting in higher body accumulation; 3) decreased Oat2 and Oat3, along with increased Oatp1 and Oat-k, may contribute to the diminished renal elimination of PFOA in pregnant rats with elevated testosterone levels.
In late pregnancy, women typically have average plasma testosterone concentrations ranging from 1.0 to 1.5 ng/mL (31-33), and rats have testosterone levels of approximately 1.2 to 1.4 ng/mL (34-36). Pregnant women with preeclampsia, however, experience 1.4- to 3.4-fold higher testosterone levels (37-39). In our study, we observed a 2-fold increase in maternal plasma testosterone levels in pregnant rats, mimicking the magnitude of testosterone elevation reported in preeclamptic pregnancies. This elevation in maternal testosterone was associated with fetal growth restriction, consistent with previous findings in rats (40) and sheep (41, 42). Therefore, investigating the impact of elevated maternal testosterone levels on PFOA elimination becomes crucial, given that PFAS remains a health hazard to vulnerable pregnant populations and their offspring.
Studies show that PFOA accumulates significantly in the human body, with an estimated half-life of 3.5 years (10). This suggests that prolonged exposure to this chemical in industrial settings may lead to its accumulation in humans, potentially causing harmful effects within biological systems. PFOA does not undergo metabolic processes within the body and is excreted in urine and feces as free carboxylic acid (25, 43, 44). Consequently, the elimination of PFOA through excretion plays a crucial role in its detoxification. Previous research has demonstrated that rapid urinary elimination of PFOA results in low hepatic concentrations of PFOA (45). Additionally, a close correlation between PFOA concentrations and the activity of peroxisomal β-oxidation in rat livers has been observed (46). These findings imply that the elimination of PFOA is pivotal in determining its biological effects in rats.
In the present study on pregnant rats, the elimination of PFOA through urine is rapid, with approximately 40% of the administered dose being eliminated within the first 24 hours. An important recent finding is that elevated maternal testosterone levels, similar to those observed in pregnant women with preeclampsia, result in reduced renal excretion of PFOA in pregnant rats. Interestingly, the amount of PFOA eliminated through feces was found to be the same in pregnant rats, regardless of whether they had elevated maternal testosterone levels or not. Therefore, the disparity in the overall elimination of PFOA from the body between pregnant rats with and without elevated testosterone levels can be attributed to differences in the kidney's excretory function for PFOA. These findings align with previous studies conducted on nonpregnant females, which indicated that the urinary excretion of PFOA is the primary route of PFOA elimination (26).
The present study shows that PFOA was widely distributed in many organs. The finding that levels of PFOA in the placenta, kidney, and liver are significantly elevated compared to tissues in healthy pregnant rats suggests that organs of higher blood flow and metabolic activity appear to accumulate more significant concentrations of PFOA. This is similar to the reports in humans where PFAS has higher accumulation in the kidney, lungs, liver, and brain (47). Also, the placenta is reported as a target organ for PFAS toxicity (17, 48, 49). Interestingly, within the placenta, PFOA was shown to have preferential accumulation in the villi than decidual tissues (50). The report that in vitro exposure of trophoblasts to PFOA leads to their intracellular accumulation further emphasizes the notion that PFOA has the potential to get accumulated within the cells of specific organs. The reason for their specificity to certain organs and the mechanism that facilitates their intracellular accumulation is unclear. PFAS is known to have a binding affinity to protein (51). Whether PFAS binds to cytoplasmic proteins or if they accumulate preferentially within specific intracellular organelles needs to be further investigated. Nevertheless, the current study provides new evidence that elevated testosterone levels in pregnant rats enhance the ability of certain organs to accumulate higher concentrations of PFOA compared to normal pregnant rats. These findings indicate that elevated maternal testosterone levels could amplify the overall presence of PFOA in the body, subjecting the tissues to substantially higher concentrations than in control cases.
The renal processes of reabsorption and elimination of naturally occurring and foreign organic anions in mammals are facilitated by the multispecific OATs located in the apical or basolateral membrane of epithelial cells (52). Histochemical analyses have revealed the presence of the OAT family of transporters in the basolateral membranes of tubular cells, responsible for the basolateral uptake of organic anions from the bloodstream into tubular cells (53). Interestingly, the expression of Oat2 and Oat3 in the kidney was significantly reduced by testosterone, indicating a potential decrease in the secretion of PFOA through these transporters in pregnant rats with elevated testosterone levels. Consistent with this finding, previous studies have demonstrated that testosterone directly downregulates Oat1 and Oat3 through androgen receptor-mediated transcriptional pathways (54, 55). Furthermore, both human and rat Oat1 and Oat3 are shown to exhibit high affinities for PFOA and facilitate its transport through the basolateral membrane of proximal tubular cells in vivo (56). On the other hand, Oatp1 and Oat-k, which are expressed in the brush border membrane, play a role in the reabsorption of organic anions (53, 57). In the present study, testosterone significantly increased the expression of oatp1 and Oat-k. This indicates that pregnant rats with elevated testosterone levels have a greater potential for reabsorption of PFOA. Collectively, these findings indicate that Oat1 and Oat3 may be involved in the renal secretion of PFOA, while Oatp1a1 and Oat-k could contribute to its reabsorption, similar to what has been reported for perfluorinated carboxylates (58). Thus, the observed regulatory effect on the expression of PFOA transporters in the kidneys could explain the higher accumulation and reduced elimination of PFOA in pregnant rats with elevated testosterone levels. However, further investigations involving the specific overexpression or knockdown of these transporters are necessary to confirm their precise involvement in PFOA elimination.
Perspectives
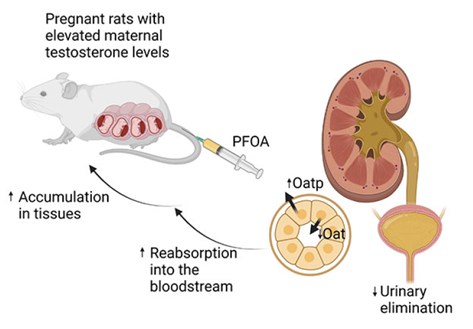
Previous research indicated elevated maternal testosterone levels induce preeclampsia-like manifestations, including gestational hypertension and feto-placental growth restriction. This study uncovers an additional consequence of elevated maternal testosterone levels—an augmented PFOA accumulation within the body, potentially due to impeded urinary excretion (Figure 6). Since PFOA is shown to induce gestational hypertension, placental dysfunction and restrict fetal growth, the presence of elevated maternal testosterone levels may amplify adverse maternal outcomes by induced PFOA accumulation. Intriguingly, investigating whether testosterone amplifies the accumulation of other environmental chemicals and exacerbates detrimental maternal health effects holds promise for future exploration.
Acknowledgment:
This study was funded by National Institutes of Health (NIH) grants (R01HL134779 and R01ES033345 to SK)
Conflicts of interest:
None
References
- Domingo JL, Nadal M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ Res 177 (2019): 108648.
- Olsen GW, Mair DC, Lange CC, et al. Per- and polyfluoroalkyl substances (PFAS) in American Red Cross adult blood donors, 2000-2015. Environ Res 157 (2017): 87-95.
- Hanssen L, Dudarev AA, Huber S, et al. Partition of perfluoroalkyl substances (PFASs) in whole blood and plasma, assessed in maternal and umbilical cord samples from inhabitants of arctic Russia and Uzbekistan. Sci Total Environ 447 (2013): 430-437.
- Calafat AM, Kuklenyik Z, Reidy JA, et al. Serum concentrations of 11 polyfluoroalkyl compounds in the u.s. population: data from the national health and nutrition examination survey (NHANES). Environ Sci Technol 41 (2007): 2237-2242.
- Sunderland EM, Hu XC, Dassuncao C, et al. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29 (2019): 131-147.
- Houde M, De Silva AO, Muir DC, et al. Monitoring of perfluorinated compounds in aquatic biota: an updated review. Environ Sci Technol 45 (2011): 7962-7973.
- Houde M, Martin JW, Letcher RJ, et al. Biological monitoring of polyfluoroalkyl substances: A review. Environ Sci Technol 40 (2006): 3463-3473.
- Zafeiraki E, Vassiliadou I, Costopoulou D, et al. Perfluoroalkylated substances in edible livers of farm animals, including depuration behaviour in young sheep fed with contaminated grass. Chemosphere 156 (2016): 280-285.
- Barbarossa A, Gazzotti T, Zironi E, et al. Short communication: Monitoring the presence of perfluoroalkyl substances in Italian cow milk. J Dairy Sci 97 (2014): 3339-43.
- Olsen GW, Burris JM, Ehresman DJ, et al. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115 (2007): 1298-1305.
- Groisman L, Berman T, Quinn A, et al. Levels of PFAS concentrations in the placenta and pregnancy complications. Ecotoxicol Environ Saf 262 (2023): 115165.
- Li Y, Yu N, Du L, et al. Transplacental Transfer of Per- and Polyfluoroalkyl Substances Identified in Paired Maternal and Cord Sera Using Suspect and Nontarget Screening. Environ Sci Technol 54 (2020): 3407-3416.
- Pan Y, Zhu Y, Zheng T, et al. Novel Chlorinated Polyfluorinated Ether Sulfonates and Legacy Per-/Polyfluoroalkyl Substances: Placental Transfer and Relationship with Serum Albumin and Glomerular Filtration Rate. Environ Sci Technol 51 (2017): 634-644.
- Cariou R, Veyrand B, Yamada A, et al. Perfluoroalkyl acid (PFAA) levels and profiles in breast milk, maternal and cord serum of French women and their newborns. Environ Int 84 (2015): 71-81.
- Martin J, Rodriguez-Gomez R, Zafra-Gomez A, et al. Validated method for the determination of perfluorinated compounds in placental tissue samples based on a simple extraction procedure followed by ultra-high performance liquid chromatography-tandem mass spectrometry analysis. Talanta 150 (2016): 169-176.
- Zhang T, Sun H, Lin Y, et al. Distribution of poly- and perfluoroalkyl substances in matched samples from pregnant women and carbon chain length related maternal transfer. Environ Sci Technol 47 (2013): 7974-81.
- Mamsen LS, Bjorvang RD, Mucs D, et al. Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ Int 124 (2019): 482-492.
- Chain EPoCitF, Schrenk D, Bignami M, et al. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J 18 (2020): 06223.
- Manea S, Salmaso L, Lorenzoni G, et al. Exposure to PFAS and small for gestational age new-borns: A birth records study in Veneto Region (Italy). Environ Res 184 (2020): 109282.
- Luo D, Wu W, Pan Y, et al. Associations of Prenatal Exposure to Per- and Polyfluoroalkyl Substances with the Neonatal Birth Size and Hormones in the Growth Hormone/Insulin-Like Growth Factor Axis. Environ Sci Technol 55(2021): 11859-11873.
- Birru RL, Liang HW, Farooq F, et al. A pathway level analysis of PFAS exposure and risk of gestational diabetes mellitus. Environ Health 20 (2021): 63.
- Ou Y, Zeng X, Lin S, et al. Gestational exposure to perfluoroalkyl substances and congenital heart defects: A nested case-control pilot study. Environ Int 154 (2021): 106567.
- Starling AP, Liu C, Shen G, et al. Prenatal Exposure to Per- and Polyfluoroalkyl Substances, Umbilical Cord Blood DNA Methylation, and Cardio-Metabolic Indicators in Newborns: The Healthy Start Study. Environ Health Perspect 128 (2020): 127014.
- Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol 13 (2017): 161-173.
- Vanden Heuvel JP, Kuslikis BI, Van Rafelghem MJ, et Tissue distribution, metabolism, and elimination of perfluorooctanoic acid in male and female rats. J Biochem Toxicol 6 (1991): 83-92.
- Kudo N, Katakura M, Sato Y, et al. Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem Biol Interact 139 (2002): 301-316.
- Vanden Heuvel JP, Davis JW, Sommers R, et al. Renal excretion of perfluorooctanoic acid in male rats: inhibitory effect of testosterone. J Biochem Toxicol 7 (1992): 31-46.
- Kumar S, Gordon GH, Abbott DH, et al. Androgens in maternal vascular and placental function: implications for preeclampsia pathogenesis. Reproduction 156 (2018): 155-167.
- Varlamov O, Bethea CL, Roberts CT, et al. Sex-specific differences in lipid and glucose metabolism. Front Endocrinol (Lausanne) 5 (2014): 241.
- Mishra JS, Blesson CS, Kumar S. Testosterone Decreases Placental Mitochondrial Content and Cellular Bioenergetics. Biology (Basel) 9 (2020).
- Saez JM, Forest MG, Morera AM, et al. Metabolic clearance rate and blood production rate of testosterone and dihydrotestosterone in normal subjects, during pregnancy, and in hyperthyroidism. J Clin Invest 51 (1972): 1226-1234.
- Mizuno M, Lobotsky J, Lloyd CW, et al. Plasma androstenedione and testerone during pregnancy and in the newborn. J Clin Endocrinol Metab 28 (1968): 1133-1142.
- Rivarola MA, Forest MG, Migeon CJ. Testosterone, androstenedione and dehydroepiandrosterone in plasma during pregnancy and at delivery: concentration and protein binding. J Clin Endocrinol Metab 28 (1968): 34-40.
- Chinnathambi V, Balakrishnan M, Ramadoss J, et al. Testosterone alters maternal vascular adaptations: role of the endothelial NO system. Hypertension 61 (2013): 647-654.
- Sathishkumar K, Elkins R, Chinnathambi V, et al. Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reprod Biol Endocrinol 9 (2011): 110.
- Sathishkumar K, Elkins R, Yallampalli U, et al. Fetal programming of adult hypertension in female rat offspring exposed to androgens in utero. Early Hum Dev 87 (2011): 407-414.
- Ghorashi V, Sheikhvatan M. The relationship between serum concentration of free testosterone and preeclampsia. Endokrynol Pol 59 (2008): 390-312.
- Salamalekis E, Bakas P, Vitoratos N, et al. Androgen levels in the third trimester of pregnancy in patients with preeclampsia. Eur J Obstet Gynecol Reprod Biol 126 (2016): 16-19.
- Acromite MT, Mantzoros CS, Leach RE, et al. Androgens in preeclampsia. Am J Obstet Gynecol 180 (1999): 60-73.
- Chinnathambi V, Blesson CS, Vincent KL, et al. Elevated testosterone levels during rat pregnancy cause hypersensitivity to angiotensin II and attenuation of endothelium-dependent vasodilation in uterine arteries. Hypertension 64 (2014): 405-414.
- Steckler T, Wang J, Bartol FF, et al. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology 146 (2005): 3185-3193.
- Manikkam M, Crespi EJ, Doop DD, et al. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology 145 (2004): 790-798.
- Kuslikis BI, Vanden Heuvel JP, Peterson RE. Lack of evidence for perfluorodecanoyl- or perfluorooctanoyl-coenzyme A formation in male and female rats. J Biochem Toxicol 7 (1992): 25-39.
- Ophaug RH, Singer L. Metabolic Handling of perfluorooctanoic acid in rats. Proc Soc Exp Biol Med 163 (1980): 19-23.
- Kudo N, Suzuki E, Katakura M, et al. Comparison of the elimination between perfluorinated fatty acids with different carbon chain length in rats. Chem Biol Interact 134 (2001): 203-216.
- Kudo N, Bandai N, Suzuki E, et al. Induction by perfluorinated fatty acids with different carbon chain length of peroxisomal beta-oxidation in the liver of rats. Chem Biol Interact 124 (2000): 119-132.
- Perez F, Nadal M, Navarro-Ortega A, et al. Accumulation of perfluoroalkyl substances in human tissues. Environ Int 59 (2013): 354-362.
- Hutcheon JA, McNamara H, Platt RW, et al. Placental weight for gestational age and adverse perinatal outcomes. Obstet Gynecol 119 (2012): 1251-1258.
- Hall SM, Zhang S, Hoffman K, et al. Concentrations of per- and polyfluoroalkyl substances (PFAS) in human placental tissues and associations with birth outcomes. Chemosphere 295 (2022): 133873.
- Pascali JP, Piva E, Bonasoni MP, et al. Analysis and distribution of per- and polyfluoroalkyl substances in decidua and villi placenta explants. Environ Res 229 (2023): 115955.
- Qin W, Henneberger L, Huchthausen J, et al. Role of bioavailability and protein binding of four anionic perfluoroalkyl substances in cell-based bioassays for quantitative in vitro to in vivo extrapolations. Environ Int 173 (2023): 107857.
- Emami Riedmaier A, Nies AT, Schaeffeler E, et al. Organic anion transporters and their implications in pharmacotherapy. Pharmacol Rev 64 (2012): 421-449.
- Inui KI, Masuda S, Saito H. Cellular and molecular aspects of drug transport in the kidney. Kidney Int 58 (2000): 944-958.
- Ljubojevic M, Balen D, Breljak D, et al. Renal expression of organic anion transporter OAT2 in rats and mice is regulated by sex hormones. Am J Physiol Renal Physiol 292 (2007): 361-372.
- Ljubojevic M, Herak-Kramberger CM, Hagos Y, et al. Rat renal cortical OAT1 and OAT3 exhibit gender differences determined by both androgen stimulation and estrogen inhibition. Am J Physiol Renal Physiol 287 (2004): 124-138.
- Nakagawa H, Hirata T, Terada T, et al. Roles of organic anion transporters in the renal excretion of perfluorooctanoic acid. Basic Clin Pharmacol Toxicol 103 (2008): 1-8.
- Bergwerk AJ, Shi X, Ford AC, et al. Immunologic distribution of an organic anion transport protein in rat liver and kidney. Am J Physiol 271 (1996): 231-238.
- Weaver YM, Ehresman DJ, Butenhoff JL, et al. Roles of rat renal organic anion transporters in transporting perfluorinated carboxylates with different chain lengths. Toxicol Sci 113 (2010): 305-314.