Effect of Handing a Written Document to Share Concerns and Values with Physicians When Making Decisions about Starting Drug Treatment among Patients: A Pre-post Quasi-Experimental Study with Propensity Score Matching
Article Information
Seiji Bito1*, Tomomi Iioka1, Yasuhiro Yamada1, Eiji Hiraoka2, Taiju Miyagami3, Naoki Nago4, Takahiro Mori5, Masamichi Sato6, Tadao Okada7, Tetsuro Hayashi1, Atsushi Asai8
1Division of Clinical Epidemiology/Department of General Internal Medicine, National Hospital Organization Tokyo Medical Center, 2-5-1 Higashigaoka, Meguro-ku, Tokyo-to, Japan, 152-8902
2Department of Internal Medicine, Tokyo Bay Urayasu Ichikawa Medical Center, 3-4-32 Todaijima, Urayasu-shi, Chiba-ken, Japan, 279-0001
3Department of General Medicine, Juntendo University Faculty of Medicine, 3-1-3 Hongo, Bunkyo-ku, Tokyo-to, Japan, 113-0033
4Musashi Kokubunji Park Clinic, 2-16-34-127 Nishimotomachi, Kokubunji-shi, Tokyo-to, Japan, 185-0023
5Department of General Internal Medicine, National Hospital Organization Nagasaki Medical Center, 2-1001-1 Kubara, Omura-shi, Nagasaki-ken, Japan, 856-0835
6Department of General Medicine, National Hospital Organization Takasaki General Medical Center, 36 Takamatsu-cho, Takasaki-shi, Gunma-ken, Japan, 370-0829
7Tesshoukai Kameda Family Clinic Tateyama, 4304-9 Masaki, Tateyama-shi, Chiba-ken, Japan, 294-0051
8Department of Medical Ethics, Tohoku University Graduate School of Medicine, 2-1 Seiryo-machi, Aoba-ku, Sendai-shi, Miyagi-ken, Japan, 980-8575
*Corresponding author: Seiji Bito, Division of Clinical Epidemiology/Department of General Internal Medicine, National Hospital Organization Tokyo Medical Center, 2-5-1 Higashigaoka, Meguro-ku, Tokyo-to, 152-8902, Japan.
Received: 18 May 2023; Accepted: 23 May 2023; Published: 05 June 2023
Citation: Seiji Bito, Tomomi Iioka, Yasuhiro Yamada, Eiji Hiraoka, Taiju Miyagami, Naoki Nago, Takahiro Mori, Masamichi Sato, Tadao Okada, Tetsuro Hayashi, Atsushi Asai. Effect of Handing a Written Document to Share Concerns and Values with Physicians When Making Decisions about Starting Drug Treatment among Patients: A Pre-post Quasi-Experimental Study with Propensity Score Matching. Journal of Biotechnology and Biomedicine. 6 (2023): 235-243.
Share at FacebookAbstract
Background: There is limited evidence on the effectiveness of decision aids in promoting clinicians' understanding of patients' subjective perceptions. The aim of this study was to assess the impact of using a standard patient-completed template to express patients' views, preferences or concerns to their clinicians in a clinical decision-making setting on patients' perceived decisional conflict and post-decision regret.
Methods: A pre-post quasi-experimental study with a six-month control period followed by a 12-month intervention period was conducted. Participants were recruited from six teaching hospitals and two clinics in Japan. The target population included 150 patients with diabetes mellitus, hypertension, and/or dyslipidaemia whose physicians had recently suggested drug treatment as a medical option. In the control period, a general informed consent booklet was distributed, and in the intervention period, a shared decision-making template was also provided. Patients were asked to complete the template, which was then attached to their electronic medical records.
Results: Two months after enrolment, the decision conflict and regret scales were mailed to patients. Three months after enrolment, the decision status for starting drug treatment and the concordance between the patients' decision statuses and the initial medical recommendations were observed. Seventy-nine and seventy-one participants assigned to the control and intervention groups were enrolled. Fifty-five patient pairs generated by propensity score matching were analysed. No significant difference was observed between the two groups in the subscale scores of the decision conflict scale and the decision regret scale. The relative risk of patients with a decision status of ‘still considering’ starting drug treatment was 2.2 (95% CI, 1.02-4.9) in the intervention group. The concordance rate between the physicians' recommendation to start drug treatment at enrolment and the patients' actual decision was 78.2% and 90.9% in the control and intervention groups, respectively (relative risk = 1.5; 95% CI, 1.05-2.2).
Conclusions: The use of a template document to express patients' personal values and feelings to their physicians is associated with longer deliberation times when making decisions about starting drug treatment; this may lead to decisions that are closer to medical recommendations.
Keywords
Decision tool; Shared decision making; Patient preferences; Patient-professional relations
Decision tool articles Decision tool Research articles Decision tool review articles Decision tool PubMed articles Decision tool PubMed Central articles Decision tool 2023 articles Decision tool 2024 articles Decision tool Scopus articles Decision tool impact factor journals Decision tool Scopus journals Decision tool PubMed journals Decision tool medical journals Decision tool free journals Decision tool best journals Decision tool top journals Decision tool free medical journals Decision tool famous journals Decision tool Google Scholar indexed journals Shared decision making articles Shared decision making Research articles Shared decision making review articles Shared decision making PubMed articles Shared decision making PubMed Central articles Shared decision making 2023 articles Shared decision making 2024 articles Shared decision making Scopus articles Shared decision making impact factor journals Shared decision making Scopus journals Shared decision making PubMed journals Shared decision making medical journals Shared decision making free journals Shared decision making best journals Shared decision making top journals Shared decision making free medical journals Shared decision making famous journals Shared decision making Google Scholar indexed journals Patient preferences articles Patient preferences Research articles Patient preferences review articles Patient preferences PubMed articles Patient preferences PubMed Central articles Patient preferences 2023 articles Patient preferences 2024 articles Patient preferences Scopus articles Patient preferences impact factor journals Patient preferences Scopus journals Patient preferences PubMed journals Patient preferences medical journals Patient preferences free journals Patient preferences best journals Patient preferences top journals Patient preferences free medical journals Patient preferences famous journals Patient preferences Google Scholar indexed journals Patient-professional relations articles Patient-professional relations Research articles Patient-professional relations review articles Patient-professional relations PubMed articles Patient-professional relations PubMed Central articles Patient-professional relations 2023 articles Patient-professional relations 2024 articles Patient-professional relations Scopus articles Patient-professional relations impact factor journals Patient-professional relations Scopus journals Patient-professional relations PubMed journals Patient-professional relations medical journals Patient-professional relations free journals Patient-professional relations best journals Patient-professional relations top journals Patient-professional relations free medical journals Patient-professional relations famous journals Patient-professional relations Google Scholar indexed journals medical treatment articles medical treatment Research articles medical treatment review articles medical treatment PubMed articles medical treatment PubMed Central articles medical treatment 2023 articles medical treatment 2024 articles medical treatment Scopus articles medical treatment impact factor journals medical treatment Scopus journals medical treatment PubMed journals medical treatment medical journals medical treatment free journals medical treatment best journals medical treatment top journals medical treatment free medical journals medical treatment famous journals medical treatment Google Scholar indexed journals antihypertensive articles antihypertensive Research articles antihypertensive review articles antihypertensive PubMed articles antihypertensive PubMed Central articles antihypertensive 2023 articles antihypertensive 2024 articles antihypertensive Scopus articles antihypertensive impact factor journals antihypertensive Scopus journals antihypertensive PubMed journals antihypertensive medical journals antihypertensive free journals antihypertensive best journals antihypertensive top journals antihypertensive free medical journals antihypertensive famous journals antihypertensive Google Scholar indexed journals hypertension† articles hypertension† Research articles hypertension† review articles hypertension† PubMed articles hypertension† PubMed Central articles hypertension† 2023 articles hypertension† 2024 articles hypertension† Scopus articles hypertension† impact factor journals hypertension† Scopus journals hypertension† PubMed journals hypertension† medical journals hypertension† free journals hypertension† best journals hypertension† top journals hypertension† free medical journals hypertension† famous journals hypertension† Google Scholar indexed journals diabetes mellitus articles diabetes mellitus Research articles diabetes mellitus review articles diabetes mellitus PubMed articles diabetes mellitus PubMed Central articles diabetes mellitus 2023 articles diabetes mellitus 2024 articles diabetes mellitus Scopus articles diabetes mellitus impact factor journals diabetes mellitus Scopus journals diabetes mellitus PubMed journals diabetes mellitus medical journals diabetes mellitus free journals diabetes mellitus best journals diabetes mellitus top journals diabetes mellitus free medical journals diabetes mellitus famous journals diabetes mellitus Google Scholar indexed journals drug articles drug Research articles drug review articles drug PubMed articles drug PubMed Central articles drug 2023 articles drug 2024 articles drug Scopus articles drug impact factor journals drug Scopus journals drug PubMed journals drug medical journals drug free journals drug best journals drug top journals drug free medical journals drug famous journals drug Google Scholar indexed journals sex and age articles sex and age Research articles sex and age review articles sex and age PubMed articles sex and age PubMed Central articles sex and age 2023 articles sex and age 2024 articles sex and age Scopus articles sex and age impact factor journals sex and age Scopus journals sex and age PubMed journals sex and age medical journals sex and age free journals sex and age best journals sex and age top journals sex and age free medical journals sex and age famous journals sex and age Google Scholar indexed journals
Article Details
Background
The style of clinical decision-making is evolving from a model of informed consent, which enhances patient autonomy, to a style of shared decision-making (SDM), in which patients and clinicians reach a decision through mutual engagement [1-4]. For patients to make an optimal decision through an SDM process, they should have a thorough understanding of their health conditions and available medical options; similarly, their healthcare professionals should have a deep understanding of their values and preferences for treatment [5-7]. However, a problematic issue arises when decision-making conversations are usually conducted by healthcare professionals (e.g., doctors) who are not sufficiently aware of patients' subjective perceptions of their own priorities, such as values and goals, and the difficulties they face in making decisions [8,9]. Patients are often reluctant to express their concerns or worries about the medical options recommended by their doctors [10,11]. This poor communication may prevent patients from making an optimal decision [10,12]. Furthermore, decisions about medical treatment made by patients who feel that their doctors do not understand their values may affect subsequent adherence to the chosen option [13]. There is a large body of evidence on the effectiveness of decision aids, which have been developed to improve patients' understanding of their own health status and treatment content, in creating an ideal environment for SDM [14-20]. Most of the decision aids that have been found to be effective have helped patients to better understand the medical rationale behind their decisions. However, there is a lack of evidence on the effectiveness of decision aids designed to help healthcare professionals understand their patients' situation and values. While Henselmans et al [21] reported that interventions aimed at providing communication training to physicians to enhance SDM could improve the SDM process, there is very little empirical evidence on how the messages physicians receive from their patients may actually affect the SDM process. The aim of this study was to assess the impact of using a standard template completed by patients to express their views, preferences or concerns to their clinicians in a clinical decision-making setting on their perceived decisional conflict, post-decision regret or divergence between their clinician's recommendations and their own preferences.
Methods
Study settings
The study was conducted in six teaching hospitals (two in Tokyo, one in Chiba, one in Gunma, one in Nagasaki, and one in Saitama) and two teaching clinics (one in Tokyo and one in Chiba) in Japan.
Study population and participants
The target population included patients with diabetes mellitus, hypertension and/or dyslipidaemia who had recently been advised by their physicians to start drug treatment. Inclusion criteria were as follows: 1) patients aged 20 years or older who were receiving outpatient care in a centre where the study was conducted and who were newly considered by a physician for treatment with antidiabetic, antihypertensive, or antidyslipidemic drugs (including patients who had previously been continuously treated with drugs other than those used in the study), and 2) patients with a time interval of one week or more between the physician's recommendation and their decision.
Study design
We conducted a pre-post quasi-experimental study. During the pre-intervention period, patients received a booklet called ‘Guide to Shared Informed Consent Between Patients and Healthcare Professionals’ (hereafter referred to as the ‘guide’), which explained the principles of informed consent. After data collection for the six-month pre-intervention period was completed, a 12-month intervention period began, during which the intervention was delivered to enrolled study participants.
Intervention
Similar to the pre-intervention period, during the intervention period, patients were given the participant's guide. At the same time, patients were asked to complete a 'medical decision support template' (hereafter referred to as the ‘template’) to express their preferences and values in writing to the physician in charge of making a decision about starting drug treatment. Patients were asked to express themselves freely on five items. An example of how to complete a support template is shown in Figure 1, along with the five items.

Figure 1: Medical decision-making support template.
Patients could either complete the template at the outpatient clinic on the day they received it, or complete it at home and return it to the clinic within two weeks of consent. The researcher attached the completed template to the patient's electronic medical record. The patient's doctor was then advised to review the contents of the template before the next consultation.
Outcomes and independent variables
The primary outcomes used to assess the effect of the intervention were the decision conflicts and regrets generated by the patients' decisions. The Decision Conflict Scale (DCS), developed by O'Connor [22] and translated into Japanese by Kawaguchi et al. [23], was used to assess the patients' decisional conflicts. The Decision Regret Scale (DRS), developed by Brehaut et al. [24] and translated into Japanese by Tanno et al. [25], was used to assess decision regret. We assessed two decision statuses three months after study enrolment as secondary outcomes. First, we assessed the percentage of patients who had already started or decided to start drug treatment, those who had refused to start, and those who had postponed their decision. Based on a chart review of patients' medical records, patients' decision status was classified as 'decided to start' if they had already started or decided to start the prescribed drug. Conversely, it was classified as 'decided NOT to start drugs' if they refused to start treatment. Patients who were still undecided were classified as 'still considering'. Second, we looked at the agreement between the doctors' recommendation to start drug treatment at the time of enrolment and the patients' actual decision three months after enrolment. At enrolment, physicians were asked to rate their recommendation to start drug treatment by selecting one of the following five options: 1) ‘strongly recommend’, 2) ‘would rather recommend’, 3) ‘medically neutral’, 4) ‘would rather not recommend’, and 5) ‘do not recommend’. Based on the responses given at enrolment, cases in which options 1 or 2 were selected were listed as 'recommend drug', whereas cases in which options 3, 4 or 5 were selected were listed as 'do not recommend drug'. Three months after enrolment, the doctors' recommendation recorded at enrolment and the patients' actual decision recorded three months later were defined as 'concordant' if the patients' medical records for cases listed as 'recommend drug' had a status of 'decided to start drugs', or if their medical records for cases listed as 'not recommend drug' had a status of 'decided NOT to start drugs' or 'still considering'. Otherwise, the physician's initial recommendation and the patient's actual decision were defined as 'discordant'. The following information was collected at enrolment: patients' sex and age, whether they lived alone, whether they attended the outpatient clinic with family members, the name of the disease for which drug treatment was to be started, and whether they were still taking other drugs in addition to the newly recommended drug. We also collected data on patients' health locus of control as a confounding variable that could affect the primary outcome, as reported in previous literature [26,27]. The Japanese version of the health locus of control (LOC) scale was used to measure this [28,29].
Data collection
We used three methods of data collection: a patient questionnaire survey, a medical record survey, and a physician survey. These surveys were administered to both patients and their physicians by the researchers at each institution at the time of enrolment. Patients were asked to complete a questionnaire that included a baseline DCS and the Japanese version of the Health LOC scale [28,29]. Physicians were asked to rate their level of recommendation for prescribing the target drug. Two months after enrolment, the researchers mailed questionnaires containing the DCS and DRS to patients. Patients returned their completed questionnaires to the study site. Baseline LOC scale scores were calculated for the six domains included, with a minimum score of 5 points and a maximum of 30 points. The scores of the five subscales and the total score of the DCS measured at baseline and two months later were scaled from 0 to 100 points. Three months after enrolment, investigators at each site collected data on secondary endpoints based on a chart review of patients' medical records. Each site began enrolling the pre-intervention group after receiving ethics committee approval. After completion of the six-month pre-intervention period, the 12-month intervention group was established. The pre-intervention enrolment period was from September 2016 to August 2017, and the intervention enrolment period was from March 2017 to March 2018.
Statistical analysis
After the completion of data entry and collection, propensity score matching was performed after logistic regression analysis on patients' age, sex, whether they lived alone, whether they were accompanied, and whether they were already prescribed medication. LOC/DCS subscale scores at baseline were confounding variables and control/intervention was the dependent variable. The tolerance level for comparisons was set at 0.05. A Student's t-test was performed to compare the mean DRS and DCS scores between the two matched groups. The two secondary outcomes were analysed for frequency comparison using a chi-squared test. The primary outcome used for sample size estimation was the comparison of mean DRS scores. Based on the Japanese version of the DRS, the clinically expected effect size was set at 0.5. The required sample size was estimated using a two-tailed test with a power of 0.8 and a significance level of 0.05. Consequently, we estimated that 128 patients (64 each in the control and intervention groups) would be required.
Results
Of the 175 participants who gave informed consent, a total of 88 (control group) and 87 (intervention group) patients were enrolled during the pre-intervention and intervention periods, respectively. Of these, 25 participants who did not respond to the baseline questionnaire were excluded from the analysis. After data entry and collection, propensity score matching was performed and data from 55 patients for each group (total n = 110), adjusted for background factors, were selected for analysis. Of these, questionnaire data were also collected from 50 and 40 patients in the control and intervention groups, respectively (Figure 2).
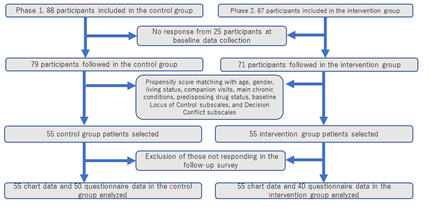
Figure 2: Flow diagram of patients' enrolment.
Table 1 shows the background factors of the two sample groups extracted by propensity score matching. There were no significant differences between the two groups in the frequency distribution of patients' sex, age, whether they lived alone, whether they were accompanied, whether they were already prescribed a medication at the time of enrolment, and the prevalence of major illnesses in relation to their decision to start drug treatment. In addition, no significant differences were observed between the groups in the mean scores of the health LOC and decision conflict subscales, nor in the mean total scores at enrolment.
|
Variable |
No. (%) |
||
|
Control Group |
Intervention Group |
P value |
|
|
No. |
55 |
55 |
|
|
Gender (Women) |
29(52.7) |
27(49.1) |
0.70 |
|
Age group |
|||
|
20-49 |
16(29.1) |
15(27.3) |
0.93 |
|
50-64 |
20(36.4) |
22(40.0) |
0.93 |
|
65- |
19(34.5) |
18(32.7) |
0.93 |
|
Living alone |
9(16.4) |
8(14.5) |
0.79 |
|
Consultation with a companion |
8(14.5) |
8(14.5) |
1.00 |
|
Is there any drug at enrollment? |
24(43.6) |
21(38.2) |
0.56 |
|
Comorbidity |
|||
|
Diabetes |
8(14.5) |
6(10.9) |
0.57 |
|
Hypertension |
27(49.1) |
26(47.3) |
0.85 |
|
Hyperlipidemia |
25(45.5) |
26(47.3) |
0.85 |
|
Baseline Locus of Control Scale (5-30) score |
|||
|
Supernatural, mean±SD |
11.1±3.6 |
11.1±4.3 |
0.95 |
|
Internal, mean±SD |
23.4±2.7 |
23.3±3.1 |
0.84 |
|
Family, mean±SD |
23.2±3.5 |
23.4±5.0 |
0.82 |
|
Chance, mean±SD |
14±4.1 |
14.6±4.4 |
0.50 |
|
Professional, mean±SD |
21±3.3 |
21±4.0 |
1.00 |
|
Baseline Decision Conflict Scale (0-100 score) |
|||
|
Informed, mean±SD |
46.3±15.9 |
46.2±18.8 |
0.99 |
|
Values clarity, mean±SD |
52.1±16.2 |
51.5±18.6 |
0.86 |
|
Support, mean±SD |
41.2±16.2 |
40.8±13.8 |
0.87 |
|
Uncertainty, mean±SD |
54.1±18.0 |
55.5±19.9 |
0.71 |
|
Effective decision, mean±SD |
43.9±14.9 |
43.4±16.0 |
0.88 |
|
Total score, mean±SD |
47.2±13.0 |
47.2±13.7 |
0.98 |
|
Physicians' recommendation at enrollment |
|||
|
Strongly recommend drug treatment |
14(25.5) |
17(30.9) |
0.40 |
|
Would rather recommend drug treatment |
21(38.2) |
16(29.1) |
0.40 |
|
Medically neutral about drug treatment |
15(27.3) |
11(20.0) |
0.40 |
|
Would rather not recommend drug treatment |
3(5.5) |
8(14.5) |
0.40 |
|
Not recommend drug treatment |
2(3.6) |
3(5.5) |
0.40 |
Table 1: Patient characteristics and baseline variables.
A comparison of the mean DCS and DRS scores between the two groups two months after enrolment is shown in Table 2. The mean DCS subscale scores for both groups and the 95% confidence interval (CI) of the difference between the mean scores were as follows 32.4 and 39.2 (95% CI -14.5-0.9) for the informed subscale, 39.9 and 43.1 (95% CI -11.0-4.6) for the values clarity subscale, 31.6 and 34.1 (95% CI -9.9-4.8) for the support subscale, 37.9 and 43.2 (95% CI -13.0-2. 5) for the uncertainty subscale, 31.1 and 36.5 (95% CI -13.1-2.3) for the effective decision subscale, and 34.3 and 38.5 (95% CI -10.8-2.6) for the effective decision subscale, respectively; none of them showed a statistically significant difference. The mean DRS scores for both groups and the 95% CI of the difference between the mean scores were 21.6 and 21.7 (95% CI -5.7-5.5), respectively, showing no statistically significant difference.
|
Variable |
mean±SD |
|
|
|
Control (N=55) |
Intervention (N=55) |
||
|
DCS score |
|||
|
Informed |
32.4±14.5 |
39.2±21.7 |
-14.5 - 0.9 |
|
Values clarity |
39.9±15.8 |
43.1±21.1 |
-11.0 - 4.6 |
|
Support |
31.6±13.8 |
34.1±20.6 |
-9.9 - 4.8 |
|
Uncertainty |
37.9±15.5 |
43.2±20.7 |
-13.0 - 2.5 |
|
Effective decision |
31.1±14.1 |
36.5±21.4 |
-13.1 - 2.3 |
|
Total score |
34.3±12.2 |
38.5±18.5 |
-10.8 - 2.6 |
|
DRS score |
21.6±12.0 |
21.7±14.3 |
-5.7 - 5.5 |
Table 2: Decision conflict and decision regret two months after starting observation.
The frequency distribution of the decision status three months after enrolment and the concordance between the physicians' recommendation for drug treatment at enrolment and the patients' actual decisions three months later are shown in Figure 3. In the control group, the percentages of events requiring a decision with a status of ‘decided to start drugs’, ‘decided not to start drugs’, and ‘still considering’ were 49.1% (n = 27), 41.8% (n = 23), and 9.1% (n = 5), respectively, and in the intervention group they were 43.6% (n = 24), 29.1% (n = 16), and 27.3% (n = 15), respectively (P = 0.040). The relative risk of the percentage of events requiring a decision with a status of ‘still considering’ in the intervention group was 2.2 (95% CI 1.02-4.86). The percentages of concordance between the physicians' recommendation for drug treatment at enrolment and the actual decisions made by patients three months later in the control and intervention groups were 78.2% and 90.9%, respectively (P = 0.065), with a relative risk of 1.5 (95% CI 1.05-2.2; Figure 4).
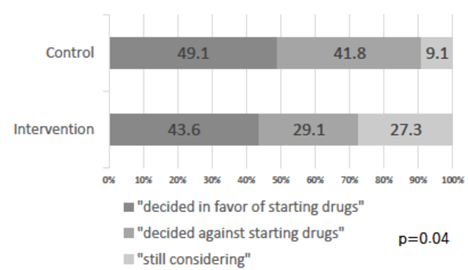
Figure 3: Decision status three months after starting observation.
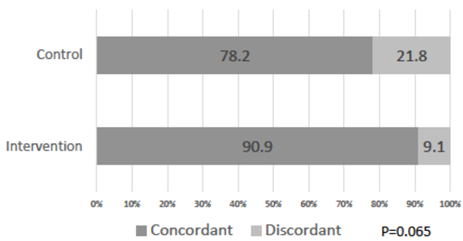
Figure 4: Concordance between the initial physicians’ recommendation and decision-making status after three months.
Discussion
Interpretations and generalisability
In the decision-making model based on informed consent, patients are the ultimate autonomous agents on whom this process is based, including the medical rationale, which relies entirely on patient understanding [30,31]. In contrast, in the SDM model, the decision-making process is characterised by 'team talk', with the patient remaining the final decision maker [2,3,32,33]. However, the progress of the conversation prior to the actual deliberation process may be hindered by circumstances such as patients not properly verbalising their thoughts to the healthcare professional or psychological barriers that prevent patients from expressing their preferences and needs, which is common in East Asian cultures [34-37]. In addition to the decisional conflict felt at the time of the decision, results from previous studies have identified concerns about the treatment, trust in the physician, and post-decision changes in one's health status as potential factors that may influence post-decision regret [38-40]. Our results showed that patients' expression of their preferences and beliefs to their doctors did not lead to a statistically significant difference in the level of conflict and regret caused by their own decisions, as measured by rating scales. However, although there was no statistically significant difference in decisional conflict, there was a trend towards higher mean scores for all DCS subscales in the intervention group. This may be due to a relative increase in patients' awareness of participating in the decision-making process, brought about by the opportunity to express their own views to their doctors, rather than uncritically following the doctors' recommendations. In terms of the actual decision, there was a significant difference between the group that used the template and the group that did not in two key areas. First, the percentage of patients who postponed their decision three months after the event that required a decision was higher in the intervention group. This suggests that using the template may make doctors more sensitive to patients' concerns and values, help them to avoid making hasty decisions, and encourage them to engage in a discussion about the decision. In clinical practice, it is usually acceptable for patients to take some time to consider all the possible medical options before deciding whether to start drug treatment for a chronic condition. In addition, the use of the template in the intervention group tended to result in a higher rate of agreement between what the doctors thought was the best option and the patients' final decision. Our results suggest that patients who were willing to express their feelings were also more likely to understand and respect the views of their healthcare providers; patients expressing their values and concerns to their doctors is conducive to more clinically appropriate decisions. Furthermore, when explaining and recommending medical options to their patients, doctors should be aware of their preferences and values so that their patients are more likely to understand and accept their recommendations.
Study limitations
There were several limitations to this study. First, given the influence of potential confounding variables in the evaluation of the study hypothesis and the complexity of the behavioural conceptual framework in which the study was based, the results should be interpreted within a complex framework that takes into account the inherent influence of various potential factors between intervention and effect. Second, the internal validity of the study is limited compared with a randomised controlled trial. Third, as the outcome that was found to be statistically significant was the one that we set as a secondary outcome, it may be necessary to evaluate the statistical results using stricter criteria than the 95% CI. Finally, because the study had insufficient statistical power, the results of a single study alone could not be used to develop a general theory.
Conclusions
Our results showed that the use of a structured template, completed by patients, to express their values and concerns to their doctors when making decisions about starting drug treatment for chronic diseases did not affect their decisional conflict and regret. Conversely, the same intervention was suggested to give patients more time to reflect, resulting in a final decision that was more in line with medical recommendations. Expressing patients' subjective views to doctors in a written document can effectively promote SDM between patients and healthcare providers.
Declarations
Ethical approval and consent to participate
Ethical approval and consent to participate were collected prior to the start of the study. All participating institutions applied to the National Tokyo Medical Center Institutional Review Board and received approval to conduct the research (Ethics Committee Authorisation No. R17-163). For both the pre-intervention and intervention periods, patients who met the eligibility criteria were given a written explanation of the purpose and protocol of the study, the content of the intervention and the method of data collection, and verbal consent was obtained. As the trial was not designed as a two-arm clinical trial, it was not registered in the UMIN Clinical Trials Registry.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analysed in the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
This study was supported by funding from JSPS Grants-in-Aid for Scientific Research (KAKENHI), grant number JP26460623 for the conduct of the research and JP 20H03922 for data analysis and manuscript writing. The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Author contributions
SB had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors were responsible for the study’s concept and design. YY, TH, EH, Taju Miyagmani, NN, Takahiro Mori, and MS acquired data and TO analyzed and interpretated the data. SB drafted the manuscript; managed the data; received the funding; and provided administrative, technical, and material support. TI conducted the statistical analysis and supervised the study. All authors read and approved the final manuscript.
Acknowledgments
Besides the authors, we would like to thank Arifumi Takazawa for their help in collecting the data for our analysis. We would like to thank Editage (www.editage.com) for English language editing.
References
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: What does it mean? (or it tak es at least two to tango).Soc Sci Med 44 (1997): 681-692.
- Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: Multistage consultation process.BMJ 359 (2017): j4891.
- Elwyn G, Frosch D, Thomson R, et al. Shared decision making: A model for clinical practice.J Gen Intern Med 27 (2012): 1361-1367.
- Slim K, Bazin JE.From informed consent to shared decision-making in surgery.J Visc Surg 156 (2019): 181-184.
- Elwyn G, Frosch DL, Kobrin S.Implementing shared decision-making: Consider all the consequences.Implement Sci 11 (2016): 114.
- Kambhampati S, Ashvetiya T, Stone NJ, et al.Shared decision-making and patient empowerment in preventive cardiology.Curr Cardiol Rep 18 (2016):
- Megregian M, Nieuwenhuijze M.Choosing to decline: Finding common ground through the perspective of shared decision making.J Midwifery Women’s Health 63 (2018): 340-346.
- Charles C, Gafni A, Whelan T.Decision-making in the physician–patient encounter: Revisiting the shared treatment decision-making model.Soc Sci Med 49 (1999): 651-661.
- Stiggelbout AM, Pieterse AH, De Haes JC.Shared decision making: Concepts, evidence, and practice. Patient Educ Couns 98 (2015): 1172-1179.
- Covvey JR, Kamal KM, Gorse EE, et al. Barriers and facilitators to shared decision-making in oncology: A systematic review of the literature.Support Care Cancer 27 (2019): 1613-1637.
- Faiman B, Tariman JD.Shared decision making: Improving patient outcomes by understanding the benefits of and barriers to effective communication.Clin J Oncol Nurs 23 (2019): 540-542.
- Visser M, Deliens L, Houttekier D.Physician-related barriers to communication and patient- and family-centred decision-making towards the end of life in intensive care: A systematic review.Crit Care 18 (2014): 604.
- Bukstein DA.Patient adherence and effective communication. Ann Allergy Asthma Immunol 117 (2016): 613-619.
- Coronado-Vázquez V, Canet-Fajas C, Delgado-Marroquín MT, et al.Interventions to facilitate shared decision-making using decision aids with patients in Primary Health Care: A systematic review.Med 99 (2020): e21389.
- Sunjaya AP, Bao L, Martin A, et al. Systematic review of effectiveness and quality assessment of patient education materials and decision aids for breathlessness. BMC Pulm Med 22 (2022): 237
- LeBlanc A, Herrin J, Williams MD, et al.Shared decision making for antidepressants in primary care: A cluster randomized trial.JAMA Intern Med 175 (2015): 1761-1770.
- Perestelo-Pérez L, Rivero-Santana A, Boronat M, et al. Effect of the statin choice encounter decision aid in Spanish patients with type 2 diabetes: A randomized trial. Patient Educ Couns 99 (2016): 295-299.
- Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Sys Rev 4 (2017): CD001431.
- Weymiller AJ, Montori VM, Jones LA, et al. Helping patients with type 2 diabetes mellitus make treatment decisions: Statin choice randomized trial.Arch Intern Med 167 (2007): 1076-1082.
- Grüne B, Kriegmair MC, Lenhart M, et al. Decision Aids for Shared Decision-making in Uro-oncology: A Systematic Review. Eur Urol Focus 8 (2022): 851-869.
- Henselmans I, van Laarhoven HWM, van Maarschalkerweerd P, et al. Effect of a skills training for oncologists and a patient communication aid on shared decision making about palliative systemic treatment: A randomized clinical trial.Oncol 25 (2020): e578-e588.
- O’Connor AM.Validation of a decisional conflict scale.Med Decis Mak 15 (1995):25-30.
- Kawaguchi T, Azuma K, Yamaguchi T, et al.Development and validation of the Japanese version of the Decisional Conflict Scale to investigate the value of pharmacists’ information: A before and after study.BMC Medical Inform Decis Mak 13 (2013): 50.
- Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale.Med Decis Mak 23 (2003): 281-292.
- Tanno K, Bito S, Isobe Y, et al. Validation of a Japanese version of the decision regret scale.J Nurs Meas 24 (2016): E44-E54.
- Metz MJ, Veerbeek MA, van der Feltz-Cornelis CM, et al. Decisional conflict in mental health care: A cross-sectional study.Soc Psychiatry Psychiatric Epidemiology 53 (2018): 161-169.
- Schneider A, Körner T, Mehring M, et al. Impact of age, health locus of control and psychological co-morbidity on patients’ preferences for shared decision making in general practice. Patient Educ Couns 61 (2006): 292-298.
- Kuwahara A, Nishino Y, Ohkubo T, et al.Reliability and validity of the Multidimensional Health Locus of Control Scale in Japan: Relationship with demographic factors and health-related behavior.Tohoku J Exp Med 203 (2004): 37-45.
- Wallston KA.The validity of the multidimensional health locus of control scales.J Health Psychol 10 (2005): 623-631.
- Lustig BA.Informed consent as a tool for medical management: A review of Informed Consent: Patient Autonomy and Physician Beneficence within Clinical Medicine by Stephen Wear.J Med Philos 21 (1996): 101-109.
- Whitney SN, McGuire AL, McCullough LB.A typology of shared decision making, informed consent, and simple consent.Ann Intern Med 140 (2004): 54-59.
- Beers E, Lee Nilsen M, Johnson JT.The role of patients: Shared decision-making.Otolaryngol Clin North Am 50 (2017): 689-708.
- Hashim MJ.Patient-centered communication: Basic skills.Am Fam Physician 95 (2017): 29-34.
- Claramita M, Utarini A, Soebono H, et al.Doctor–patient communication in a Southeast Asian setting: The conflict between ideal and reality.Adv Health Sci Educ Theory Pract 16 (2011): 69-80.
- Ohtaki S, Ohtaki T, Fetters MD.Doctor–patient communication: A comparison of the USA and Japan.Fam Pract 20 (2003): 276-282.
- Torishima M, Urao M, Nakayama T, et al.Negative recollections regarding doctor–patient interactions among men receiving a prostate cancer diagnosis: A qualitative study of patient experiences in Japan.BMJ Open 10 (2020): e032251.
- Koyama T, Nawa N, Itsui Y, et al. Facilitators and barriers to implementing shared decision making: A cross-sectional study of physicians in Japan. Patient Educ Couns 105 (2022): 2546-2556.
- Becerra Pérez MM, Menear M, Brehaut JC, et al.Extent and predictors of decision regret about health care decisions: A systematic review.Med Decis Mak 36 (2016): 777-790.
- Elidor H, Adekpedjou R, Zomahoun HTV, et al. Extent and predictors of decision regret among informal caregivers making decisions for a loved one: A systematic review.Med Decis Mak 40 (2020): 946-958.
- Tanno K, Bito S.Patient factors affecting decision regret in the medical treatment process of gynecological diseases.J Patient-Rep Outcomes 3 (2019): 43.

 95%CI
95%CI