Diagnosis and Management of Hyperkalemia
Article Information
Mohammad Tinawi*
1Department of Internal Medicine and Nephrology, Nephrology Specialists, Munster, IN, USA
*Corresponding author: Mohammad Tinawi, Department of Internal Medicine and Nephrology, Nephrology Specialists, P.C., 801 MacArthur Blvd., Ste. 400A, Munster, IN 46321, USA
Received: 28 April 2020; Accepted: 18 May 2020; Published: 21 May 2020
Citation: Mohammad Tinawi. Diagnosis and Management of Hyperkalemia. Archives of Clinical and Biomedical Research 4 (2020): 153-168.
Share at FacebookAbstract
Potassium (K+) is the most abundant intracellular cation. Intracellular K+ concentration is around 150 mEq/l, while its extracellular concentration is 3.5-5 mEq/l. Serum K+ is determined by intake, excretion, and transcellular distribution. The kidneys and the adrenal glands regulate K+ excretion. Hyperkalemia is mainly seen in patient with impaired renal function (acute kidney injury or advanced chronic kidney disease) or in patients with decreased mineralocorticoid (aldosterone) activity. Certain medications can result in hyperkalemia in patients with impaired renal K+ excretion. Aldosterone is the key regulator of renal K+ excretion. Hyperkalemia (serum K+ ≥ 5.5 mEq/l) has multiple manifestations; however, cardiac arrhythmias are the most serious. Detailed history and basic laboratory tests are required to diagnose hyperkalemia. Hyperkalemia is treated medically; hemodialysis is reserved for severe and emergency cases.
Keywords
Hyperkalemia, Potassium disorders, Electrolyte abnormalities
Hyperkalemia articles, Potassium disorders articles, Electrolyte abnormalities articles
Article Details
1. Potassium Homeostasis
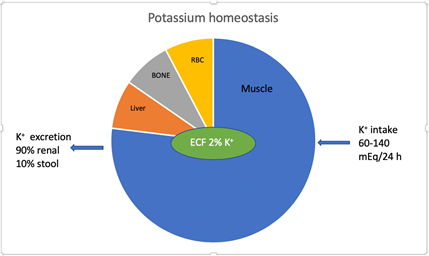
Hyperkalemia is defined as serum K+ concentration ≥ 5.5 mEq/l. K+ physiology including homeostasis and renal transport has been described in detail in a recent article by the author [1]. The average intake of K+ in individuals on a western diet is 60-140 mEq/day. The kidneys excrete 90% of K+ and the rest is excreted in the stool [2]. Figure 1. K+ excretion in stool increases in stage 4 and 5 chronic kidney disease (CKD) and in patients on renal replacement therapy (RRT). K+ homeostasis is maintained by the kidneys [3]. Large shifts in extracellular K+ do not occur because K+ moves into or outside the skeletal muscles maintaining extracellular K+ within a tight range. Insulin, catecholamines (via β2 receptors) and alkalemia result in intracellular shift of K+. Increase in serum osmolality and normal anion-gap hyperchloremic metabolic acidosis result in extracellular K+ shift. After a meal, hyperkalemia is prevented due to insulin secretion which shifts K+ intracellularly until it is renally excreted.
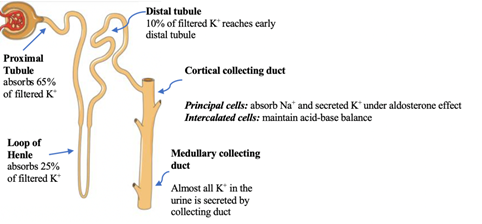
1.1 Potassium transport in the kidney [4], Figure 2.
Figure 2: The Nephron. Courtesy of Servier Medical Art licensed under a Creative Commons Attribution 3.0 Unported License. https://smart.servier.com Labels added to illustrate K+ transport in the nephron.
There are two types of K+ channels in the cortical collecting duct [5]. Table 1.
|
K+ Channel |
Renal Outer Medullary K+ Channel |
Maxi-K+ Channel |
|
Abbreviation |
ROMK |
BK |
|
Activator |
Aldosterone |
High flow rate through collecting duct |
|
Location |
Principal cells |
Both principal and intercalated cells |
|
Role in K+ secretion |
Major |
Minor |
Table 1: Types of K+ channels in the cortical collecting duct.
There are four major factors that determine K+ secretion in the collecting duct [6]:
- Aldosterone: aldosterone is the major determinant of K+ It activates the Na+-K+-ATPase pump, and the epithelial sodium channel (ENaC). Na+-K+-ATPase pump is located on the basolateral membrane of the principal cell while the ENaC is located on the apical membrane of the principal cell of the collecting duct.
- Distal flow rate: a decrease in distal flow rate decreases K+ secretion in the collecting duct, while a high flow rate activates the Maxi-K+ (BK channel).
- Serum K+: hyperkalemia increases aldosterone secretion from the zona glomerulosa, and vice versa.
- Delivery of anions to the collecting duct: anions such as bicarbonate and nafcillin increase lumen negativity and K+
2. Hyperkalemia
2.1 Prevalence
Hyperkalemia is seen in hospitalized and community dwelling subjects. A study in about 5000 community subjects aged 55 years or older (the Rotterdam Study) found hyperkalemia (defined as K+ > 6) in 0.7% of patients aged 75 and older [7]. Other electrolyte abnormalities such as hyponatremia and hypokalemia were not restricted to older age groups. Therefore, the prevalence of hyperkalemia increases significantly with age. In the same study, hyperkalemia was associated with adjusted mortality rate of 1.42 (0.71-2.86). A study in about 8000 patients admitted to the emergency department (ED) found hyperkalemia in 1.8% [8]. Hyperkalemia is seen in about 3% of hospitalized patients [9].
2.2 Etiology
Increased intake of K+ can rarely result in hyperkalemia in absence of a renal or adrenal abnormality because of the ability of the normal kidneys to adapt and excrete K+. It has been shown that urinary K+ excretion can rise to almost 400 mEq/24 h to match increased oral intake of K+ with only a small rise in serum K+ [10]. The focus of the differential diagnosis is on impaired renal K+ excretion due to renal disease, decreased mineralocorticoid (aldosterone) activity, and certain medications. Transcellular shift of K+ is seen in some conditions, while pseudohyperkalemia is uncommon. It is important to know that several factors can simultaneously contribute to hyperkalemia, for example, hyperkalemia in a patient with stage 5 CKD due to diabetic nephropathy with underlying hyporeninemic hypoaldosteronism syndrome, who is on an angiotensin converting enzyme inhibitor (ACEI) and spironolactone. Table 2.
2.2.1 Pseudohyperkalemia: Pseudohyperkalemia is seen in patients with thrombocytosis (platelets > 500 x 109 /L), and severe leukocytosis (>75 x 109/L). It can also be seen due to release of K+ from RBCs due to trauma resulting from tourniquet application and specimen transportation [11].
2.2.2 Hyperkalemia due to transcellular shift of K+: As mentioned above an increase in serum osmolality (as in hyperglycemia) and normal anion-gap hyperchloremic metabolic acidosis (mineral acidosis) can result in transient hyperkalemia due to extracellular K+ shift. High anion-gap (organic) metabolic acidosis does not result in significant hyperkalemia. Therefore, hyperkalemia in diabetic ketoacidosis (DKA) is the result of insulin deficiency which shifts K+ extracellularly by reducing the activity of the Na+-K+-ATPase pump, impaired renal K+ secretion due to reduced kidney function associated with volume depletion and hyperosmolality [12]. The role of organic acidosis (ketoacidosis) is minor. Severe transient hyperkalemia can result from cell damage as in tumor lysis syndrome, rhabdomyolysis [13] and severe hemolysis [14]. Hyperkalemic periodic paralysis results in transient hyperkalemia and subsequent severe muscle weakness or paralysis. Attacks last less than 24 h (usually < 2 h) and is more common in the morning and after ingesting K+ rich food products. Most patients are young (< 20 years old). The cause is a mutation in the sodium (Na+) channel gene SCN4A [15].
2.2.3 Hyperkalemia due to impaired renal excretion:
Acute kidney injury (AKI): hyperkalemia is common in AKI [16] especially in oliguric patients and in patients with cell damage such as rhabdomyolysis and tumor lysis syndrome [17]. Hyperkalemia results from direct injury to the principal cells in the collecting tubule.
Chronic kidney disease (CKD): [18] hyperkalemia is seen in patients with advanced stage 4 and stage 5 CKD (glomerular filtration rate or GFR < 15-20 ml/min) [19], and in patients on RRT. Hyperkalemia is uncommon in earlier CKD stages (CKD stages 1-3) unless there is a concomitant reduced aldosterone secretion (as in hyporeninemic hypoaldosteronism syndrome in patients with diabetic kidney disease or chronic interstitial nephritis) [20], or high K+ intake. Medications that impair renal K+ excretion such as K+ sparing diuretics, ACEI and angiotensin receptor blockers (ARBs) can cause severe hyperkalemia in patients with advanced CKD. Salt substitutes containing K+ can result in severe hyperkalemia in CKD patients.
Hyperkalemic renal tubular acidosis (RTA): in this RTA, impaired distal Na+ reabsorption results in hyperkalemia hyperchloremic normal anion-gap metabolic acidosis. Two types of hyperkalemic RTA are recognized: voltage-dependent RTA and type 4 RTA or hypoaldosteronism [21].
- Voltage-dependent RTA [22]: some authorities prefer the name voltage-dependent hyperkalemic distal RTA. Na + reabsorption in the principal cells is impaired, this decreases the lumen electronegativity and subsequent K+ and H+ This is seen in patients with sickle cell disease, lupus nephritis and obstructive uropathy. Voltage-dependent RTA can also be the result of severe decrease in distal delivery of Na+ due to dehydration, AKI, liver cirrhosis or heart failure (HF).
- Type 4 RTA (Hypoaldosteronism): this disorder can be due to reduced aldosterone secretion or reduced aldosterone responsiveness.
- Reduced aldosterone secretion is commonly seen in diabetic nephropathy due to hyporeninemic hypoaldosteronism syndrome. Other common causes include [23] the use of renin-angiotensin-aldosterone (RAAS) inhibitors (ACEI, ARBs, and direct renin inhibitors), calcineurin inhibitors (such as cyclosporine and tacrolimus), and non-steroidal anti-inflammatory medications (NSAIDs) [24]. Adrenal insufficiency is much less common, but an accurate diagnosis is critical. Chronic heparin therapy is an uncommon cause of hyperkalemia [25]. Pseudohypoaldosteronism type II or familial hyperkalemic hypertension also known as Gordon’s syndrome [26] is characterized by hyperkalemia, hypertension, volume expansion and normal renal function. It is a rare cause of hyperkalemia. It is autosomal dominant and is usually due to mutations in the WNK4 and WNK1 kinases. Treatment is with thiazide diuretics.
- Reduced aldosterone responsiveness (mineralocorticoid resistance) is seen in patients on K+ sparing diuretics, trimethoprim, and pentamidine. Pseudohypoaldosteronism type I [27] is a rare cause of mineralocorticoid resistance. In this syndrome hyperkalemia and metabolic acidosis are associated with salt wasting nephropathy. The autosomal recessive type of this syndrome is due to mutations in the 3 subunits of the epithelial Na+ channel (ENaC). It is worth mentioning that proximal (type 2) and distal (type 1) RTA cause hypokalemia.
|
1.Pseudohyperkalemia: as in thrombocytosis (platelets > 500 x 109 /L), severe leukocytosis (>75 x 109/L) and trauma resulting from tourniquet application 2.High K+ intake (diet, supplements, or K+ containing salt substitutes): hyperkalemia is uncommon unless the patient has advanced CKD, hyperkalemic RTA or on RRT. Certain herbal supplements such as alfalfa and dandelion can cause hyperkalemia. 3.Hyperkalemia due to transcellular shift of K+: as in DKA, serum hyperosmolality (hyperglycemia, hypertonic mannitol), normal anion-gap hyperchloremic metabolic acidosis (mineral acidosis), digoxin toxicity, somatostatin, octreotide, succinylcholine, cell damage in patients with tumor lysis syndrome, rhabdomyolysis or severe hemolysis, and hyperkalemia periodic paralysis (rare). 4. Hyperkalemia due to impaired renal excretion: Primary renal disease: Acute kidney injury (especially severe oliguric AKI), advanced CKD (GFR < 15-20) and RRT. Hyperkalemic renal tubular acidosis (RTA): I. Voltage-dependent RTA: as in sickle cell disease, lupus nephritis, amyloidosis, obstructive uropathy and severe decrease in distal delivery of Na+ (dehydration, HF, liver cirrhosis, and AKI) II. Type 4 RTA (Hypoaldosteronism): is due to reduced aldosterone secretion or reduced aldosterone responsiveness. · Reduced aldosterone secretion: v Hyporeninemic hypoaldosteronism syndrome (diabetic kidney disease and chronic interstitial nephritis) v RAAS inhibitors: ACEI, ARBs, direct renin inhibitors v Medications that inhibit renin secretion from the juxtaglomerular cells: β blockers, calcineurin inhibitors (such as cyclosporine and tacrolimus), and NSAIDs v Aldosterone secretion inhibitors: heparin, ketoconazole v Adrenal insufficiency (primary or secondary) v Pseudohypoaldosteronism type II also known as familial hyperkalemic hypertension (Gordon’s syndrome) · Reduced aldosterone responsiveness (mineralocorticoid resistance): v Mineralocorticoid receptor blockers: spironolactone, eplerenone and drospirenone (a progestin medication used in some contraceptive pills) v Collecting tubule Na+ channel blockers (ENaC): amiloride, triamterene, trimethoprim, pentamidine v Pseudohypoaldosteronism type I (rare) |
Table 2: Causes of hyperkalemia.
2.3 Symptoms and complications
Hyperkalemia has multiple manifestations, of which cardiac conduction abnormalities are the most serious.
- Muscle weakness is the most common manifestation, it can rarely progress into ascending flaccid paralysis [28].
- Fasciculations and paresthesias in the extremities
- Palpitations, nausea, or vomiting
- Metabolic acidosis (hyperkalemia decreases renal ammonia production)
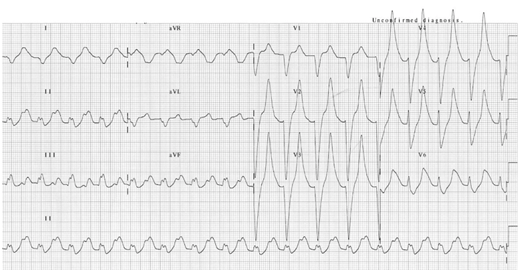
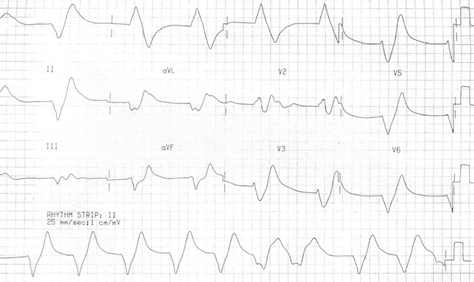
- ECG changes correlate poorly with the severity of hyperkalemia. In one study [29] abnormal ECG changes in hyperkalemia had poor sensitivity and specificity. ECG changes in hyperkalemia are due to the depolarizing effect on the myocardium [30]. The earliest ECG sign of hyperkalemia is usually peaked T waves, followed by small or absent P waves, and PR-interval prolongation, more severe changes include wide QRS complexes, high-grade AV block and sinus bradycardia. Appearance of sine waves [31] is ominous. Finally, cardiac arrest results from ventricular tachycardia, ventricular fibrillation, asystole or pulseless electrical activity (PEA). Figures 3 and 4.
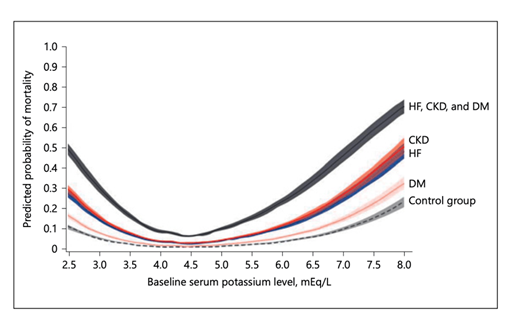
2.4 Mortality
The association between serum K+ and mortality is U-shaped. A study based on medical records review of > 900,000 patients showed that adjusted all-cause
mortality increased for every 0.1 mEq/l change in K+ < 4.0 mEq/l and ≥5.0 mEq/l. Mortality was greater in patients with one or more the following conditions: CKD, DM or HF (32). See Figure 5.
Figure 3: ECG in a patient with a serum K+ of 9.3 mEq/l. It shows peaked T waves and wide QRS complexes. ECG is courtesy of #FOAMed Medical Education Resources by LITFL is licensed under a Creative Commons Attribution-Noncommercial-ShareAlike 4.0 International License. Based on a work at https://litfl.com.
Figure 4: Sine waves in a patient with K+ 9.9 mEq/l. ECG is courtesy of #FOAMed Medical Education Resources by LITFL is licensed under a Creative Commons Attribution-Noncommercial-ShareAlike 4.0 International License. Based on a work at https://litfl.com.
Figure 5: Serum K is a continuous variable with all-cause mortality. Courtesy of Am J Nephrol 2017;46:213–221 DOI: 10.1159/000479802 This article is licensed under the Creative Commons Attribution- NonCommercial-NoDerivatives 4.0 International License (CC BY- NC-ND) (http://www.karger.com/Services/OpenAccessLicense).
2.5 Diagnosis
When approaching a patient with hyperkalemia, the following principles should be kept in mind:
- Recognizing pseudohyperkalemia requires a high index of suspicion [33]. A tourniquet should not be applied for more than a minute. Fist clenching is not recommended. Since intracellular K+ concentration is more than 30 times its extracellular concentration, even a small release of intracellular K+ will lead to significant hyperkalemia. If hemolysis in the sample is recognized, K+ measurement should be repeated. Clues to pseudohyperkalemia include absence of symptoms, offending medications, or underlying conditions such as CKD, diabetes mellitus (DM) or hyperkalemic RTA. An unexpected hyperkalemia in an individual with a known history of normokalemia is a potential clue to the presence of pseudohyperkalemia.
- Hyperkalemia is usually divided into mild (K+ 5.5-5.9), moderate (K+ 6-6.9), and severe (K+≥ 7 mEq/l). Many patients are asymptomatic especially if hyperkalemia is mild. Muscle weakness is the most common symptom. Cardiac conduction abnormalities should be immediately recognized.
- History and physical exam are critical. History of chronic kidney disease, DM, heart failure and adrenal insufficiency will aid the diagnosis. The presence of muscle weakness, ascending paralysis, ectopic beats may lead to a diagnosis of hyperkalemia. Medications review is critical. Oftentimes certain medications lead to significant hyperkalemia in the presence of an underlying renal or aldosterone abnormality [34]. The clinician should also inquire about the use of supplements and K+ containing salt substitutes.
- The diagnosis of hyperkalemia is readily made upon review of a basic chemistry profile. The profile will provide useful information regarding renal function, other electrolytes, acid-base status and serum glucose. If adrenal insufficiency is suspected, ACTH stimulation test should be done [35]. Primary adrenal insufficiency (Addison’s disease) may manifest with hypotension, hyponatremia, hyperkalemia and metabolic acidosis. In addition to management of hyperkalemia, patients require infusion of 0.9 NaCl and IV hydrocortisone [36]. Creatine kinase (CK) is ordered if rhabdomyolysis is suspected.
- Many clinicians do an ECG in case of moderate or severe hyperkalemia (K+≥ 6 mEq/l). The presence of ECG changes such as peaked T waves or wide QRS complexes should prompt emergency management of hyperkalemia in a cardiac monitoring unit [37]. Rapid rise in K+ as in rhabdomyolysis, DKA or tumor lysis syndrome should also trigger prompt treatment of hyperkalemia. Therefore, a hyperkalemic emergency is hyperkalemia associated with ECG changes, rapid rise of K+, muscle weakness or paralysis.
- A 24 h urine collection for K+ is not helpful in the diagnosis of hyperkalemia. The use of transtubular K+ (TTKG) gradient is no longer recommended [38].
- Detailed review of medications is critical in patients suspected of having hyperkalemic RTA (see Table 2). A clue to the diagnosis is persistent chronic hyperkalemia in absence of advanced CKD. These patients are acidotic due to hyperkalemia-induced reduction in urinary ammonium excretion [39]. Hyperkalemic RTA is a form of normal anion gap, hyperchloremic metabolic acidosis. HIV is an important cause of hypoaldosteronism [40]. Measurement of plasma renin activity, plasma aldosterone and serum cortisol may be required for the diagnosis. A specialist consultation is recommended.
- Voltage-dependent RTA is differentiated from type 4 RTA (hypoaldosteronism) based on the etiology and medications review. Patients with voltage-dependent RTA often cannot reduce urine pH below 5.5, while patients with type 4 RTA can. Further differentiation requires sophisticated testing [41] which is not indicated because it has little impact on management.
2.6 Treatment
The clinician should distinguish between hyperkalemic emergencies and nonemergent hyperkalemia.
2.6.1 Treatment of hyperkalemic emergencies: Hyperkalemic emergencies are seen in patients with rapidly rising K+ as in rhabdomyolysis, patients presenting with symptoms related to hyperkalemia such as muscle weakness or paralysis, or in hyperkalemia associated with cardiac conduction abnormalities. Serum K+ is usually ≥6.0 mEq/l. The focus of treatment is on myocardium membrane stabilization, cellular uptake of K+, and finally K+ elimination, in that order. All patients with hyperkalemic emergencies should be placed on cardiac monitoring.
Myocardium membrane stabilization: This is the first priority in patients with ECG changes. It is achieved by giving calcium gluconate 10 ml of 10% solution over 5-10 minutes [42]. This will antagonize the effect of K+ on myocardial cell membrane but will not shift or eliminate K+. Onset of action is immediate with a duration of 30-60 minutes. Intravenous calcium should be avoided if the patient is on digitalis due to several case reports of sudden cardiac death in this patient population [43].
Intracellular shift of K+: [44] Patients should be first given insulin (10 units of regular insulin intravenously with 1 ampule of D50 if serum glucose is > 250 mg/dl). The onset of action is 15 minutes with a duration of about 2 h. A retrospective study recommended the use of only 5 units of IV regular insulin in patients with CKD and ESRD to lower the risk of hypoglycemia [45]. Insulin is followed by albuterol nebulized treatment 10 mg. The onset of action is 30 minutes with a duration of about 2 h. This is a higher dose than the customary 2.5 mg used for respiratory purposes. Sodium bicarbonate can be considered in patients with metabolic acidosis [46]. Many clinicians give one ampule of Na bicarbonate containing 50 mEq (50 ml of 8.4% Na bicarbonate 1 mEq/ml). Some give Na bicarbonate in a continuous drip (150 mEq of Na bicarbonate in 1 L of 5% dextrose), the rate will vary depending on clinical circumstances. Na bicarbonate is not effective for treatment of hyperkalemia in patients with end-stage renal disease (ESRD) [47].
Elimination of K+: Loop diuretics can be utilized to eliminate K+ in patients with adequate urine output [42]. K + free intravenous fluids can be given simultaneously to prevent volume depletion and facilitate further K+ elimination. Hemodialysis (HD) is the definitive management option for severe and recalcitrant hyperkalemia [48]. K+ elimination starts immediately in HD. The above treatment options should precede HD due to the logistics related to obtaining dialysis access (if the patient is not already on chronic HD) and preparing the HD machine. K+ binders may be used in addition to the measures mentioned above. Time of onset can be hours. K+ binders should never be the sole treatment in hyperkalemic emergencies. Until further data is available, K+ binders should not be used in hyperkalemia in the immediate post-operative period. Other important considerations include treatment of underlying causes of hyperkalemia (e.g. insertion of a Foley catheter in a patient with obstructive uropathy, and K+ free intravenous fluids in patients with volume depletion), initiation of low K+ diet, and discontinuation of mediations or supplements that are contributing to hyperkalemia. Serum K+ should be checked every 2 h following the above measures to document adequate reduction. If hyperkalemia persists, the above measures should be repeated, and hemodialysis initiated.
2.6.2 Treatment of nonemergent hyperkalemia:
Patients in this category usually have mild and chronic hyperkalemia. The focus is on dietary counseling, medications and supplements review, and the use of K+ binders. Further benefit may be derived from the addition of loop diuretics (thiazide diuretics may be used in patients with eGFR > 30 ml/min/1.73 m2) and oral sodium bicarbonate tablets (in case of metabolic acidosis). Fludrocortisone is a consideration in patients with hyperkalemic RTA, however, hypertension and volume overload limit its usefulness [49].
Low K+ diet: It should be advised to all patients with chronic hyperkalemia. The recommended amount of K+ is 2 g (50 mEq or mmol) in 24 h. Detailed information on how to advise patients on low K+ diet is available [50].
Medications and supplements review: Patients should stop taking K+ containing salt substitutes. K+ supplements such as potassium chloride or potassium gluconate should be stopped as well.
Potassium binders: K+ binders should be considered in patients with persistent chronic hyperkalemia despite dietary interventions and in patients with strong indications for certain medications associated with hyperkalemia. Examples are patients with chronic systolic heart failure who require RAAS inhibition and patients with proteinuric CKD such as diabetic nephropathy who require an ACEI or an ARB. Newer K+ binders (patiromer and sodium zirconium cyclosilicate) are good options for these patients because giving a suboptimal dose of RAAS blockers is associated with increased mortality [51]. Sodium polystyrene sulfonate should not be used on a chronic basis [11].
2.6.3 Potassium binders:
Sodium polystyrene sulfonate (SPS): SPS is an exchange resin [52]. It exchanges Na+ for K+ and binds K+ and Mg+2. It was approved by the FDA in 1958. Onset takes several hours. It is usually given with sorbitol and can cause volume overload. There are rare reports of colon necrosis [53]; therefore, SPS should never be used postoperatively or in patients with ileus or severe constipation. The dose is 15-30 g orally or 30-50 g as a retention enema. The FDA recommends that SPS is administered separately from other medications by 3 hours.
Patiromer: It was approved in 2015 by the FDA. This nonabsorbed polymer exchanges Ca+2 for K+ and Mg+2. Onset can take several hours. Patients should be monitored for hypomagnesemia. Constipation is the most common adverse effect [54]. Patiromer should be administered separately from other medications by 3 hours. Clinicians should be aware that significant binding occurs with metformin, levothyroxine and ciprofloxacin [55]. The starting dose is 8.4 g daily; the dose is adjusted weekly up to 25.2 g per day to achieve target K+ level. Patiromer was effective in patients with hyperkalemia and diabetic kidney disease on RAAS inhibitors for up to 52 weeks [56].
Sodium zirconium cyclosilicate (SZC): SZC was approved by the FDA in 2018. It binds K+ in exchange for Na+ and H+ [57]. Time of onset is 1 h. SZC should be administered separately from other medications by 2 hours. Volume overload does not seem to be a major concern [58]. The starting dose is 10 g three times a day for up to 48 h. The maintenance dose is 5-15 g daily.
2.7 Clinical vignettes
- 60-year-old man with a known history of CKD-3 due to diabetic nephropathy, his serum creatinine is 1.9 mg/dl, baseline serum K+ is 4.7 mEq/l. He takes lisinopril 10 mg daily. He was instructed on a low Na+ diet as part of his BP management. Three weeks later he presents with generalized muscle weakness, K+ 7.1 mEq/l and peaked T waves on his ECG. What is the etiology of his severe hyperkalemia? Answer: This patient is prone to hyperkalemia. He has CKD-3 due to diabetic nephropathy which is commonly associated with hyporeninemic hypoaldosteronism syndrome. He is already on an ACEI. After he was instructed on a low Na+ diet, he started using a K+ containing salt substitute. KCl salt substitutes contain 13.6 mEq/g. Taking 3 g (about 1⁄2 teaspoon) is equivalent to 40 mEq of KCl daily [1].
- 40-year-old woman with a serum K+ of 5 mEq/l, normal renal function, body weight 70 kg and an estimated ECF K+ content of 70 mEq. She eats a large bowl of fruits, 2 h later her K+ is 5.2 mEq/l. If K+ content of the fruits she ate is 35 mEq/l, explain why her serum K+ did not rise by 50% to 7.5 mEq/l, which would have resulted in life-threatening hyperkalemia. Answer: Carbohydrates in fruits will increase insulin release from the pancreas, this will drive K+ intracellularly until it is excreted by the kidneys, this prevents severe hyperkalemia in individual with normal kidney function and high K+ intake. This is not the case in patients with advanced chronic kidney disease and patients on RRT [59].
- 45-year-old man presents with a K+ of 6.1 mEq/l due to rhabdomyolysis. He was given insulin/glucose/albuterol/Na bicarbonate/IVF/furosemide. 2 h later his serum K+ has risen to 8 mEq/l, ECG shows peaked T waves. What should be done next? Answer: The above measures should be repeated, and emergency hemodialysis is indicated. Hemodialysis is life-saving in this scenario.
- 72-year-old woman with chronic systolic heart failure. Ejection fraction is 25%. She is on lisinopril 40 mg daily, spironolactone 25 mg daily, furosemide 40 mg twice daily, and carvedilol 25 mg twice daily. Her BP control is optimal, serum creatinine is stable at 2.4 mg/dl, serum K+ is 6 mEq/l despite adherence to low K+ diet. Should her spironolactone be discontinued and lisinopril dose reduced? Answer: She is on an optimal medical regimen for her chronic systolic heart failure. Dose reduction or medication discontinuation is associated with increased mortality [51]. This patient is a good candidate for the newer K+ binders (patiromer or sodium zirconium cyclosilicate).
- 47-year-old man with CKD-4 secondary to diabetic nephropathy was seen in the second post-operative day following an open cholecystectomy. He remains NPO due to post-operative ileus. He is on lactated ringer IV solution. Serum creatinine is stable at 3.1 mg/dl, serum K is 6.1 mEq/l. He is asking whether he should be given a dose of sodium polystyrene sulfonate (SPS), which he has taken in the past with good results. Answer: Post-operative hyperkalemia should never be treated with SPS. Newer binders should not be used for post-operative hyperkalemia until further data are available. In this patient, lactated ringer should be discontinued because it contains K+. 0.9 NS is a good IVF choice. Other measures for treatment of hyperkalemia should be utilized. Due to reports of intestinal and colon necrosis, the FDA has warned against the use of SPS in patients who do not have normal bowel function including post-operative patients who have not had a bowel movement. This also applies to patients who are at risk for constipation or fecal impaction. Repeated doses should be avoided in patients who have not had a bowel movement [60].
- 61-year-old woman with CKD-3 secondary to diabetic nephropathy. She has chronic hyperkalemia due to hyporeninemic hypoaldosteronism syndrome. Her BP is 152/91. She is on a calcium channel blocker, beta blocker, loop diuretic and hydralazine. Her K+ has been in the range of 5.8-6.2 mEq/l while on a strict low K + diet. Should fludrocortisone be added to her regimen? Answer: Fludrocortisone may lead to improvement in her hyperkalemia. It is rarely used in patients such as this one with hyporeninemic hypoaldosteronism syndrome. It will aggravate hypertension and increase fluid retention, making it a poor choice. Newer K+ binders such as patiromer and SZC should be considered in this patient. If optimal K+ control is achieved, she can be started on an ACEI or ARB which is indicated for CKD due to diabetic nephropathy and may result in optimal BP control. Always start with a low dose ACEI/ARB and slowly uptitrate with close K+ monitoring.
- Conclusion
- Hyperkalemia is common in the inpatient and outpatient settings especially in patients with CKD.
- Hyperkalemia is associated with increased mortality.
- Musculoskeletal symptoms are the most common presenting symptoms, while cardiac conduction abnormalities can be life-threatening.
- Most cases of hyperkalemia are diagnosed based on history and physical exam, basic laboratory testing and ECG.
- Hyperkalemia is treated medically; hemodialysis is reserved for severe and recalcitrant cases.
- New K+ binders are now available and may be appropriate for managing patients with chronic hyperkalemia.
References
https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/011287s026lbl.pdf