Effects of Extruded and Conventional Sorghum Flour on Postprandial Plasma Amino Acid and Glucose Patterns in Adult Men
Article Information
Prae Charoenwoodhipong1, Xiang Li1, Nasim Hedayati2, Roberta R Holt1, Carl L Keen1,3, Robert M Hackman1*
1Department of Nutrition, University of California Davis, Davis, CA 95616 USA
2Department of Surgery, University of California Davis, Sacramento, CA, 95817 USA
3Department of Internal Medicine, University of California Davis, Sacramento, CA 95817 USA
*Corresponding Author: Robert M Hackman, PhD, Department of Nutrition, University of California, Davis, One Shields Avenue, 3135 Meyer Hall, Davis, CA 95616 USA
Received: 28 May 2021; Accepted: 05 June 2021; Published: 18 August 2021
Citation:
Prae Charoenwoodhipong, Xiang Li, Nasim Hedayati, Roberta R Holt, Carl L Keen, Robert M Hackman. Effects of Extruded and Conventional Sorghum Flour on Postprandial Plasma Amino Acid and Glucose Patterns in Adult Men. Journal of Food Science and Nutrition Research 4 (2021): 213-226.
Share at FacebookAbstract
Sorghum is a nutrient-rich grain shown to improve growth and alleviate malnutrition in clinical studies; however, starch-protein interactions can limit its protein digestibility. Extrusion can help to improve protein availability from some foods. Three probe feeding studies were conducted to assess amino acid availability from extruded sorghum flour using postprandial plasma amino acid concentrations. For each study, a randomized crossover design with a one-week washout period was used to determine responses in healthy men aged 21-34 yr following intake of either extruded (EX) or conventional (CON) sorghum flour. In probe 1 (P1) and probe 2 (P2), men consumed 34 g (n=2) or 68 g (n=3) of flour, with plasma amino acid concentrations determined every 30 min for 180 min. A third probe (P3) provided 68 g (n=4) of flour, and samples for both plasma amino acids and glucose were collected every 15 min for 90 min. Responses were calculated as both the area-under-the-curve (AUC) and the incremental AUC (iAUC). In all three probes, amino acid responses were similar between the flours. The plasma glucose AUC was significantly greater from EX compared to CON, but the iAUCs between them were not significantly different. In these initial probe trials, a small sample size, along with individual variability in responses may explain the lack of differences in patterns of postprandial amino acids. Additional research on extrusion techniques and response measures is warranted.
Keywords
Sorghum, Extrusion, Plasma amino acids, Protein availability, Plasma glucose, Gluten-free
Sorghum articles; Extrusion articles; Plasma amino acids articles; Protein availability articles; Plasma glucose articles; Gluten-free articles
Sorghum articles Sorghum Research articles Sorghum review articles Sorghum PubMed articles Sorghum PubMed Central articles Sorghum 2023 articles Sorghum 2024 articles Sorghum Scopus articles Sorghum impact factor journals Sorghum Scopus journals Sorghum PubMed journals Sorghum medical journals Sorghum free journals Sorghum best journals Sorghum top journals Sorghum free medical journals Sorghum famous journals Sorghum Google Scholar indexed journals Extrusion articles Extrusion Research articles Extrusion review articles Extrusion PubMed articles Extrusion PubMed Central articles Extrusion 2023 articles Extrusion 2024 articles Extrusion Scopus articles Extrusion impact factor journals Extrusion Scopus journals Extrusion PubMed journals Extrusion medical journals Extrusion free journals Extrusion best journals Extrusion top journals Extrusion free medical journals Extrusion famous journals Extrusion Google Scholar indexed journals Plasma amino acids articles Plasma amino acids Research articles Plasma amino acids review articles Plasma amino acids PubMed articles Plasma amino acids PubMed Central articles Plasma amino acids 2023 articles Plasma amino acids 2024 articles Plasma amino acids Scopus articles Plasma amino acids impact factor journals Plasma amino acids Scopus journals Plasma amino acids PubMed journals Plasma amino acids medical journals Plasma amino acids free journals Plasma amino acids best journals Plasma amino acids top journals Plasma amino acids free medical journals Plasma amino acids famous journals Plasma amino acids Google Scholar indexed journals Protein availability articles Protein availability Research articles Protein availability review articles Protein availability PubMed articles Protein availability PubMed Central articles Protein availability 2023 articles Protein availability 2024 articles Protein availability Scopus articles Protein availability impact factor journals Protein availability Scopus journals Protein availability PubMed journals Protein availability medical journals Protein availability free journals Protein availability best journals Protein availability top journals Protein availability free medical journals Protein availability famous journals Protein availability Google Scholar indexed journals Plasma glucose articles Plasma glucose Research articles Plasma glucose review articles Plasma glucose PubMed articles Plasma glucose PubMed Central articles Plasma glucose 2023 articles Plasma glucose 2024 articles Plasma glucose Scopus articles Plasma glucose impact factor journals Plasma glucose Scopus journals Plasma glucose PubMed journals Plasma glucose medical journals Plasma glucose free journals Plasma glucose best journals Plasma glucose top journals Plasma glucose free medical journals Plasma glucose famous journals Plasma glucose Google Scholar indexed journals Gluten-free articles Gluten-free Research articles Gluten-free review articles Gluten-free PubMed articles Gluten-free PubMed Central articles Gluten-free 2023 articles Gluten-free 2024 articles Gluten-free Scopus articles Gluten-free impact factor journals Gluten-free Scopus journals Gluten-free PubMed journals Gluten-free medical journals Gluten-free free journals Gluten-free best journals Gluten-free top journals Gluten-free free medical journals Gluten-free famous journals Gluten-free Google Scholar indexed journals peanut articles peanut Research articles peanut review articles peanut PubMed articles peanut PubMed Central articles peanut 2023 articles peanut 2024 articles peanut Scopus articles peanut impact factor journals peanut Scopus journals peanut PubMed journals peanut medical journals peanut free journals peanut best journals peanut top journals peanut free medical journals peanut famous journals peanut Google Scholar indexed journals barley articles barley Research articles barley review articles barley PubMed articles barley PubMed Central articles barley 2023 articles barley 2024 articles barley Scopus articles barley impact factor journals barley Scopus journals barley PubMed journals barley medical journals barley free journals barley best journals barley top journals barley free medical journals barley famous journals barley Google Scholar indexed journals porridge articles porridge Research articles porridge review articles porridge PubMed articles porridge PubMed Central articles porridge 2023 articles porridge 2024 articles porridge Scopus articles porridge impact factor journals porridge Scopus journals porridge PubMed journals porridge medical journals porridge free journals porridge best journals porridge top journals porridge free medical journals porridge famous journals porridge Google Scholar indexed journals multivitamin articles multivitamin Research articles multivitamin review articles multivitamin PubMed articles multivitamin PubMed Central articles multivitamin 2023 articles multivitamin 2024 articles multivitamin Scopus articles multivitamin impact factor journals multivitamin Scopus journals multivitamin PubMed journals multivitamin medical journals multivitamin free journals multivitamin best journals multivitamin top journals multivitamin free medical journals multivitamin famous journals multivitamin Google Scholar indexed journals
Article Details
1. Introduction
Sorghum is currently the fifth most consumed grain in the world [1], being consumed in particularly high amounts in Africa, Asia, and South America, where the crop is a staple food and can be considered a sustainable source of protein [2,3]. On a weight basis, the grain contains an average of 70% carbohydrates, 11% protein, 11% fiber, 3% fat, a variety of vitamins and minerals and is gluten-free [4,5]. This nutrient-rich grain has been used as one of the primary ingredients in complementary foods for dietary interventions with infants and toddlers suffering from acute malnutrition [6-10]. Collectively, these studies report that a child’s recovery rate after consuming sorghum-based products has comparable responses to that of milk- or peanut-based products and show a better growth response compared to the intake of control foods such as a maize-soy mixture or rice [6-10]. However, the protein digestibility of sorghum may limit its use as a primary dietary protein source. Illustrative of this, 26 Peruvian children, six to 30 months of age, were given 6.4% protein as whole grain sorghum (11-15.63 g sorghum/100kcal) or a similar amount of protein from casein (1.86 g Casec® /100 kcal ) for a 59-day period [11]. When the children consumed the sorghum diet, they had significantly lower nitrogen absorption (46%) and retention (14%) compared to when they ingested the casein diet (81% nitrogen absorption and 38% retention) [11]. The majority of proteins in grains are found in a storage form, with prolamins and glutelins predominating [12]. Gluten is the main storage protein in wheat, rye, and barley and is responsible for triggering hypersensitivities that can result in celiac disease [12]. Sorghum is devoid of gluten and instead contains kafirin proteins that are rich in the amino acids proline and glutamine [13]. However, kafirin proteins can bind tighly to the surrounding carbohydrate and polyphenol matrices, limiting the availability of amino acids during digestion from sorghum [14,15]. Food technology has been used to improve the protein digestibility of sorghum, including malting, fermentation, and extrusion, resulting in novel products [16-18]. Extrusion is a method of food processing that employs temperature, pressure, and shear stress, and is useful in improving the protein digestibility of sorghum, as noted from in vitro models [19-21]. In humans, 27 days of daily extruded sorghum intake in Peruvian infants and toddlers showed nitrogen absorption and retention values similar to those seen for casein (81% and 21% for extruded sorghum and 84% and 27% for casein, respectively) [22]. While promising, no washout period between the two interventions was used in the above study, so residual carry-over effects between treatments could not be determined, which may have confounded the results. In addition to protein considerations, sorghum has been reported to improve glucose control. Two studies in well-nourished adults reported that sorghum intake modulated the postprandial blood glucose response and aided in weight loss more effectively than wheat [23,24]. A crossover study among overweight men noted that extruded sorghum intake (40 g/d) for two eight-week periods was significantly associated with reduced body fat compared to consumption of calorie-matched extruded wheat (38 g/d) [25]. Another crossover study that assessed the response to muffins made with either whole wheat or conventional sorghum flours (50 g each) reported that lower mean glucose and insulin responses were observed over a 180-minute period following ingestion of the sorghum, but not the whole wheat product [24]. To our knowledge, no published studies have compared the effects of extruded versus non-extruded sorghum on blood glucose concentrations. The primary aim of the current study was to determine if ingestion of extruded sorghum flour could increase protein availability to a greater extent than flour produced from a conventional milling process. Plasma amino acid levels were used as markers of protein availability [26]. The second aim of this study was to compare the postprandial blood glucose responses from the two sorghum flours. Three probe studies were conducted, with the blood glucose concentrations being measured only in the third probe. The conventionally milled sorghum was not cooked as porridge like in previous studies, as many in vitro reports note that wet cooking decreases digestibility and absorption of sorghum, which could potentially affect the availability of the protein [14,15,27-29].
2. Methods
2.1 Study participants
Healthy men, 21 to 50 years old, were recruited through flyers at the University of California, Davis (UC Davis) and via the Department of Nutrition website. Before enrollment, all volunteers were interviewed by telephone. Inclusion criteria required a body mass index between 18.5 and 36 kg/m2 and weights greater than 49.9 kg (110 pounds). Exclusion criteria included fruit consumption greater than two cups per day, vegetable consumption greater than three cups per day, fatty fish intake greater than three servings per week, coffee or tea intake greater than three cups per day, dark chocolate intake higher than 75 g (three ounces)/day, consumption of a non-traditional diet (e.g., vegetarian, vegan, gluten-free, intermittent fasting), alcohol intake of more than three drinks/week (one drink defined as one bottle of beer, one glass of wine, or one shot of distilled spirits), and dislike of, or allergy to, sorghum. Additional exclusion factors included self-reports for use of daily anticoagulation agents including aspirin or other non-steroidal anti-inflammatory drugs, restriction of physical activity due to a chronic health condition, routine high-intensity exercise, diabetes, blood pressure ≥ 140/90 mm Hg, renal or liver disease, heart disease (including cardiovascular events and stroke), malabsorption or cancer within the past five years, currently taking prescription drugs except for a stable amount of thyroid medication for at least six months, use of a multivitamin and mineral supplement other than a general formula providing up to a maximum of 100% of the US Daily Value, use of botanical or oil supplements within one month prior to study enrollment, indications of substance or alcohol abuse within the last three years, or current participation in another clinical research study. Volunteers were excluded if they had abnormal liver values outside of the reference range from a comprehensive metabolic panel (CMP), if determined to be clinically significant by the study physician.
2.2 Study designs
Three probe studies were conducted using randomized, two-treatment crossover designs to investigate the postprandial plasma amino acid and glucose patterns, as well as short-term safety and tolerability of extruded and non-extruded sorghum flour consumed under fasting conditions. Each probe employed either varying amounts of sorghum flour or assessed amino acid levels over different time courses. A one-week washout was included in all designs. Extruded (EX) and non-extruded conventional (CON) sorghum flours were produced in a licensed food-grade facility (GHL International, Cedarberg, WI). The microbial and heavy metal concentrations were below acceptable upper limits. Extrusion processed the sorghum at 12-14% water content with no additional water added, and used a single screw and pressure >1,000 PSI [30]. The final product was 4-10% water and became water soluble at <60 degree Celsius [30]. For probe one (P1), two participants consumed 34 g (0.25 cup) of each sorghum flour, with plasma amino acid levels assessed at baseline and every 30 minutes for 180 minutes. For probe two (P2), three participants consumed 68 g (0.5 cup) of each flour, and plasma amino acid levels were assessed every 30 minutes for 180 minutes. For probe three (P3), four participants consumed 68 g (0.5 cup) of each flour, and plasma amino acid levels were assessed every 15 minutes for 90 minutes. For P3, a CMP was also collected. Since proline and glutamic acid are major amino acids in the kafirin proteins in sorghum, the plasma levels of these two amino acids were of particular interest and were used as primary indicator amino acids. Additionally, essential amino acids were measured. For each probe, the final amount of flour was based on a 70-kg male as the standard and then adjusted according to each participant’s metabolic size, (body weight in kilograms to the three quarter power [kg3/4]).
2.3 Study Procedures
The intervention was conducted at the Ragle Human Nutrition Research Center, Department of Nutrition on the UC Davis campus. The protocol, forms, and advertisements were approved by the UC Davis Institution Review Board and all participants provided written informed consent prior to entry. At each study visit, participants arrived at the facility after an overnight 12-hour fast. Anthropometric data (height, weight, body mass index) was collected, seated blood pressure was measured, and baseline blood collection was performed by a registered nurse using an indwelling catheter placed in the antecubital vein. Participants then consumed a mixture of the extruded or non-extruded sorghum flour along with 237 ml (one cup) of bottled water. Serial blood samples were collected at the time points described above. Blood samples were centrifuged at 4oC for 15 minutes at 3,500 g. The resultant plasma was combined with 6% sulfosalicylic acid (1:1) for deproteinization and the mixture was centrifuged at 16,100 g for 25 minutes. The supernate was processed through a 0.45 mm syringe drive PTFE filter and the final solution was analyzed with a Biochrom 30 amino acid analyzer at the Amino Acid Laboratory, UC Davis School of Veterinary Medicine. The CMP analysis, including blood glucose, sodium, potassium, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and other liver and kidney markers was performed at the UC Davis Department of Pathology and Laboratory Medicine.
3. Statistical Analysis
Data were calculated as the area-under-the-curve (AUC) and incremental AUC (iAUC) and are presented as their mean and standard deviation (SD). The AUC values were calculated from the plasma amino acid levels over the time course of the assessment by summing the trapezoid area between each time point on the absorption curve. The iAUC was calculated by considering only the area in which the plasma concentration was higher than the baseline value for each trapezoid between the time points on the absorption curve. Evaluating the AUC alone could overestimate the actual responses since the plasma concentration from some participants went below their baseline levels following the flour intake. As the baseline values may be varied among the participants, the mean baseline was used to check for the differences at baseline and reported as mean(SD). Additionally, the maximum concentration (Cmax) was determined for plasma glucose. The RStudio statistical package version 4.0.3 (RStudio, Boston, Massachusetts, USA) was used to calculate the AUC and iAUC values for individual plasma amino acids and glucose. SigmaPlot version 14.0 (Systat Software, Inc., San Jose, California, USA) was used to perform statistical analyses including paired t-tests to assess the differences in AUC and iAUC of plasma amino acids between EX and CON in P1, P2, and P3, and to assess the differences in the AUC and iAUC of plasma glucose in P3. The Wilcoxon signed rank test was used for data that was not normally distributed.
4. Results
Complete plasma amino acid data was obtained from eight participants and complete CMP data was obtained from four participants in probe 3. Data from one participant was removed from the amino acid calculations due to analytical errors, but was included in the glucose analysis. The participant demographics are shown in Table 1. No adverse events were reported.
|
Demographics |
mean(SD; range) |
|
Age (years) |
28(4; 22-34) |
|
Weight (kg) |
77(20; 61.5-115.8) |
|
Height (cm) |
174(8; 163.1-180.4) |
|
BMI* (kg/m2) |
25(5; 20.7-35.5) |
|
Race |
N (%) |
|
White |
4 (50) |
|
Asian |
3 (37.5) |
|
African/Black |
1 (12.5) |
Table 1: Participant characteristics, n=8
4.1 Plasma amino acids
There were no significant differences for any of the measured plasma amino acids at baseline for all three studies. In P1, P2, and P3, all AUCs were similar between EX and CON [Tables 2-4]. In P2, the baseline level of proline was significantly higher in the CON group than the EX group (p=0.004), which was reflected in a trend of higher AUC of CON relative to EX (p=0.073) [Table 3]. For a number of amino acids, iAUC plasma levels were not detectable with the intake of 34 g of either flour (Table 2). This includes the primary indicator amino acid proline. Apart from tryptophan in P2, all plasma amino acids achieved detectable levels after 68 g of flour intake. However, even with 68 g of flour intake, the iAUCs of EX and CON were not significantly different.
|
Amino acids |
Calculation Method |
CON Mean(SD) |
EX Mean(SD) |
p-value (two-tailed) |
|
Primary indicator AAs |
||||
|
Proline |
Baseline |
252 (49) |
240 (70) |
0.57 |
|
AUC |
38,153(15,220) |
37,440(10,607) |
0.86 |
|
|
iAUC |
0 |
0 |
1.00† |
|
|
Glutamic acid |
Baseline |
27 (7) |
22 (2) |
0.36 |
|
AUC |
6,773(689) |
6,398(499) |
0.22 |
|
|
iAUC |
1,973(668) |
2,468(201) |
0.57 |
|
|
EAAs |
||||
|
Histidine |
Baseline |
86 (3) |
84 (1) |
0.34 |
|
AUC |
15,180(615) |
14,895(191) |
0.52 |
|
|
iAUC |
0 |
90(42) |
0.21 |
|
|
Isoleucine |
Baseline |
76 (7) |
62 (1) |
0.18 |
|
AUC |
11,895(1,358) |
10,463(711) |
0.51 |
|
|
iAUC |
195(106) |
45(64) |
0.43 |
|
|
Leucine |
Baseline |
142 (12) |
128(6) |
0.18 |
|
AUC |
24,833(2,386) |
23,865(148) |
0.69 |
|
|
iAUC |
1,095(1,039) |
300(382) |
0.57 |
|
|
Lysine |
Baseline |
172 (41) |
176 (45) |
0.40 |
|
AUC |
29,752(7,881) |
29,745(5,282) |
1.00 |
|
|
iAUC |
270(42) |
0 |
0.07 |
|
|
Methionine |
Baseline |
29 (4) |
30 (1) |
0.50 |
|
AUC |
4,598(562) |
4,635(424) |
0.77 |
|
|
iAUC |
0 |
0 |
1.00† |
|
|
Phenylalanine |
Baseline |
66 (4) |
57 (2) |
0.10 |
|
AUC |
10,905(615) |
9,923(817) |
0.09 |
|
|
iAUC |
75(106) |
15(21) |
0.63 |
|
|
Threonine |
Baseline |
134 (10) |
131 (10) |
0.87 |
|
AUC |
21,608(32) |
21,765(870) |
0.84 |
|
|
iAUC |
15 (21) |
15 (21) |
1.00† |
|
|
Tryptophan |
Baseline |
64 (1) |
54 (6) |
0.28 |
|
AUC |
10,448(435) |
10,208(435) |
0.50† |
|
|
iAUC |
15(21) |
758(1,071) |
0.51 |
|
|
Valine |
Baseline |
194 (24) |
252 (16) |
0.09 |
|
AUC |
48,255(5,240) |
44,400(1,782) |
0.36 |
|
|
iAUC |
225(149) |
345(488) |
0.83 |
|
Table 2: Probe 1 area-under-the-curve and incremental area under the curve values for plasma amino acids after consuming 34 g and assessing the responses every 30-minute over a 180-minute time course; † Wilcoxon signed rank test was used; n=2
|
Amino acids |
Calculation Method |
CON Mean (SD) |
EX Mean (SD) |
p-value (two-tailed) |
|
Primary indicator AAs |
||||
|
Proline |
Baseline |
164 (28) |
132 (25) |
0.004* |
|
AUC |
29,360(6,017) |
25,540(4,817) |
0.073 |
|
|
iAUC |
625(394) |
1,945(1080) |
0.14 |
|
|
Glutamic acid |
Baseline |
29 (12) |
37 (3) |
0.25† |
|
AUC |
5,505(861) |
4,715(1,137) |
0.25 |
|
|
iAUC |
855(977) |
10(17) |
0.27 |
|
|
EAAs |
||||
|
Histidine |
Baseline |
72 (8) |
71 (9) |
0.50† |
|
AUC |
13,045(1,592) |
13,110(1,581) |
0.84 |
|
|
iAUC |
380(114) |
470(48) |
0.36 |
|
|
Isoleucine |
Baseline |
73 (5) |
69 (10) |
0.68 |
|
AUC |
11,645(906) |
11,015(1,605) |
0.62 |
|
|
iAUC |
200(46) |
120(104) |
0.43 |
|
|
Leucine |
Baseline |
132 (21) |
145 (9) |
0.48 |
|
AUC |
27,415(1,439) |
25,315(2,997) |
0.39 |
|
|
iAUC |
1,700(293) |
1,790(686) |
0.83 |
|
|
Lysine |
Baseline |
185 (65) |
171 (51) |
0.22 |
|
AUC |
32,765(10,110) |
31,735(8,905) |
0.29 |
|
|
iAUC |
650(693) |
1,230(676) |
0.40 |
|
|
Methionine |
Baseline |
28 (7) |
26 (6) |
0.46 |
|
AUC |
4,685 (862) |
4,550 (831) |
0.67 |
|
|
iAUC |
80 (114) |
110 (121) |
0.48 |
|
|
Phenylalanine |
Baseline |
60 (8) |
58 (4) |
0.62 |
|
AUC |
10,940 (630) |
11,075 (775) |
0.80 |
|
|
iAUC |
400 (568) |
635 (372) |
0.31 |
|
|
Threonine |
Baseline |
120 (25) |
115 (21) |
0.21 |
|
AUC |
21,165 (4,173) |
20,625 (3,500) |
0.30 |
|
|
iAUC |
210 (182) |
260 (171) |
0.83 |
|
|
Tryptophan |
Baseline |
55 (5) |
52 (6) |
0.45 |
|
AUC |
8,390 (98) |
7,715 (1,315) |
0.47 |
|
|
iAUC |
0 |
0 |
1.00† |
|
|
Valine |
Baseline |
281 (31) |
255 (45) |
0.52 |
|
AUC |
48,905 (4,974) |
44,285 (6,319) |
0.45 |
|
|
iAUC |
610 (348) |
440 (75) |
0.47 |
|
Table 3: Probe 2 area-under-the-curve and incremental area under the curve values for plasma amino acids after consuming 68 g and assessing responses every 30-minute over a 180-minute time course; *p<0.05; † Wilcoxon signed rank test was used; n=3
|
Amino acids |
Calculation Method |
CON mean (SD) |
EX mean (SD) |
p-value (two-tailed) |
|
Primary indicator AAs |
||||
|
Proline |
Baseline |
143 (5) |
145 (42) |
0.93 |
|
AUC |
14,050 (2,112) |
13,735 (2,927) |
0.72 |
|
|
iAUC |
1,513 (1,671) |
780 (727) |
0.64 |
|
|
Glutamic acid |
Baseline |
30 (20) |
41 (33) |
0.28 |
|
AUC |
2,928 (2,188) |
3,410 (2,501) |
0.15 |
|
|
iAUC |
358 (380) |
38 (46) |
0.29 |
|
|
EAAs |
||||
|
Histidine |
Baseline |
66 (3) |
47 (42) |
0.49 |
|
AUC |
6,113 (67) |
6,330 (469) |
0.45 |
|
|
iAUC |
355 (180) |
2,178 (3,199) |
0.41 |
|
|
Isoleucine |
Baseline |
67 (21) |
70 (25) |
0.80 |
|
AUC |
6,283 (1,866) |
6,073 (1,695) |
0.74 |
|
|
iAUC |
388 (123) |
168 (264) |
0.43 |
|
|
Leucine |
Baseline |
116 (40) |
125 (41) |
1.00† |
|
AUC |
11,725 (3,531) |
11,870 (2,740) |
0.92 |
|
|
iAUC |
1,398 (236) |
723 (927) |
0.41 |
|
|
Lysine |
Baseline |
135 (28) |
154(47) |
0.40 |
|
AUC |
13,265 (3,237) |
13,800 (3,452) |
0.39 |
|
|
iAUC |
1,360 (1094) |
345 (379) |
0.35 |
|
|
Methionine |
Baseline |
22 (2) |
22 (1) |
1.00† |
|
AUC |
2,093 (133) |
1,970 (178) |
0.08 |
|
|
iAUC |
135 (40) |
65 (113) |
0.48 |
|
|
Phenylalanine |
Baseline |
44 (20) |
63 (6) |
0.15 |
|
AUC |
5,202 (607) |
5,848 (96) |
0.10 |
|
|
iAUC |
1,284 (1,212) |
243 (394) |
0.16 |
|
|
Threonine |
Baseline |
97 (14) |
109 (24) |
0.61 |
|
AUC |
9,813 (1,847) |
9,782 (1,596) |
0.98 |
|
|
iAUC |
1,328 (1,622) |
248 (278) |
0.42 |
|
|
Tryptophan |
Baseline |
54 (7) |
49 (10) |
0.57 |
|
AUC |
5,165 (604) |
4,768 (288) |
0.35 |
|
|
iAUC |
453 (397) |
392 (615) |
0.92 |
|
|
Valine |
Baseline |
171 (138) |
269 (90) |
0.39 |
|
AUC |
22,250 (5,410) |
24,435 (6,722) |
0.32 |
|
|
iAUC |
7,130 (10,212) |
698 (1,041) |
0.41 |
|
Table 4: Probe 3 area-under-the-curve and incremental area under the curve values for plasma amino acids after consuming 68 g and assessing responses every 15-minutes over a 90-minute time course; n=3
4.2 Plasma glucose
Baseline fasting plasma glucose levels in P3 were in the normal range (<100 mg/dL), and were not significantly different between EX and CON. Postprandial glucose levels for three of the four participants had a similar pattern with EX generally higher than CON (Figure 1). The mean AUC of plasma glucose after EX intake was significantly greater than CON (Table 5). The mean iAUC of plasma glucose between EX and CON was not significantly different. The plasma glucose levels for most participants reached their maximum concentration (Cmax) at 30 to 45 minutes following consumption of either flour (Table 5, Figure 1). The maximum concentration of plasma glucose following EX intake was significantly greater than CON (149±14 vs. 121±11, respectively; p=0.011; Table 5).
|
Glucose |
CON Mean (SD) |
EX Mean (SD) |
p-value (two-tailed) |
|
Baseline |
88 (8) |
91 (4) |
0.61 |
|
AUC |
9,559 (716) |
11,239 (1,304) |
0.012* |
|
iAUC |
1,646 (948) |
3,107 (932) |
0.073 |
|
Cmax |
121 (11) |
149 (14) |
0.011* |
|
Tmax |
45 (21) |
34 (8) |
0.44 |
Table 5: Probe 3 mean AUC, mean Cmax, and Tmax of plasma glucose following EX and CON intake; *p<0.05; n=4.
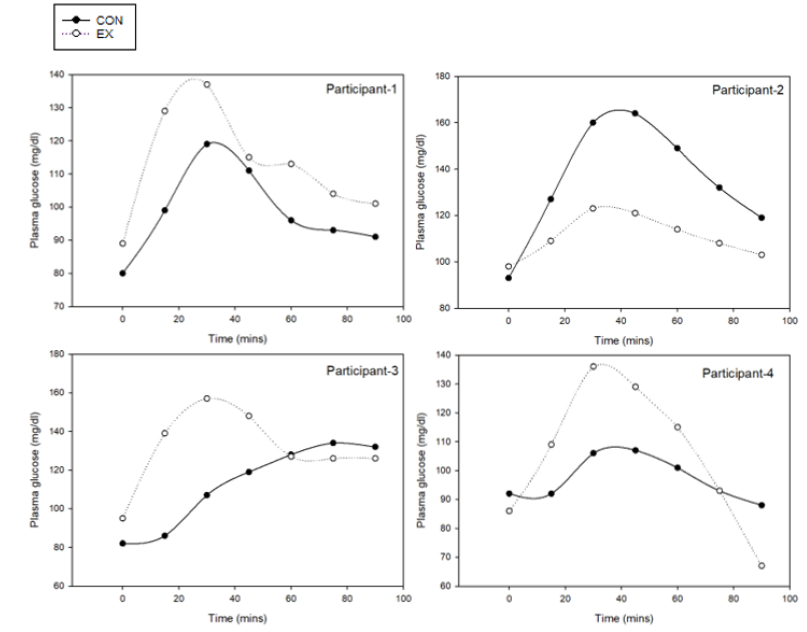
Figure 1: Plasma glucose patterns over 90 minutes for each of four individuals in P3
5. Discussion
Three probe studies were designed to provide exploratory data on the relative changes in plasma levels of indicator and essential amino acid levels following the intake of conventionally processed and extruded sorghum flours. The AUCs and iAUCs of postprandial plasma amino acid levels of EX and CON in P1, P2, and P3 were similar. A significant increase in plasma glucose AUC was noted for EX compared to CON, and a similar, non-significant trend was also observed for the iAUC values. This may be due to an increase in starch digestibility and glucose availability after the extrusion process [31]. Most of the elevated glucose values returned to a normal range within the 90 minute test period. The glucose response to sorghum intake can be influenced by numerous factors. Lower plasma glucose and insulin responses were reported after ingestion of sorghum porridge compared to sorghum flatbread [32]. When comparing sorghum intake to other grains such as wheat and maize, plasma glucose and insulin AUC levels tended to be lower for sorghum [24,33]. The polyphenol content of sorghum may also influence postprandial glucose responses. In a trial comparing three different extruded sorghum formulas of varying polyphenol compositions (proanthocyanidins [PAC] and 3-deoxyanthocyanidins [3-DXAs]; 3-DXAs; sorghum control [no PAC and 3-DXAs]), postprandial glucose AUCs were significantly lower with PAC- and 3-DXAs-rich sorghum compared to the control [34]. Since the extrusion conditions and type of sorghum used in this study were different from the current project, a direct comparison of the results is limited. Although AUCs were considered as the primary outcome measures, iAUCs were also assessed to account for the differences in individual baseline values. As the AUC does not account for the difference in each person’s baseline, a higher baseline value generally resulted in a greater AUC, which may not represent the actual change throughout the measurement period. The differences at baseline can be reflected in the AUC differences as seen in a trend towards significance of proline AUCs, while the proline iAUCs were not affected [Table 2]. Differences between AUC and iAUC calculations have also been reported for plasma glucose results among men following an exercise regimen [35]. Another study concluded that iAUC was a more representative calculation than AUC for triglyceride responses to an oral fat load in healthy and diabetic individuals [36]. A report investigating the effects of whole grain sorghum intake on plasma amino acid levels in preschool children observed a significant increase in plasma essential amino acids three hours after consumption [11]. The trends returned toward baseline values by four hours. However, the researchers noted that sorghum had a lower digestibility compared to that of wheat, rice, potato, maize, or casein [11]. Direct comparison of these results to the current study is difficult, due in part to differences in calculation methods. Moreover, the study in children averaged individual plasma amino acids at each time point, while the current study used AUC and iAUC. A number of factors limit the comparison of our results with other published work, including differences in the population assessed, the use of uncooked versus cooked sorghum flour, and specific details on the methods of extrusion, and the controls used in most trials utilized high-quality, complete protein sources (e.g., beef, dairy, and dish) [37,38]. Compared to maize, sorghum contains a higher amount of phenolic and polyphenolic compounds, [14], which often bind to and precipitate the kafirin proteins present in sorghum [39]. The extrusion process typically generates heat, and some in vitro studies report that an increase in temperature during extrusion can reduce the digestibility of sorghum protein by 40 to 60% compared to unprocessed sorghum [14,21]. This may be explained, in part, by the Maillard reaction, which increases the cross-links between amino acids and glucose, thus reducing protein digestibility and availability [40]. Differeces in other extrusion conditions, such as pressure and pore size of the sieve can also contribute to different properties of the final product [19,40]. Future studies are needed to investigate the effect of extrusion on bioavailability of other nutrients including vitamins, minerals, and polyphenols, which are known to have health benefits. Previous studies reported that different extrusion conditions can result in products with different polyphenol profiles [33,34,41] and while the present study did not assess the polyphenolic profiles of extruded and non-extruded sorghum flour, such investigation would be of interest. Sorghum polyphenols have been reported to improve markers of diseases such as glucose intolerance, inflammation, and cancer in several in vitro [42], animal [43,44] and human studies [34,45]. More clinical trials about extruded sorghum and markers of disease are clearly warranted. Sorghum is drought tolerant, environmentally sustainable, and an affordable food source, as well as a gluten-free grain [46]. Consumer demand for gluten-free products will encourage advances in food technology to enhance the nutritional and sensory attributes of sorghum [13,47]. When tested among patients with celiac disease using both in vitro organ culture of duodenal biopsies as well as a five-day feeding trial of baked goods created with sorghum flour, no evidence of immunological reactivity was found [48]. The unique composition and properties of different sorghum cultivars provide a range of new options for the creation of food products [49]. Novel processing methods designed to improve the nutritional value and sensory attributes of sorghum will further promote the consumption of this important grain. More clinical trials about extruded sorghum and markers of disease are clearly warranted
6. Conclusion
Probe studies exploring differences in postprandial plasma amino acid patterns following intake of extruded and conventional sorghum flour showed no statistically significant changes under the conditions tested. Plasma glucose levels were generally greater after extruded sorghum intake compared to conventional sorghum flour. Future studies are indicated to explore different extrusion methods in order to produce improved protein availability.
Acknowledgements
We thank Professor Andrea Fascetti and Dr. Joshua Zengshou Yu for their advice about blood sample collection and processing, and for conducting the plasma amino acid analyses.
Funding disclosure
Supported in part by an unrestricted gift from GHL International, Cedarberg, WI
References
- Zhao ZY, Dahlberg J. Sorghum: Methods and Protocols. Methods in Molecular Biology. 1931 (2019): 121-135.
- USDA Foreign Agricultural Service - World Agricultural Production. Circular Series. (2020)
- Mundia CW, Secchi S, Akamani K, Wang G. A Regional Comparison of Factors Affecting Global Sorghum Production: The Case of North America, Asia and Africa’s Sahel. Sustainability 11 (2019): 2135.
- Taylor JRN, Awika JM. Gluten-Free Ancient Grains: Cereals, Pseudocereals, and Legumes: Sustainable, Nutritious, and Health-Promoting Foods for the 21st (Taylor, John R.N., Awika J, ed.). Woodhead Publishing (2017).
- Singh P, Arora A, Strand TA, et al. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clinical Gastroenteroly and Hepatology 16 (2018): 823-836.E2
- Bahwere P, Balaluka B, Wells JCK, et al. Cereals and Pulse-Based Ready-to-Use Therapeutic Food as An Alternative to The Standard Milk- and Peanut Paste-Based Formulation for Treating Severe Acute Malnutrition: A Noninferiority, Individually Randomized Controlled Efficacy Clinical Trial. Amercian Journal of Clinical Nutrition 103 (2016): 1145-1161.
- Bahwere P, Akomo P, Mwale M, et al. Soya, Maize, and Sorghum–Based Ready-to-Use Therapeutic Food with Amino Acid is as Efficacious as The Standard Milk and Peanut Paste- Based Formulation for The Treatment of Severe Acute Malnutrition in Ahildren: A Noninferiority Individually Randomized Con. American Journal of Clinical Nutrition 106 (2017): 1100-1112.
- Kohlmann K, Callaghan-Gillespie M, Gauglitz JM, et al. Alternative Ready-to-Use Therapeutic Food Yields Less Recovery Than The Standard for Treating Acute Malnutrition in Children from Ghana. Global Heallth: Science and Practice 7 (2019): 203-214.
- Kajjura RB, Veldman FJ, Kassier SM. Effect of A Novel Supplementary Porridge on The Nutritional Status of Infants and Young Children Diagnosed with Moderate Acute Malnutrition in Uganda: A Cluster Randomised Control Trial. Journal of Human Nutrition and Dietetics 32 (2019): 295-302.
- Prasad MP R, Benhur D, Kommi K, et al. Impact of Sorghum Supplementation on Growth and Micronutrient Status of School Going Children in Southern India - A Randomized Trial. Indian Journal of Pediatrics 83 (2016): 9-14.
- Maclean WC, RomaÑa GL de, Placko RP, et al. Protein Quality and Digestibility of Sorghum in Preschool Children: Balance Studies and Plasma Free Amino Acids. Journal of Nutrition 111 (1981): 1928-1936.
- Schalk K, Lexhaller B, Koehler P, et al. Isolation and Characterization of Gluten Protein Types from Wheat, Rye, Barley and Oats for Use as Reference Materials. PLoS One 12 (2017): e0172819
- de Mesa-Stonestreet NJ, Alavi S, Bean SR. Sorghum Proteins: The Concentration, Isolation, Modification, and Food Applications of Kafirins. Journal of Food Science 75 (2010): R90-R104
- Duodu KG, Taylor JRN, Belton PS, et al. Factors Affecting Sorghum Protein Digestibility. Journal of Cereal Science 38 (2003): 117-131.
- Duodu KG, Nunes A, Delgadillo I, et al. Effect of Grain Structure and Cooking on Sorghum and Maize In Vitro Protein Digestibility. Journal of Cereal Science 35 (2002): 161-174.
- Taylor JRN, Dewar J. Developments in Sorghum Food Technologies. Advances in Food and Nutrition Research 43 (2001): 217-264.
- Adebo OA. African Sorghum-Based Fermented Foods: Past, Current and Future Prospects. Nutrients 12 (2020): 1111.
- Benhur DR, Bhargavi G, Kalpana K, et al. Development and Standardization of Sorghum Pasta Using Extrusion Technology. Journal of Food Science and Technology 52 (2015): 6828-6833.
- Holay SH, Harper JM. Influence of the Extrusion Shear Environment on Plant Protein Texturization. Journal of Food Science 47 (1982): 1869-1874.
- Hamaker BR, Mertz ET, Axtell JD. Effect of Extrusion on Sorghum Kafirin Solubility. Cereal Chemistry 71 (1994): 515-517.
- Mertz ET, Hassen MM, Cairns Whittern C. Pepsin Digestibility of Proteins in Sorghum and Other Major Cereals. Proceeding of National Academy of Sciences USA 81 (1984): 1-2.
- MacLean WC, Lopez de Romana G, Gastanaduy A, et al. The Effect of Decortication and Extrusion on The Digestibility of Sorghum by Preschool Children. Journal of Nutrition 113 (1983): 2071-2077.
- Stefoska-Needham A, Beck EJ, Johnson SK, et al. Flaked Sorghum Biscuits Increase Postprandial GLP-1 and GIP Levels and Extend Subjective Satiety in Healthy Subjects. Molecular Nutrition and Food Research 60 (2016): 1118-1128.
- Poquette NM, Gu X, Lee SO. Grain Sorghum Muffin Reduces Glucose and Insulin Responses in Men. Food and Function Journal 5 (2014): 894-899.
- Anunciação PC, Cardoso L de M, Alfenas R de CG, et al. Extruded Sorghum Consumption Associated with A Caloric Restricted Diet Reduces Body Fat in Overweight men: A Randomized Controlled Trial. Food Research International 119 (2019): 693-700.
- Burke LM, Winter JA, Cameron-Smith D, et al. Effect of Intake of Different Dietary Protein Sources on Plasma Amino Acid Profiles at Rest and after Exercise. International Journal of Sport Nutrition and Exercise Metabolism 22 (2012): 452-462.
- Llopart EE, Drago SR, De Greef DM, et al. Effects of Extrusion Conditions on Physical and Nutritional Properties of Extruded Whole Grain Red Sorghum (Sorghum spp). International Journal of Food Sciences and Nutrition 65 (2014): 34-41.
- Joye I. Protein Digestibility of Cereal Products. Foods 8 (2019): 199.
- Vu TH, Bean S, Hsieh CF, Shi YC. Changes in Protein and Starch Digestibility in Sorghum Flour during Heat–Moisture Treatments. Journal of the Science of Food and Agriculture 97 (2017): 4770-4779.
- Lipscomb JM, Rodriguez OI, Berge CC. Functionally Enhancing Flours and Methods of Making and Using Same. May 2020. https://uspto.report/patent/app/20200296975.
- Ratnavathi CV PJ. Sorghum Utilization as Food. Journal of Nutrition and Food Sciences 4 (2014):1000247
- Abdelgadir M, Abbas M, Järvi A, Elbagir M, Eltom M, Berne C. Glycaemic and Insulin Responses of Six Traditional Sudanese Carbohydrate-Rich Meals in Subjects with Type 2 Diabetes Mellitus. Diabet Medicine 22 (2005): 213-217.
- Salazar Lopez NJ, Loarca-Piña G, Campos-Vega R, et al. The Extrusion Process as an Alternative for Improving the Biological Potential of Sorghum Bran: Phenolic Compounds and Antiradical and Anti-Inflammatory Capacity. Evidence-based Complementary and Alternative Medicine 2016 (2016): 8387975.
- Anunciação PC, Cardoso L de M, Queiroz VAV, et al. Consumption of A Drink Containing Extruded Sorghum Reduces Glycaemic Response of The Subsequent Meal. European Journal of Nutrition 57 (2018): 251-257.
- Potteiger JA, Jacobsen DJ, Donnelly JE. A Comparison of Methods for Analyzing Glucose and Insulin Areas Under The Curve Following Nine Months of Exercise in Overweight Adults. International Journal of Obesity 26 (2002): 87-89.
- Carstensen M, Thomsen C, Hermansen K. Incremental Area Under Response Curve More Accurately Describes The Triglyceride Response to An Oral Fat Load in Both Healthy and Type 2 Diabetic Subjects. Metabolism 52 (2003): 1034-1037.
- Fuchs CJ, Hermans WJH, Holwerda AM, et al. Branched-Chain Amino Acid and Branched-Chain Ketoacid Ingestion Increases Muscle Protein Synthesis Rates In Vivo in Older Adults: A Double-Blind, Randomized Trial. Amercian Journal of Clinical Nutrition 110 (2019): 862-872.
- Ottosson F, Ericson U, Almgren P, et al. Postprandial Levels of Branch Chained and Aromatic Amino Acids Associate with Fasting Glycaemia. Journal of Amino Acids 2016 (2016):8576730
- Emmambux NM, Taylor JRN. Sorghum Kafirin Interaction with Various Phenolic Compounds. Journal of the Science of Food and Agriculture 83 (2003): 402-407.
- Camire ME. Protein Functionality Modification by Extrusion Cooking. Journal of the American Oil Chemists' Society 68 (1991): 200-205.
- Ortiz-Cruz RA, Ramírez-Wong B, Ledesma-Osuna AI, et al. Effect of Extrusion Processing Conditions on the Phenolic Compound Content and Antioxidant Capacity of Sorghum (Sorghum bicolor (L.) Moench) Bran. Plant Foods for Hum Nutrition 75 (2020): 252-257.
- de Sousa AR, de Castro Moreira ME, Grancieri M, et al. Extruded Sorghum (Sorghum bicolor L.) Improves Gut Microbiota, Reduces Inflammation, and Oxidative Stress in Obese Rats Fed a High-Fat Diet. Journal of Functional Foods 58 (2019): 282-291.
- Salazar-López NJ, González-Aguilar GA, Rouzaud-Sández O, et al. Sorghum Bran Supplementation Ameliorates Dyslipidemia, Glucose Dysregulation, Inflammation and Stress Oxidative Induced by A High-Fat Diet in Rats. CYTA - Journal of Food 18 (2020): 20-30.
- Moraes ÉA, Marineli R da S, Lenquiste SA, et al. Whole Sorghum Flour Improves Glucose Tolerance, Insulin Resistance and Preserved Pancreatic Islets Function in Obesity Diet-Induced Rats. Journal of Functional Foods 45 (2018): 530-540.
- Ayuba GI, Jensen GS, Benson KF, et al. Clinical Efficacy of A West African Sorghum Bicolor-Based Traditional Herbal Preparation Jobelyn Shows Increased Hemoglobin and CD4+ T-Lymphocyte Counts in HIV-Positive Patients. Journal of Alternative and Complementary Medicine 20 (2014): 53-56.
- Gorgitano MT, Sodano V. Gluten-Free Products: From Dietary Necessity to Premium Price Extraction Tool. Nutrients 11 (2019): 1997.
- Palavecino PM, Curti MI, Bustos MC, et al. Sorghum Pasta and Noodles: Technological and Nutritional Aspects. Plant Foods for Human Nutrition 75 (2020): 326-336.
- Ciacci C, Maiuri L, Caporaso N, et al. Celiac disease: In Vitro and In Vivo Safety and Palatability of Wheat-Free Sorghum Food Products. Clinical Nutrition 26 (2007): 799-805.
- Souilah R, Djabali D, Belhadi B, et al. In Vitro Starch Digestion in Sorghum Flour from Algerian Cultivars. Food Science & Nutrition 2 (2014): 251-259.
