Effect of Optical Correction on Choroid Structure in Children with Amblyopia
Article Information
Kholoud Ahmad Bokhary1, Ahad Walaa Alsaif1, Rania Fahmy1,2*
1Optometry Department, College of Applied Medical Science, King Saud University, KSA
2Department of Ophthalmology, Faculty of Medicine, Cairo University, Giza, Egypt
*Corresponding author: Rania Fahmy, Optometry Department, College of Applied Medical Science, King Saud University, KSA, Department of Ophthalmology, Faculty of Medicine, Cairo University, Giza, Egypt.
Received: 02 September 2023; Accepted: 09 September 2023; Published: 21 September 2023.
Citation: Kholoud Ahmad Bokhary, Ahad Walaa Alsaif, Rania Fahmy. Effect of Optical Correction on Choroid Structure in Children with Amblyopia. Journal of Ophthalmology and Research. 6 (2023): 50-56.
Share at FacebookAbstract
Purpose: To compare the choroidal thickness (ChT) in strabismic and non-strabismic amblyopic children undergoing or recovered amblyopia treatment.
Methods: Subjects were recruited from pediatric ophthalmology and optometry clinics at King Fahad Medical City. Measurements include visual acuity, ocular alignment tests, refraction, and retinal ChT by optical coherence tomography depth (EDl-OCT).
Results: This study findings showed a statistically significant difference in mean scores of foveal and temporal ChT between amblyopic and non-amblyopic eyes for non-strabismus amblyopia group with a mean difference (28.5, 39.7 μm) respectively and a p-value =0.007 <0.05, while, nasal ChT of amblyopic eyes was not statistically significant in all study groups eyes. The foveal and temporal ChT in amblyopic eyes of children recovered from amblyopia was significantly thinned (299.62 ± 30.24 μm ,271.85 ± 25.54 μm) respectively and within the average choroidal thickness of children p value= (P = 0.030) <0.05 and (P = 0.038) <0.05.
Conclusion: The present findings suggested that myopic anisometropic amblyopes had thinner nasal ChT and thicker foveal and temporal ChT. The ChT in children recovered from amblyopia were significantly decreased after optical correction compared to those undergoing amblyopia treatment and controls. Such a reduction was to compensate for refractive error and improve visual acuity.
Keywords
Amblyopia, Strabismus, Optical correction, Choroidal thichness, Optical coherence tomography.
Amblyopia articles; Strabismus articles; Optical correction articles; Choroidal thichness articles; Optical coherence tomography articles
Article Details
1. Background
Amblyopia was defined as a reduction in the best-corrected visual acuity in one or both eyes with the presences of amblyogenic factors[1,2]. The condition is caused by many predisposing visual abnormalities such as strabismus, refractive errors, and stimulus deprivation (e.g., congenital cataract) [1].
The incidence of amblyopia is considered high in individuals with risk factors including prematurity, low birth weight, nutritional status, smoking during pregnancy, and family history [3]. Poor depth perception (difficulty in judging object in a given space) is a symptom of amblyopia. While signs of amblyopia are mostly associated with unilateral cases including ptosis, inward or outward eye deviation, closing one eye, and head tilting [1].
The treatment of amblyopia is most effective during the critical period of the visual development, which is from the age of 3- to 7-years-old [4]. Treatment is effective if cause of ambyliopia is identified and treatment is prescribed accordingly, inducing a clearer retinal image in both eyes and obtaining normal vision. One of the most important amblyopia treatments is inspecting for any stimulus deprivation conditions such as congenital cataract treated by surgical intervention early in life. Other strategies of treatment are prescribing optical correction, patching and penalization for the dominant eye [5].
Amblyopia may induce retinal abnormalities in the affected eye such as retinal choroid thickness. The choroid is a vascular structure located between the sclera (outer layer) and the retina (inner layer) that is responsible for supplying the outer retinal structure with blood [6].The retinal choroid is primarily supplied by nerve fibres, parasympathetic, sympathetic, and trigeminal sensory nerves. The choroid innervations are significantly essential to regulate the blood flow based on retinal activity needs [7].
A previous study assessed the choroid structure changes before and after anisohypermetropic amblyopia treatment for Asian children aged between of 3- to 8-years using (ED-OCT). The results revealed significant differences in the choroid thickness before and after initiating the treatment plan and lower mean choroid thickness after amblyopia treatment [8]. This study did not include subjects with strabismic amblyopia thus, it is not clear if the choroidal structure affected by the types of amblyopia. Another study assessed choroid thickness in unilateral amblyopia subjects and found thicker choroid in both amblyopic eye and fellow eye in amblyopic children compared to normal subjects [9]. In addition, another study evaluated choroid thickness in anisometropic amblyopia and strabismic amblyopia in comparison to the normal age-matched group and found thicker choroid thickness in strabismic amblyopia subjects than that in anisometropic amblyopia and significantly reduced in control subjects [10]. The findings of previous studies may not be representative as they were limited to particular type of amblyopia or specific treatment (i.e., optical correction). Besides, previous studies did not discuss the correlation between choroid thickness and all amblyopia types. Controls included in previous studies had normal vision without refractive error. This is necessary to confirm that change in choroidal thickness and structures following treatment are related to the refractive status or amblyopia itself.
A prospective study was conducted to examine the correlation between the choroid and the axial eye growth in children with refractive errors and controls aged between 10 to 15 years. The results exhibited a significant increase in choroidal thickness in controls compared to myopic children, who had shown less thickening and faster axial length growth in 18 months. It was suggested that the findings supported existing phenomena, which stated that choroid mechanisms contribute to the regulation of children's eye growth [11]. Thus, there is a need for a study to confirm wither the changes in the choroidal thickness is related to types of amblyopia or its treatment.
2. Procedure
A total of 42 children participated in this observational, cross-sectional prospective study. They were recruited from Optometry and pediatric ophthalmology clinics at tertiary hospital. They were divided into three groups: Non strabismus ambyliopia group, comprising 12 children, Strabismus ambyliopia group, comprising 12 children and age and sex matched control group in good general and ocular health, comprising 18 children. Amblyopic children aged between 3 to 12 years with reduction in best corrected visual acuity by two lines in Log MAR between both eyes. [2] Amblyopia in children is caused by strabismus [12], refractive errors [13] and deprivation [14]. The severity of amblyopia was classified based on visual acuity of the amblyogenic eye as: mild amblyopia of 20/30 to 20/40 (log MAR 0.20 to 0.30 ), moderate amblyopia worse than 20/40 to 20/100 (log MAR 0.30 to 0.70) , and sever amblyopia being worse than 20/125 (log MAR 0.80) [15]. Children control groups with refractive errors (i.e. Myopia or hyperopia, astigmatism) were included in this study to compare axial length and choroidal structure between amblyopic children and controls and investigate the cause of changes, which could be related to amblyopia or its treatment. Children that are not age matched or with glaucoma, ocular hypertension, prior refractive surgery, laser treatment, retinal diseases, or neurological diseases or following existing treatment plans with prescribed regular medications were excluded from this study.
2.1 Ocular examination
Each child underwent a full ophthalmic examination, including visual acuity using log MAR chart and choroidal thickness using optical coherence tomography. General ocular assessment including extraocular muscles test, binocular function, and fundus assessment with prescription of amblyopia treatment were performed by pediatric ophthalmologist. Children’ medical history was obtained from the parents/caregiver [16].
2.2 Choroid thickness measurement
The choroid thickness was assessed in both amblyopic and control subjects using enhanced depth optical coherence tomography ED-OCT [17]. The resulting images were evaluated to determine the luminal, stromal, and total choroidal area changes in both subjects by comparing the ED-OCT images obtained before treating amblyopic subjects and after six months of treatment by optical corrections and patching therapy.
2.3 Ethical consideration
Ethical approval was obtained from the scientific research deanship at tertiary hospital. Its protocol was explained to each participant at the time of recruitment and informed consent was obtained from the caregivers/parents according to the Declaration of Helsinki.
2.4 Statistical analysis
The statistical software used to conduct the analysis is IBM-SPSS Statistics version 17.0, which suits the type of variables for the collected data and targeted analysis. Normality assumption tested using Normal Q-Q Plot in order to compare the choroid thickness measurements between the study groups. One-way Analysis of Variance (ANOVA) statistical test was used to check significant differences (at α=0.05) between study groups. The results of the retinal choroid thickness change in amblyopic and non-amblyopic eyes were analyzed by paired samples t-test, we considered there was a significant difference when P-value less than (P < 0.05).
3. Results
A total of 42 children were enrolled in the study and divided into three groups. Table 1 showed demographic characteristics and clinical ocular parameters of studied groups. There is no significant difference in age and gender between groups. Fixation pattern results revealed that all participants from controls and non-strabismus amblyopia groups had a central pattern, while children within the strabismus amblyopia group had an eccentric pattern. In addition, the degree of amblyopia severity was about 41.7% of patients within the non-strabismus amblyopia group with a higher degree of severity compared to the strabismus amblyopia group. Non-strabismus amblyopia group reported full extraocular muscles compared to the strabismus amblyopia group that exhibited limited abduction or adduction of extra ocular muscles. Furthermore, a higher percentage of strabismus children had limited abduction than limited adduction of extra ocular muscles.
Non-strabismus amblyopia group reported higher positive VA-log MAR mean scores for both eyes compared to other groups. On the other hand, strabismus amblyopia group reported higher positive spherical equivalent SE mean scores for both eyes compared to other groups. A statistically significant difference in visual acuity measurements mean scores between amblyopic and non-amblyopic eyes was found for patients within the two amblyopia groups (n=24) with a P-value <0.001. Moreover, there were significant differences between amblyopic and non-amblyopic eyes in spherical equivalent measurements mean scores with P-value <0.001.
Table 1: Descriptive analysis of demographic characteristics and ocular parameters of studied groups
|
Variable |
Group |
Total |
||
|
Non-Strabismus Amblyopia (n=12) |
Strabismus Amblyopia (n=12) |
Control (n=18) |
||
|
Gender Male n (%) |
7(58.3%) |
6 (50.0%) |
10 (55.6%) |
23 (54.8%) |
|
Age Mean±SD |
9.8 ± 1.8 |
8.3 ± 2.7 |
9.4 ± 2.0 |
9.2 ± 2.2 |
|
Amblyopia Treatment Status |
||||
|
Undergoing |
8 (66.7%) |
3 (25.0%) |
9 (50.0%) |
20 (47.6%) |
|
Recovered |
4 (33.3%) |
9 (75.0%) |
9 (50.0%) |
22 (52.4%) |
|
Fixation Pattern |
||||
|
Central |
12 (100%) |
0 (0.0%) |
18 (100%) |
23 (71.0%) |
|
Eccentric |
0 (0.0%) |
12 (100%) |
0 (0.0%) |
13 (29.0%) |
|
Degree of Severity |
||||
|
Mild |
4 (33.3%) |
6 (50.0%) |
0 (0.0%) |
10 (41.7%) |
|
Moderate |
3 (25.0%) |
6 (50.0%) |
0 (0.0%) |
9 (37.5%) |
|
Severe |
5 (41.7%) |
0 (0.0%) |
0 (0.0%) |
5 (20.8%) |
|
Extra Ocular Muscles |
||||
|
Limited Adduction |
0 (0.0%) |
3 (25.0%) |
0 (0.0%) |
3 (7.0%) |
|
Limited Abduction |
0 (0.0%) |
9 (90.3%) |
0 (0.0%) |
10 (25.2%) |
|
Full |
12 (95.7%) |
0 (0.0%) |
18 (100%) |
29 (67.8%) |
|
Visual acuity Mean ± SD |
- |
|||
|
Amblyopic |
0.65 ± 0.36 |
0.43 ± 0.17 |
0.15 ± 0.15 |
|
|
Non amblyopic |
0.31 ± 0.33 |
0.18 ± 0.14 |
0.13 ± 0.15 |
- |
|
Spherical equivalent |
||||
|
Amblyopic |
-1.06 ± 7.24 |
1.33 ± 3.54 |
0.42 ± 3.40 |
- |
|
Non amblyopic |
0.79 ± 4.57 |
2.54 ± 4.12 |
0.21 ± 3.49 |
- |
3.1 Comparing Choroidal Thickness between Study Groups
ANOVA test used to compare choroidal thickness measurements within study groups showed that there are no statistically significant differences between the three groups in mean scores with p-values greater than (.05). Moreover there were insignificant differences between amblyopic or non-amblyopic eyes choroidal thickness with p-values greater than (.05) (Table 2).
|
Group |
Amblyopic eye |
Non amblyopic eye |
||||
|
ChT-Nasal |
ChT-Foveal |
ChT-Temporal |
ChT-Nasal |
ChT-Foveal |
ChT-Temporal |
|
|
Strabismus Amblyopia (n=12) |
259.5 ± 25.9 |
305.2 ± 37.2 |
278.3 ± 27.4 |
266.2 ± 22.4 |
318.3 ± 31.3 |
289.3 ± 28.0 |
|
Non-Strabismus Amblyopia (n=12) |
263.0 ± 19.3 |
329.0 ± 36.2 |
300.5 ± 45.7 |
245.5 ± 28.8 |
300.5 ± 45.7 |
258.5 ± 44.0 |
|
Control Right-Left eye (n=18) |
250.29 ± 23.63 |
302.92 ± 33.19 |
273.63 ± 27.08 |
245.4 ± 23.4 |
296.8 ± 29.8 |
268.9 ± 26.8 |
|
P-value* |
0.647 |
0.559 |
0.586 |
0.151 |
0.396 |
0.353 |
Table 2: Correlation between Mean scores of Choroidal Thickness of amblyopic and control groups.
3.2 Comparing Choroidal Thickness between Amblyopic and non-Amblyopic Eyes
There are no statistically significant differences in choroidal thickness (Nasal, Foveal, and Temporal) measurements mean scores between amblyopic eye and non-amblyopic eye for patients within strabismus amblyopia group with p-values >.05 (significance level). A statistically significant difference was found in ChT-Foveal mean scores between amblyopic and non-amblyopic eyes for patients within the non-strabismus amblyopia group with p-value =.007 <.05 and mean difference of (M=28.5) µm where amblyopic eyes had higher ChT-Foveal mean score. Moreover, a statistically significant difference in ChT-Temporal mean scores between amblyopic and non-amblyopic for patients within the non-strabismus amblyopia group with p-value =.007 <.05 and mean difference of (M=39.7) µm (Table 3).
Table 3: Correlation between mean scores of Choroidal Thickness of Amblyopia group
* Comparison of mean scores of ChT between amblyopic and non-amblyopic eyes within each Amblyopia group using Paired Samples T-test (at significance level of .05)
3.3 Comparing Choroidal Thickness based on Amblyopia Status
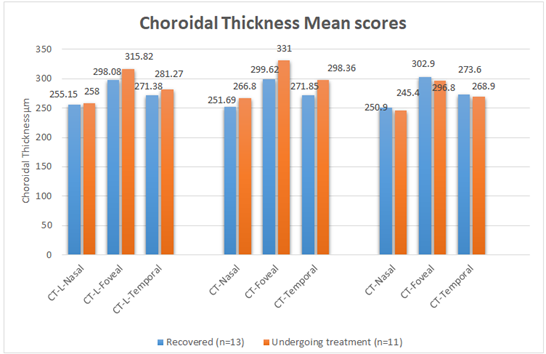
The results showed no significant difference between the recovered and undergoing amblyopia treatment groups in mean scores for nasal choroidal thickness measurements within the amblyopic eye with (P = .196) > .05. However, there was statistically significant differences in ChT (foveal, and temporal) mean scores within amblyopic eye between recovered patients and undergoing treatment patients with (P = .030) <.05 and (P = .038) <.05, and mean differences (M=31.38) µm, (M=26.51) µm respectively. Mean scores for both foveal and temporal choroidal thickness measurements for amblyopic eye were higher within undergoing treatment patients group compared to recovered patients (Table 4).
Results demonstrated that ChT-Foveal measurement mean scores were higher compared to nasal and temporal choroidal thickness measurements within the amblyopic eye for both groups, where within undergoing treatment amblyopia status group, patients reported highest ChT-Foveal; mean scores for amblyopic eye compared to other areas of the choroidal layer. Furthermore, results of Two independent samples T-test comparing choroid thickness measurements in non-amblyopic eye showed that there were no statistically significant differences between the (recovered and undergoing treatment) groups in mean scores for (nasal, foveal, and temporal) choroidal thickness measurements in non-amblyopic eye. Despite that, patients within undergoing treatment amblyopia group reported highest ChT (Nasal, Foveal, and Temporal) mean scores in the non-amblyopic compared to recovered patient group (Table 4 & Figure 1).
Table 4: Comparison between mean scores of Choroidal Thickness Mean for the Recovered and Undergoing amblyopia Treatment Groups
|
Amblyopia Status |
Amblyopic Eye Mean ± SD |
Non-Amblyopic Eye Mean ±DS |
||||
|
CT-Nasal |
CT-Foveal |
CT-Temporal |
CT-Nasal |
CT-Foveal |
CT-Temporal |
|
|
Recovered (n=13) |
251.69 ± 24.54 |
299.62 ± 30.24 |
271.85 ± 25.54 |
255.15 ± 23.33 |
298.0 ± 830.26 |
271.38 ± 32.00 |
|
Undergoing treatment (n=11) |
266.8 ± 231.02 |
331.00 ± 35.94 |
298.36 ± 33.38 |
258.00 ± 28.85 |
315.82 ± 39.31 |
281.27 ± 28.88 |
|
Controls Right- Left (n=18) |
250.29 ± 23.63 |
302.92 ± 33.19 |
273.63 ± 27.08 |
245.4 ± 23.4 |
296.8 ± 29.8 |
268.9 ± 26.8 |
|
P-value* |
0.196 |
0.03 |
0.038 |
0.792 |
0.225 |
0.439 |
4. Discussion
The mean scores of temporal and foveal ChT between amblyopic and non-amblyopic eyes for the non-strabismus amblyopia group were compared in this study. The present findings showed that nasal choroidal area in amblyopic eyes were not statistically significant between controls, recovered, and undergoing amblyopia treatment subjects. Vincent et al.'s proved that thinnest choroid is in nasal area and thickest in foveal &temporal regions in myopic anisometropic eyes. It has been proposed that thinner choroid nasal to the fovea is the most vulnerable to alteration during myopia development. A temporal crescent at the optic nerve head (peripapillary atrophy) is typical in myopic individuals and is assumed to involve chorioretinal stretching during axial elongation, exposing the underlying sclera [18]. Previous study was in agreement with our results because non-strabismic subjects recruited were myopic anisometropic amblyopic children.
Our findings showed that foveal and temporal choroidal areas in amblyopic eye of children recovered from amblyopia were significantly narrowed and within the average choroidal thickness of the children (Table 4 and Figure 1).
Foveal and temporal choroidal areas in the amblyopic eye were significantly decreased after wearing optical correction compared to those undergoing amblyopia treatment and controls. Previous research had shown that nonvascular smooth muscle cells in the stroma had a role in accommodation by varying choroidal thickness. The accommodation was impaired in amblyopic eyes than in normal eyes due to a choroidal compensation mechanism. Previous studies assumed that amblyopic subjects, after commencing amblyopia therapy, their eyes will initiate an alternative compensatory mechanism that reduce stromal areas and nonvascular smooth muscle cells, to compensate for refractive error and improve visual acuity. According to our findings, only amblyopic eyes showed a reduction in stromal area after therapy as a compensatory mechanism [8,19].
The study was limited by small sample size. Future, larger studies are recommended to investigate ambyliopia treatment more thoroughly.
5. Conclusion
The present findings suggested that choroidal thickness could be altered by different types of amblyopia. Myopic anisometropic amblyopia; could exhibit thin nasal CT area due to myopic development alterations (axial length elongation); with thick foveal and temporal regions. The foveal and temporal choroidal areas in amblyopic recovered eyes were significantly decreased after wearing optical correction compared to those undergoing amblyopia treatment and controls. Such a reduction compensated for refractive error and improved the best-corrected visual acuity.
Author Disclosure Statement
The authors declare no potential conflicts of interest with respect to the authorship, and/or publication of this article.
Disclosure:
This study has no financial or proprietary interests.
Acknowledgment:
This research project was supported by a grant from the Research Center of the Centre for Female Scientific and Medical Colleges Deanship of Scientific Research, King Saud University.
References
- Blair K, Cibis G & Gulani AC. Amblyopia. StatPearls 8 (2021).
- DeSantis D. Amblyopia. Pediatr Clin North Am 61 (2014): 505–518.
- Roger Chou, Tracy Dana MLS and Christina Bougatsos B. Screening for Visual Impairment in Children Ages 1-5 Years. Agency Healthc. Res. Qual 81 (2011).
- Holmes JM, Lazar EL, Melia BM, et al. Effect of age on response to amblyopia treatment in children. Arch Ophthalmol 129 (2011): 1451–1457.
- Park S H. Current Management of Childhood Amblyopia. Korean J. Ophthalmol 33 (2019): 557-568.
- Nickla D L & Wallman J. The multifunctional choroid. Prog Retin Eye Res 29 (2010): 144–168.
- Reiner A, Fitzgerald M EC, Del Mar N, et al. Neural control of choroidal blood flow. Prog Retin Eye Res 64 (2018): 96–130.
- Nishi T, Ueda T, MY, et al. Effect of optical correction on choroidal structure in children with anisohypermetropic amblyopia. Plos one 15 (2020).
- Xu J, et al. Macular choroidal thickness in unilateral amblyopic children. Invest. Ophthalmol. Vis. Sci. 55 (2014): 7361–7368.
- Aygit E D, Yilmaz I, Ozkaya A, et al. Choroidal thickness of children’s eyes with anisometropic and strabismic amblyopia. J AAPOS 19 (2015): 237–241.
- Read S A, Alonso-Caneiro D, Vincent SJ, et al. Longitudinal changes in choroidal thickness and eye growth in childhood. Investig Ophthalmol Vis Sci 56 (2015): 3103–3110.
- Helveston EM. Understanding, detecting and managing strabismus. Community Eye Heal J 23 (2010): 12–14.
- Gomez-Salazar F, Campos-Romero A, Gomez-Campaña H. et al. Refractive errors among children, adolescents and adults attending eye clinics in Mexico. Int J Ophthalmol 10 (2017): 796–802.
- Antonio-Santos A, Vedula S S, Hatt S R, et al. Occlusion for stimulus deprivation amblyopia. Cochrane Database Syst Rev 2 (2014): 005136.
- Williams C. Amblyopia. BMJ clin evid 2009 (2009): 0709.
- Tan CS, Ouyang Y, Ruiz H, et al. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Investig. Ophthalmol Vis Sci 53 (2012): 261–266.
- Bhende M, Shetty S, Parthasarathy M, et al. Optical coherence tomography: A guide to interpretation of common macular diseases. Indian J Ophthalmol 66 (2018): 20-35.
- Vincent S J, Collins M J, Read SA, et al. Retinal and Choroidal Thickness in Myopic Anisometropia. Invest Ophthalmol Vis Sci 54 (2013): 2445–2456.
- Aygit ED, Yilmaz I, Ozkaya A, et al. Choroidal thickness of children's eyes with anisometropic and strabismic amblyopia. J AAPOS 19 (2015): 237-241.