Differentiation of Human Adipose Tissue Derived Mesenchymal Stem Cells to Functional Hepatocytes like Cells: Exploring the Niche and Admscs Conditioned Media
Article Information
Rasia Sultana, Anugya Bhatt*
Division of Thrombosis Research, Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvnanthpuram, India
*Corresponding author: Anugya Bhatt, Division of Thrombosis Research, Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvnanthpuram, 695012, India
Received: 05 January 2021; Accepted: 09 March 2021; Published: 19 March 2021
Citation:
Rasia Sultana, Anugya Bhatt. Differentiation of Human Adipose Tissue Derived Mesenchymal Stem Cells to Functional Hepatocytes like Cells: Exploring the Niche and Admscs Conditioned Media. Archives of Clinical and Biomedical Research 5 (2021): 169-184.
Share at FacebookAbstract
Tissue engineered approaches have been used for hepatic regeneration and growing hepatic cells in-vitro, however it remains challenging due to the lesser proliferative capacity of the hepatic cells as well as cell source availability due to damaged tissues. Stem cells could be an alternative strategy if differentiated to functional hepatic like cells by providing suitable niche or culture conditions. In the present study we tried to explore differentiation of mesodermal origin cells to the endodermal origin hepatic cells under different culture conditions including fibrin niche and adipocytes derived mesenchymal stem cells conditioned medium. Fibrin provides better cell adhesion and proliferation. Mesenchymal stem cells were isolated and characterized. Two step induction was used for differentiating into hepatic like cells. Functionality of the differentiated hepatic like cells was analyzed using Cyto P 450, glycogen storage and urea/albumin assay at different time points. It was observed the fibrin niche supports the hepatic differentiation and differentiated cells shows hepatic functions. This approach may be used to generate alternative cell source for hepatic tissue engineering.
Keywords
Adipose derived mesenchymal stem cells; Hepatic differentiation; Glycogen storage
Adipose derived mesenchymal stem cells articles; Hepatic differentiation articles; Glycogen storage articles
Article Details
1. Introduction
Liver is one of the vital organs of the human body having critical roles to play including storage and release of glucose, conversion of ammonia to urea, removal of drugs and toxins from blood etc. Liver failure has increased in recent years and liver transplantation is becoming the only solution available. However, due to the shortage of organ donors the morbidity has increase significantly. Cell based therapy could be an alternative approach to this problem. Various types of stem cells have been used for differentiation towards hepatic cells including embryonic stem cells, adult stem cells, however ethical concerns for embryonic stem cells makes it difficult to use for therapeutic application. Mesenchymal stem cells provide a good platform for stem cell therapy. Cell-based therapeutic strategies includes various approaches like cell transplantation and bioartificial liver devices [1]. However, autologous hepatic tissue maintain the phenotype of primary hepatocytes in culture limits the application. This led to critical scarcity of human hepatocytes as a stumbling block to advancement of cell-based therapies. Alternative cell sources for hepatic differentiation could be possible way for overcoming this scarcity [2]. ADMSCs hold promise here due to its multi lineage differentiation potential as well as non immunogenic nature [3, 4]. There are many factors which contribute to the potential of MSCs in cell therapy which includes ease of accessibility, less time requirements for growth and expansion, ease of biopreservation, less chance of teratoma formation, immunomodulation and minimal ethical issues [5, 6]. However, to obtain functional hepatic like cells, homing/ niche/microenvironment is critical. Fibrin, a plasma protein is well known for enhancing cell adhesion and proliferation [7]. Thus in the current study we aimed to optimize and explore fibrin niche for differentiation of Adipose Derived Mesenchymal Stem cells (ADMSCs) into hepatic like functional cells. MSCs has been assumed to be a recruitment source at the time of liver regeneration [8]. It is reported that MSCs do not express early hepatic specific genes but can differentiate to mature hepatocyte-like cells [9]. Various researches point out that the key players in bringing about hepatic differentiation of MSCs are appropriate niche and Insulin-transferrin-selenium (ITS), human epidermal growth factor (hEGF), human hepatocyte growth factor (hHGF) and hOSM (human Oncostatin M) [10 -12]. In this study we have explored specific combination of Thrombin, Fibrinogen and Gelatin to support the hepatic differentiation of the MSCs and their survival. The use of biomimetic and biospecific molecules for coating will aid to regulate the properties and function of cells [13, 14]. It is possible to attach these bioactive molecules in specific concentrations and spatial distributions whereby we can regulate the adhesion as well as growth, viability and differentiation of the cells [15].
2. Materials and Methods
2.1 Isolation and characterization of ADMSCs
Human adipose tissue was collected from the patients undergoing bypass surgery at SCTIMST, Trivandrum and the tissue was collected after obtaining the Institute Ethics Committee approval (SCT/IEC/1231/June2018, SCT/IC-SCR/43/March 2017). ADMSCs were isolated as described by Zuk et al 2002 [16]. Briefly, the tissue obtained was washed with sterile Hank’s Balanced Salt Solution (HBSS) and was minced thoroughly. Minced tissue was washed again in HBSS and kept for digestion with 4mg/ml Collagenase type I enzyme (Merck Sigma, USA) for 45 min at 37°C with continuous shaking at 120 rpm in an incubator shaker (Kuhner Shaker, Switzerland). Undigested tissue sections were discarded and the remaining solution was sieved using a 70μm nylon mesh (BD Falcon, USA). Serum containing medium was added to sieved tissue suspension and this was centrifuged at 400g for 6 minutes to obtain cell pellet. Resuspended the pellet in 10% FBS (FBS; Gibco, USA) containing Dulbecco’s modified Eagle’s medium DMEM supplemented with 10% Fetal Bovine Serum) and Antibiotic-Antimycotic solution (ABAM; Invitrogen, USA). The cells were seeded to a 25cm2 tissue- culture polystyrene flask (TCPS; Nunc, Denmark) and incubated at 37°C under 5% CO2. Medium was changed at 3 day intervals. Upon reaching ~ 80% confluence, cells were passaged by standard trypsinization protocol using 0. 25% Trypsin- EDTA (Invitrogen, USA) for expansion of cell numbers. Cultured MSCs from passage two-five were characterized using flowcytometry. In Brief, cells (1X106) from second passage were fixed with 3. 7% paraformaldehyde for 20 min at room temperature. After fixation cells were thoroughly washed with PBS at 400g for 6 minutes three times. Cells were blocked using 3% BSA in PBS for 10 min. Washed cells were then stained with specific positive markers CD90, CD105 and negative markers CD14 and CD45. After 1hour incubation in dark, cells were washed at 400g for 6 minutes, twice in PBS. Cell pellet was resuspended in sheath fluid and flowytometric analysis was carried out using BD FACS ARIA II. Unstained cells were used to gate the cell population. Data was analyzed using FlowJo software (Tree Star Inc. , USA). Trilineage differentiation of hADMSCs was done to prove the multipotency, using StemPro medium (Life Technologies, USA), as per the standard procedure. For adipogenic induction 1×104 cells/cm2 and for osteogenic induction 5×103 cells/cm2 respectively, were seeded and cultured in respective Stem Pro induction medium for 21 days with medium change once in three days. For chondrogenic differentiation, cells were seeded at high density as microdroplets and grown for 14 days in respective medium. Differentiation to adipogenic, osteogenic and chondrogenic lineages was confirmed by staining with Oil Red O, Alizarin Red S and Toluidine blue stains respectively, as per the standard protocols. The staining pattern was observed using light microscope (Leica, Germany) and images were acquired using LAS software (Leica, Germany).
2.2 Preparation of fibrin matrix
Combination of Thrombin, Fibrinogen and Gelatin in the ratio of 1:2:0. 05 was used as a culture matrix. In-house prepared clinical grade fibrinogen and thrombin were used for the coating the culture dishes. In brief, lyophilized thrombin and fibrinogen was reconstituted in the respective solvents and then diluted with 25 mM calcium chloride and sterile water to get 5 IU/ml and 2 mg/ml concentration respectively. Culture flasks were incubated with thrombin at 37°C for 30 minutes. Gelatin was added to fibrinogen solution. After 30 minute incubation, thrombin treated plates and flasks were streaked with fibrinogen-gelatin solution such that no space was left uncovered. Multi layering was avoided during the streaking. At the end, a gentle swirl was given and the plates and flasks were incubated at 30°C for 30 minutes for clot formation to take place. Culture plates/flasks were frozen at -80°C for overnight and were lyophilized. Coated plates and flasks were stored at 4°C till the time of use.
2.3 Induction of ADMSCs to hepatic like cells
Mesenchymal stem cells in passage 2-5 were selected
for the hepatic induction. The cells were trysinized and seeded at a density of 10,000 cells/cm². Cells were induced for the differentiation using growth factors. To analyze the effect of fibrin gelatin niche, culyures without growth factor inductions were also used. ADMSCs secrete proteins /growth factors which may help in the differentiation. Thus ADMSCs conditioned medium collected from the ADMSCs culture was also analyzed. Uninduced cultures with and without fibrin matrix were used as control. Experimental groups are described below.
ADMSCs were differentiated to hepatic like cells in two step induction protocol as described by Lee et al. , 2004 [17] hepatic initiation and hepatic maturation. In brief, cells were cultured in 10% FBS for 24h and then in serum free medium for 48h with epidermal growth factor and basic fibroblast growth factor (bFGF). After the completion of 48h , cells were provided with differentiation medium (serum-free DMEM) consisting of Hepatocyte growth factor (Sigma Aldrich), basic fibroblast growth factor and Nicotinamide (Sigma Aldrich) for seven days. Following this, the cells were exposed to serum-free media supplemented with Dexamethasone (Sigma Aldrich) and Insulin-transferrin-sodium selenite (Sigma Aldrich) for a period of two weeks. The unduced groups with and without fibrin matrix were continued to be supplemented with 10% FBS containing DMEM throughout the experiment. Cells were observed morphologically using microscope at different time points.
2.4 Functional characterization of differentiated hepatocyte-like cells
Differentiated cells were analyzed for the functional hepatocytes assay.
2.4.1 Indocyanine green uptake assay: The Indocyanine green (cardiogreen) dye (Sigma Aldrich) was dissolved in sterile water to make a 1 mg/ml solution. The cells were incubated with 1 mg/ml ICG solution for 15 minutes at 37°C, and rinsed thrice with PBS. The cellular uptake of the dye was observed under a light microscope (Leica, Germany) and the images were acquired at different magnifications using LAS software (Leica, Germany) [18].
2.4.2 Glycogen storage assay: The glycogen storage by the cells was assayed using Periodic acid-Schiff staining (PAS). Cells were permeabilized with 0. 1% Triton X-100 for 10 minutes followed by fixation in 4% formaldehyde. Cells were stained with 1% periodic acid for 5 minutes at room temperature. Cells were rinsed thrice with distilled water and then incubated with Schiff’s solution for 15 minutes at room temperature. Cells were counter stain with haematoxylin for 1 to 2 minutes and then were washed thoroughly with dH?O. Cells were observed using a light microscope (Leica, Germany) and the images were captured at different magnifications using LAS software (Leica, Germany).
2.4.3 EROD assay for cyto P 450: Cells were incubated in DMEM media containing Ethoxyresorufin at 37°C for four hours and were counter stain with Hoechst. Cells were washed in PBS and were observed under a fluorescence microscope (Leica, Germany) to check for the presence of Resorufin. Images were captured at 10x magnification using LAS software (Leica, Germany).
2.4.4 Urea assay: The presence of urea in the cell cultured-media (from day 6 and day 10) was assayed using BioChain urea assay kit. Briefly, the media samples were mixed with 200μl of provided working reagent, incubated at room temperature for 20 minutes and the absorbance was measured at 470-550nm using Bio-Rad iMarkmicroplate absorbance reader. Data analysis was carried out using MPM6 software.
2.4.5 Human albumin ELISA: The presence of secreted albumin in the media (from day 6 and day 10) was assayed using Human albumin ELISA quantification set from Bethyl Laboratories [Roll &Willenbring 2010] as well as by SDS- PAGE. Briefly, 100μl of coating antibody (1:100 dilution, diluted in coating buffer) was added to each well and incubated at room temperature for one hour. Wells were incubated with blocking buffer for 30 minutes. The samples and the standards provided with the kit were added to the wells (100μl each) and incubated at room temperature for one hour. Wells were rinsed with PBS thrice and were incubated with 100μl of HRP detection antibody (1:100,000 dilution) for one hour at room temperature. After the incubation, TMB substrate solution was added and the plate was developed in the dark for 15 minutes. 100μl of stop solution/well was used to stop the reaction and the absorbance was measured at 450nm in Bio-Rad iMark microplate absorbance reader. Medium was used as blank. Analysis was done using MPM software.
2.4.6 SDS- PAGE: 12% SDS polyacrylamide gel was used to separate the proteins. The stacking gel and resolving gel were prepared using the standard protocol and the samples with equal protein concentrations (20μg; estimated with the help of standard plotted from Lowry assay) were loaded on the gel. The samples were run on 100V until it crossed the stacking and the voltage was increased to 120V upon reaching the resolving gel. Once the dye front has run out the electrophoresis was stopped and the gel was carefully removed from the glass plates and the stacking gel was cut out. The gel was washed with distilled water and incubated in freshly prepared coomassie stain for 3 hours. The gel was destained with methanol acetic acid and water solution.
2.5 Statistical analysis
All quantitative data was analyzed using t test and data was considered significant at p≤0. 05.
3. Results
3.1 MSC isolation and characterization
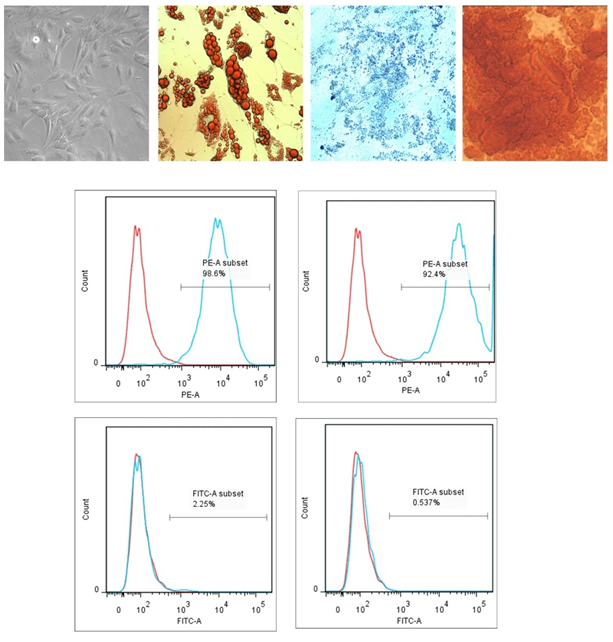
The mesenchymal stem cells were isolated from adipose tissue samples, collected from cardiac subjects of both sexes who underwent coronary artery surgeries at SCTIMST. After four to five hours, cells were washed and plastic adherent cells were cultured for 72h. Morphological observations confirmed typical elongated MSCs morphology with a bulged central region (Figure 1A). Cells were further characterized using trileneage differentiation as well as cell surface markers. Figure 1 B-D represents the adipogenic (Oil Red O stain) , chondrogenic (Alcian blue stain) and osteogenic (Alizarin Red S stain) differentiation of isolated cells. Adipocyte differentiation was evident from the red droplet staining of the oil droplets. Chondrogenic differentiation of MSCs resulted in the formation of chondrocytes along with its typical extracellular matrix. The stain was picked up by proteoglycans which are the major molecules within the cartilage matrix, and showed a dark blue color under light microscope. The mesenchymal stem cells differentiated in to Osteoblasts when osteogenic induction medium was provided for a time period of 21 days. The calcium deposits attributable to the osteocytes uptake the stain and resulted in red colour. Isolated cells showed more than 90% expression for CD90 and CD105 and were negative for CD14 and CD45. Unstained cells were used to gate the population on forward scattered and side scattered plot. Analysis was done using FlowJo software (Tree Star Inc. , USA). Figure 1 E represents the flowcytometric data. Red lines denotes the fluorescence intensity of unstained cells where as blue line denotes the stained cells.
3.2 Hepatic differentiation of MSCs
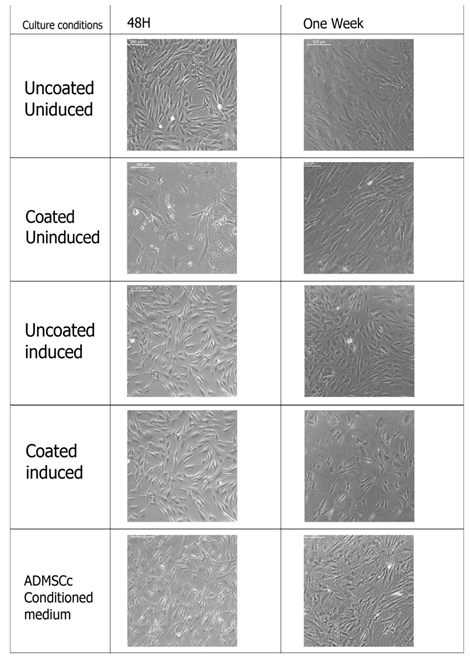
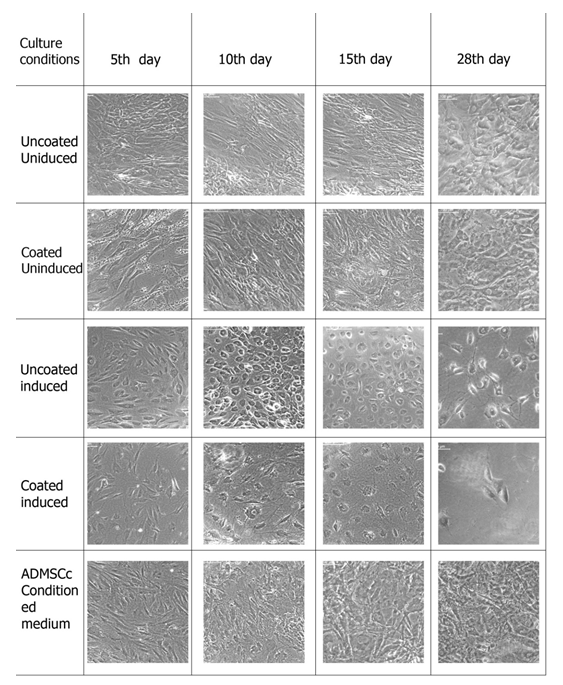
The mesenchymal stem cells were provided with the hepatic induction medium in a two stage protocol as described in the section 2. 3. Cells were first inducted for endodermal lineage and then specific hepatic lineage. Cell were analyzed morphologically after the first induction and then at different time points after second induction. Different culture conditions were compared to analyze the effect of induction media, fibrin-gelatin niche as well as ADMSCs conditioned media on hepatic differentiation. Figure 2 and Figure 3 represents the morphology of cells at endodermal lineage stage and on different days of hepatic differentiation respectively. Morphology of cells in culture changes from long elongated to small rounded cells after endodermal induction, however, this effect was most evident in induced but uncoated culture. Cells in fibrin-gelatin coated dishes as well as in ADMSCs conditioned medium showed morphological changes in terms of length reduction. Cultures were further inducted using specific growth factors to hepatic cells and microscopic observations were made on 5th day, 10th day, 14th day and 28th day. Cells in induction medium alone showed hexagonal morphology at 5th day itself and cells were differentiating till 28th day, however, cell death were observed as culture prolonged to 15th day to 28th day. ADMSCs conditioned medium culture showed dense cell population however it could not be distinct morphologically. Fibrin gelatin niche without induction medium did not support hepatic differentiation and cells were morphologically similar to the uncoated uninduced cultures. Fibrin gelatin niche cultures along with induction medium supported hepatic differentiation, however, cell growth were found to be less compare to the induction medium culture alone.
Figure 1: ADMSC Isolation and Characterization A) Morphology of plastic adhered MSCs after 72 hours in culture (10X magnification) B) MSC derived adipocytes stained with Oil Red O stain C) MSC derived chondrocytes stained with Alcian blue stain D) MSC derived osteocytes stained with Alizarin Red S stain (Images are captured at 40X magnification) E) Flowcytometric characterization of ADMSCs using two positive and two negative markers (n=4).
Figure 2: Hepatic Initiation of ADMSCs. The morphology of cells after 48 hrs in endoderm induction media and the morphology of cells after one week in hepatic initiation media. Representative images of hepatic differentiation of ADMSCs with and without induction medium and in ADMSCs conditioning medium (n=4).
3.3 Hepatic function analysis in differentiated cells
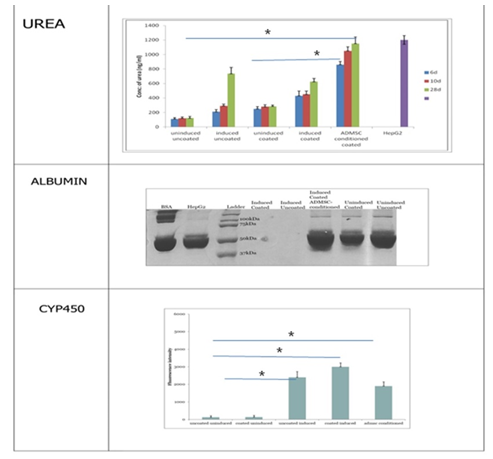
Differentiated cells were analyzed for functional hepatic markers like Indocyanine green uptake, glycogen storage, cytochrome P-450, urea and albumin.
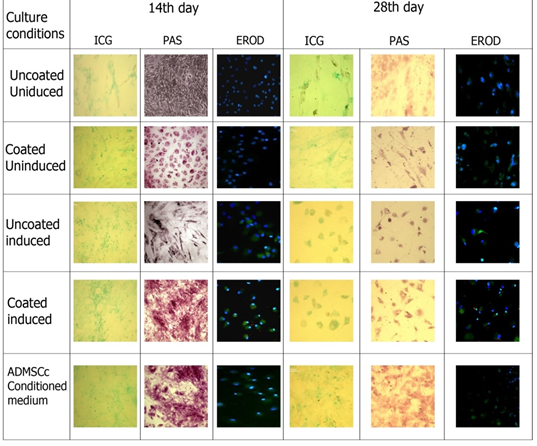
3.3.1 Indocyanine green uptake assay: The uptake of Indocyanine green/cardiogreen by the cells exposed to different culture conditions was observed at two different time points (after two weeks and after four weeks of culturing in hepatic maturation media), under a light microscope (Figure 4). The ICG uptake pattern shown by cells grown in induction culture conditions was notably high compared to the control groups. The induced cells grown in fibrin coated plate did uptake more dye than those cultured in uncoated plates which is indicative of a hepatic induction- promoting role played by the fibrin niche. ADMSCs conditioned medium showed the maximum uptake of the dye. By 28th day in culture, the cells appeared to be losing its structural integrity.
3.3.2 PAS Staining: PAS staining was done for MSCs subjected to hepatocytic differentiation conditions and glycogen storage within the cells was evaluated with the help of a light microscope (Figure 4). The comparative analysis of control MSCs and differentiated cells showed that glycogen storage was comparatively higher in the induced cells at 14th day of culture. The cells cultured in fibrin-gelatin coating had more glycogen storage in comparison with those cultured in uncoated plates.
Coated induced cultures and ADMSCs conditioned culture showed high level of glycogen storage when compared to uncoated induced. In coated induces cultures at 14th day cell showed the hexagonal morphology and though apperantly microscopically cells were less in number, PAS staining revealed better hepatic function compare to cultures with induction media alone. Thus fibrin gelatin niche may not support the cell differentiation but indicative of promoting hepatic function. At 28th day culture reduction in glycogen storage in all culture conditions were observed.
3.3.3 EROD assay: The ethoxyresorufin-O-deethylase (EROD) assay was done to study the induction of the xenobiotic-metabolizing enzyme cytochrome P-450. It is based on the microsomal conversion of ethoxyresorufin into resorufin by liver enzymes Cyto P450. The cells subjected to hepatic induction were found to be positive for resorufin (a product of CYP450 enzyme) which was absent in the control cells (Figure 4). Cells were counter stain with Hoechst nuclear stain. The CYP450 activity was similar in induced cells cultured in fibrin gelatin coated and uncoated plates and also in those cultured in ADMSC conditioned media. At 28th day of culture Cyto P 450 activity was also reduced compare to the 14th day of culture.
Quantification of Cyto P 450 activity at 28th day showed maximum in fibrin-gelatin niche induced culture followed by induced uncoated and ADMSCs conditioned cultures. Quantification of cyto P 450 activity at 28th day culture using Image J software revealed maximum levels in fibrin gelatin coated induced cultures followed by induction media alone and ADMSCs conditioned cultures. This also indicates that niche supports to maintain the functionality of the differentiated cells.
3. 4 Urea assay: The hepatocyte-like cells obtained from MSCs are expected to secrete urea since liver is the major organ involved in maintenance of amino acid levels and its conversion to the final metabolite urea. The media in which the cells were cultured was collected at different time points for each of the five experimental conditions. The media from HepG2 cell line was used as control in the experiment (Figure 5A). The data obtained from the assay indicated the presence of urea to be maximum in the media of cells cultured in ADMSC conditioned media followed by uncoated induced and coated induced cultures. The urea production by cells grown on fibrin-gelatin coating was similar to those grown on uncoated plates. The media collected on 28th day was containing significantly higher amount of urea compared to the media collected on initial day indicating fuctional hepatocytes.
3.3.5 Albumin: Albumin could not be detected in any culture conditions by SDS –PAGE as well as by ELISA. Probably long term cultures will be needed to analyze albumin secreted by the differentiated cells. Study was terminated as four weeks due to the cell death observed in the cultures.
4. Discussion
Liver, a regenerative organ, once damaged beyond regeneration or diseased, needs to be treated either by organ transplantation or cellular therapy [19]. Though most appropriate and choice of treatment, organ transplantation is not the way forward due to the huge gap between the need and supply. Thus alternative approaches are preferred and tissue engineered approaches, cell therapy could be a possible opportunity [18]. However, optimum cell source remains challenging. Hepatic cells from damages tissue may be functionally compromised as well expansion of hepatic cells in culture is limited due to its terminally differentiated nature [19]. Mesenchymal stem cells could be an alternative choice of cell source due to its multipotency and non-immunogenic behavior, thus overcoming the need of autologous cell transplantation. This can also provide off the shelf therapy for immediate need [20]. However, MSCs has to be differentiated from mesodermal origin to the endodermal origin cells and may need optimized culture conditions for obtaining functional hepatic cells. In the present study, we explored fibrin-gelatin niche as well as ADMSCs conditioned media to optimize culture conditions to obtain functional hepatic cells. ADMSCs releases growth factors, which promotes hepatic cell growth [21]. Isolated ADMSCs were plastic adherent and CD90 and CD105 positive. Trilineage differentiation of ADMSCs was also established in order to characterize the isolated cells.
The supplementation of hepatic differentiation media brought significant differences in cell morphology. At the end of the 48h, the control MSCs retained their initial morphology while the induced cells started changing its morphology. More proliferating cells were observed in induced uncoated culture dishes. Cell aggregates and multilayering was observed in ADMSCS conditioned medium supplemented group. These cells didn’t show typical cuboidal hepatocytes morphology. Indocyanine green is a cyanine dye generally used for clinical diagnostic applications. It is used as an indicator substance to assess liver functionality. The cells in culture show the metabolic potential by three weeks [22, 23]. In our study we observed that the uptake of ICG by hepatocyte-like cells derived from MSCs in induction media followed by fibrin gelatin and ADMSC conditioned cultures was notably higher than those cultured in the absence of induction media. Similar results are reported by Afshari et al. , 2020 [24]in the presence and absence of growth factors. . The control MSCs also did uptake ICG to a slight extend. A similar observation is mentioned in another study which showed that undifferentiated naïve ADMSCs do show a tendency to uptake the organic anion ICG [23]. Cell survival was maximum in cells supplemented with ADMSC conditioned media which could be attributed due to the presence anti-apoptotic components known to be secreted by mesenchymal stem cells [25, 26].
The PAS staining is done since the liver is one of the major sites of glycogen storage in the human body. The synthesis and degradation of glycogen are regulated by the hepatocytes to maintain blood-glucose levels of the organism. Glycogen storage was found to be comparatively higher in the fibrin gelatin coated induced cultures and ADMSCs conditioned cultures compared to other groups. EROD assay was done to study the induction of the enzyme cytochrome P-450. Studies have shown that addition of the substrate for CYP450 didn’t yield any fluorescence in cell cultures which were two weeks post induction, while there was slight fluorescence after four weeks and a significant increase in fluorescence by six week [27]. However in our studies, we have observed CYP 450 activity in two weeks culture. This could be attributed by niche provided, thus more functional cells could be obtained in the short duration. The data obtained from the Urea assay indicated the presence of urea to be maximum in the media of cells cultured in ADMSC conditioned media followed by uncoated induced and coated induced cultures. Reports have indicated that urea production by the differentiated cells became detectable by six weeks post induction [27]. In our study we observed urea production at 6,10 and 28 days, and a hike could be observed with time. The culture conditions and niche provided for cell differentiation might be supporting ADMSCs to become hepatocytes like cells at a early time point compared to the reported literature. However we could not trace albumin synthesis in cultures, which could be due to the early time points selected in the study. Albumin has been reported from six week cultures [28]. Our data revealed that Induced culture without fibrin-gelatin niche could promote differentiation well, however, fibrin gelatin niche supports the hepatic function which was evident from the hepatic function analysis in all the groups. ADMSCs conditioned medium also promotes hepatic function. We observe reduction in the cellular function at 28th day of culture in all the experimental groups, which could be as once differentiated to hepatic like cells, these cells could not proliferate further [29].
5. Conclusions
Mesenchymal stem cells were isolated from adipose tissue and characterized via cell surface marker analysis and tri-lineage differentiation potential. The isolated cells were differentiated into hepatocyte-like cells when provided with appropriate conditions for endoderm induction, hepatic initiation and hepatic maturation in a stage- by-stage manner. The hepatocyte-like cells showed functional properties such as ICG uptake, glycogen storage, CYP450 activity and urea synthesis significantly by the end of fourth week in induction media. Though induced culture with out niche showed maximum cell growth, fibrin gelatin niche and ADMSCs conditioned medium found to be promoting hepatic function of the differentiated cells.
Declarations Section
Ethical approval and consent to participate
All protocols in this study were approved by the Institutional Ethics Committee (IEC), SCTIMST (IEC number - SCT/IEC/1231/June2018) and by the Institutional Committee for Stem Cell Research (IC-SCR), SCTIMST (IC-SCR number - SCT/IC-SCR/43/March 2017) Thiruvananthapuram, Kerala, India. Informed consent was obtained from patients prior to the collection of adipose tissue.
Consent for publication
The submission of this manuscrip has been approved by all authors. The Director, who is the Head of the Institute has approved the publication of the manuscript, after review and recommendation by Research and Publication Cell of SCTIMST.
Availability of Supporting Data
All supporting data has been shown in the current manuscript itself.
Conflict of Interest
The authors declare no financial or non-financial conflict of interest.
Author contributions
Rasiya Sultana was responsible for data acquisition, data analysis and drafting of the manuscript
Dr. Anugya Bhatt was responsible for study conception, design, data analysis and revision of the manuscript
Acknowledgements
Authors Acknowledge the Department of Science and Technology, Govt. of India and SCTIMST Trivandrum for funding this work (TRC P8141). The authors are grateful to the Director, SCTIMST and the Head, Biomedical Technology Wing, SCTIMST for the facilities provided. The authors thank Ms. Priyanka A, Mr. Ranjith S, Mr. Anilkumar V and Ms. Deepa S for providing the clinical grade human fibrinogen and thrombin.
References
- Nussler A, Konig S, Ott M, et al. Present status and perspectives of cell-based therapies for liver diseases. Journal of Hepatology 45 (2006) :144-159.
- Strauer B E, Kornowski R. Stem cell therapy in perspective. Circulation 107 (2003): 929-934.
- Poulsom R, Alison M R, Forbes S J, et al. Adult stem cell plasticity. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland 197 (2002): 441-456.
- Wang Y, Chen X, Cao W, et al. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nature immunology 15 (2014): 1009.
- Chengzhan Zhu, Bingzi Dong, Leqi Sun, et al. Cell Sources and Influencing Factors of Liver Regeneration: A Review. Med Sci Monit 26 (2020): e929129-1-e929129-12.
- Mishra VK, Shih HH, Parveen F, et al. Identifying the Therapeutic Significance of Mesenchymal Stem Cells. Cells 9 (2020): 1145.
- Barsotti M C, Magera A, Armani C, et al. Fibrin acts as biomimetic niche inducing both differentiation and stem cell marker expression of early human endothelial progenitor cells. Cell proliferation 44 (2011): 33-48.
- Zhang Y, Bai X-F, Huang C-X. Hepatic stem cells: existence and origin. World J Gastroenterol 9 (2003): 201-204.
- Shu S-N, Wei L, Wang J-H, et al. Hepatic differentiation capability of rat bone marrow-derived mesenchymal stem cells and hematopoietic stem cells. World J Gastroenterol 10 (2004): 2818-2822.
- Kang X Q, Zang W J, Bao L J, et al. Fibroblast growth factor-4 and hepatocyte growth factor induce differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocytes. World journal of gastroenterology 11 (2005): 7461.
- Forte G, Minieri M, Cossa P, et al. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem cells 24 (2006): 23-33.
- Ong S Y, Dai H, Leong K W. Inducing hepatic differentiation of human mesenchymal stem cells in pellet culture. Biomaterials 27 (2006): 4087-4097.
- Sivan U, Jayakumar K, Krishnan L K. Constitution of fibrin-based niche for in vitro differentiation of adipose-derived mesenchymal stem cells to keratinocytes. BioResearch open access 3 (2014): 339-347.
- Catelas I, Sese N, Wu B M, et al. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue engineering 12 (2006): 2385-2396.
- Prasad Chennazhy K, Krishnan L K. Effect of passage number and matrix characteristics on differentiation of endothelial cells cultured for tissue engineering. Biomaterials 26 (2005): 5658-5667.
- Zuk PA, Zhu M, Ashjian P, et al. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol Biol Cell 13 (2002): 4279-4295.
- Lee KD, Kuo TK, Whang-Peng J, et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology 40 (2004): 1275-1284.
- Yamada T, Yoshikawa M, Kanda S, et al. In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells 20 (2002): 146-154.
- Everhart J E, Ruhl C E. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology 136 (2009): 1134-1144.
- Barry F P, Murphy J M, English K, et al. Immunogenicity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cells Dev 14 (2005): 252-265.
- Hu C, Zhao L, Li L. Current understanding of adipose-derived mesenchymal stem cell-based therapies in liver diseases. Stem Cell Res Ther 10 (2019): 199.
- Hoda El-Kehdy, Guillaume Pourcher, Wenwei Zhang, et al. Hepatocytic Differentiation Potential of Human Fetal Liver Mesenchymal Stem Cells: In Vitro and In Vivo Evaluation. Stem Cell International (2016).
- Zemel R, Bachmetov L, Ad-El D, et al. Expression of liver-specific markers in naïve adipose-derived mesenchymal stem cells. Liver International 29 (2009): 1326-1337.
- Afsoon Afshari, Sara Shamdani, Georges Uzan, et al. Different approaches for transformation of mesenchymal stem cells into hepatocyte-like cells. Stem Cell Res Ther 11 (2020): 54.
- Phelps J, Sanati-Nezhad A, Ungrin M, et al. Bioprocessing of Mesenchymal Stem Cells and Their Derivatives: Toward Cell-Free Therapeutics. Stem Cells International 2018 (2018): 9415367.
- van Poll D, Parekkadan B, Cho C H, et al. Mesenchymal stem cell–derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 47 (2008): 1634-1643.
- Kuan-Der Lee, Tom Kwang-Chun Kuo, Jacqueline Whang-Peng, et al. In Vitro Hepatic Differentiation of Human Mesenchymal Stem Cells. Hepatology 40 (2004): 1275-1284.
- Ji Ru, Ning Zhang, Nan You, et al. The differentiation of MSCs into functional hepatocyte-like cells in a liver biomatrix scaffold and their transplantation into liver-fibrotic mice. Biomaterials 33 (2012): 8995-9008.
- Irina V Kholodenko, Leonid K Kurbatov, Roman V Kholodenko, et al. Mesenchymal Stem Cells in the Adult Human Liver: Hype or Hope?. Cells 8 (2019): 1127.