Determination of Efficiency of Transcerebellar Diameter/Abdominal Circumference, Head Circumference/Abdominal Circumference, and Femur Length/ Abdominal Circumference Ratios in Diagnosing Intrauterine Growth Restriction
Article Information
Satyanarayana Kummari1, Challa Anil Kumar2, Bagadi Lava Kumar2, Karuna Priya Perumalla3*
1Assistant Professor, Department of Radiology, All India Institute of Medical Sciences (AIIMS), Nagpur
2 Assistant Professor, Department of Radiology, Great Eastern Medical School & Hospital, Srikakulam
3Assistant Professor, Department of Obstetrics and Gynaecology, Gandhi medical College, Secunderabad
*Corresponding author: Karuna Priya Perumalla, Assistant Professor Department of Obstetrics and Gynaecology Gandhi medical College, Secunderabad, India.
Received: 01 August 2024; Accepted: 07 August 2024; Published: 14 August 2024
Citation: Satyanarayana Kummari, Challa Anil Kumar, Bagadi Lava Kumar, Karuna Priya Perumalla. Determination of Efficiency of Transcerebellar Diameter/ Abdominal Circumference, Head Circumference/Abdominal Circumference, and Femur Length/ Abdominal Circumference Ratios in Diagnosing Intrauterine Growth Restriction. Obstetrics and Gynecology Research. 7 (2024): 69-75.
Share at FacebookAbstract
Background:
Intrauterine growth restriction (IUGR) is the term used to describe when a foetus does not achieve its anticipated growth. Timely diagnosis of IUGR is essential for providing the best possible obstetric treatment in order to minimise foetal complications, neonatal morbidity, and mortality.
Aim:
To determine efficiency of transcerebellar diameter (TCD)/abdominal circumference (AC), head circumference (HC)/abdominal circumference (AC), and femur length/abdominal circumference (AC) ratios in diagnosing IUGR
Methods:
A total of five hundred expectant mothers, with GA ranging from 18 to 40 weeks, were included. TCD/AC, HC/AC, and FL/AC ratios were computed. SPSS version 22.0 was utilised to perform statistical calculations. The correlation coefficients were utilised, and regression analysis was performed. Both parametric and non-parametric tests were employed.
Results:
The mean TCD/AC, HC/AC, and FL/AC ratios were 14.12±0.89, 1.05±0.04, and 22.04±0.98, respectively, with the standard deviations for each group. The cut off value (Mean±2SD) of TDC/AC ratio, HC/AC ratio and FL/AC ratio for diagnosing IUGR was15.9, 1.13 and 24.0 respectively. TCD/AC ratio demonstrated a sensitivity of 87.5%, a specificity of 93.6%, a PPV of 91.7%, and a NPV of 89.3% in diagnosing intrauterine growth restriction; for the HC/AC ratio, these values were 81.2%, 89.3%, 86.4%, and 81.5%, respectively; and for the FL/AC ratio, they were 52.4%, 88.3%, 43.6%, and 89.2%, respectively.
Conclusion:
TCD/AC, HC/AC, and FL/AC ratios were the parameters that were not affected by GA and can be used to accurately detect intrauterine growth restriction. However, TCD/AC ratio exhibited higher sensitivity, specificity, PPV, and NPV as compared to HC/AC and FL/AC ratios in diagnosing IUGR.
Keywords
Intrauterine growth restriction (IUGR), Transcerebellar Diameter (TCD), Abdominal Circumference (AC), Head Circumference (HC), Femur Length (FL)
Intrauterine growth restriction (IUGR) articles, Transcerebellar Diameter (TCD) articles, Abdominal Circumference (AC) articles, Head Circumference (HC) articles, Femur Length (FL) articles
Article Details
Introduction:
Both the pregnant woman and her health care provider strive for the birth of a healthy baby. IUGR refers to the estimated foetal weight (EFW) and/or abdominal circumference (AC) at a specific time throughout pregnancy that are less than the third percentile, or the EFW and/or AC are less than the tenth percentile for the gestational age (GA), along with abnormal doppler values. EFW is the only parameter that is used to evaluate foetal growth [1-3].
The whole population has a 10% incidence of foetal growth restriction. IUGR occurs six times more frequently in underdeveloped/developing nations than in developed nations. The three common types of IUGR are asymmetrical, symmetrical, and mixed. IUGR diagnosed at or before 32 weeks is considered early onset, whereas IUGR diagnosed after 32 weeks is considered late onset [1].
Intrauterine growth restriction is the most frequent consequence of maternal, placental, foetal, or genetic causes, and it may also be the result of any combination of these variables. IUGR increases prenatal and neonatal morbidity and mortality. IUGR causes a threefold increase in newborn mortality. Accurately identifying foetuses with growth restriction is crucial to prevent perinatal complications. Foetal hypoxia is a condition attributed to the delayed detection of IUGR. Perinatal asphyxia can result in hypoxic ischemic encephalopathy, ischaemic congestive heart failure, meconium aspiration, pneumonia, and gastrointestinal perforation. The long-term consequences include impaired neurological development in children and a higher likelihood of cardiovascular diseases, lipid abnormalities, and insulin resistance in adulthood [1,4]. Early diagnosis of intrauterine growth restriction is essential for effective obstetric management to reduce late foetal complications, perinatal morbidity, and mortality [5].
The evaluation of gestational age was traditionally carried out by health care providers using a combination of oral information and a physical examination. Prenatal ultrasonography provides a more accurate tool for evaluating foetal growth, which is essential in light of the fact that clinical evaluations of foetal growth are not always reliable [3].
A significant number of prenatal women frequently have an unreliable LMP without confirmation of GA in the 1st trimester. When there is a lack of precision regarding the GA, it will be more difficult to differentiate between foetuses that are growing normally and those who are experiencing IUGR [3-5].
Head circumference (HC), biparietal diameter (BPD), abdominal circumference (AC), and femur length (FL) are the more commonly used parameters for evaluating foetal growth. Nevertheless, the aforementioned measurements may only be precisely related when GA is accurately established. Out of all the measurements taken using ultrasonography, AC is the most accurate predictor of IUGR. Foetal growth restriction results in the rapid depletion of glycogen in the liver and subcutaneous fat, which ultimately leads to a decrease in AC. Therefore, AC is considered as an important measurement for early identification of intrauterine growth restriction. AC within the usual range for GA rules out the foetal growth restriction, while a measurement below the 5th percentile certainly represents the foetal growth restriction [3].
The term "transcerebellar diameter (TCD)" refers to the greatest horizontal measurement of the foetal cerebellar hemispheres. The developing foetal cerebellar hemispheres are contained within the posterior cranial fossa, which is resilient to external pressures and alterations in foetal growth. The measurement of TCD is a highly accurate method for evaluating the gestational age of expectant mothers in whom the LMP is uncertain and without confirmation of gestational age in the 1st trimester [6-8].
Intrauterine growth restriction is primarily caused by a decrease in the blood flow between the uterus and the placenta, or uteroplacental blood flow. In foetuses with IUGR, blood flow is essentially redirected towards central organs such as the brain, heart, and adrenal glands. During acute asphyxia, the blood flow to the cerebellum is sustained as a result of the redistribution of the cardiac output. The foetal cerebellar growth is slightly impacted by IUGR, which makes the TCD the most dependable measure for estimating gestational age. The foetal abdominal circumference is impacted at the initial stages of IUGR, causing the TCD/AC ratio to rise in cases of IUGR, while it generally stays consistent throughout a normal pregnancy. It is possible to predict intrauterine growth restriction using TCD/AC ratio, regardless of gestational age [6-8].
HC is a measurement that is slightly altered by external force, which might cause deformation of the foetal head and growth abnormalities. FL is minimally affected by the process of IUGR. AC is impacted at the initial stages of IUGR, causing the HC/AC and FL/AC ratios to rise in cases of IUGR. HC/AC and FL/AC ratios are measurements that are not influenced by gestational age and can be utilised to predict intrauterine growth restriction [8-11].
As far as we are aware, there are only a few comprehensive studies that have been published in the scientific literature that evaluate the efficiency of TCD/AC, HC/AC, and FL/AC ratios in diagnosing IUGR.
Aim of the present study was to determine the efficiency of TCD/AC, HC/AC, and FL/AC ratios in diagnosing IUGR.
Materials and Methods
The study included 500 expectant mothers who were sent for ultrasound examinations to the radiology department of GEMS Hospital, India, with GA ranging from 18 to 40 weeks. Prior to the examinations, informed and written consent was sought from each and every pregnant woman. The institutional ethics committee gave its approval to conduct the study.
Inclusion criteria
Expectant mothers with GA ranging from 18 to 40 weeks, a single live foetus in the uterus, and accurate dates were included in the research.
Exclusion criteria
Expectant mothers with an uncertain LMP, foetal anomalies, multiple gestation, polyhydramnios, a gestational age less than 18 weeks, or who did not deliver in our hospital were excluded from the study.
Research imaging methods:
Ultrasound examinations were performed using a 1-7 MHz frequency curvilinear probe. As a means of minimising the influence of inter-observer variability, the ultrasound was carried out by a single radiologist who had five years of expertise. For the purpose of minimising the variability within the observer, two measurements were averaged. Along with regular biometric measurements, TCD was also measured. Calculations were made to determine all the ratios. The antenatal women were notified of the findings of the scan and monitored until delivery, with additional scans conducted as needed; including at least one more scan at 30-34 weeks. Every newborn was evaluated by a neonatologist at birth (Figure 1).
TCD - Transcerebellar Diameter, BPD - Biparietal Diameter, HC - Head Circumference, AC - Abdominal Circumference, and FL - Femur Length
Statistical analysis
The information that was gathered was subsequently exported into the version of Microsoft Excel that was released in 2019. The SPSS Statistics for Windows, V. 22.0 was utilised in order to perform the statistical analysis. Categorical variables were represented by utilising numbers and percentages. On the other hand, quantitative variables were expressed by using the mean value in conjunction with the standard deviation (SD). In both normal and IUGR foetuses, a regression analysis was carried out to compare GA of the foetus with each ultrasonography-recorded parameter, including TCD, BPD, HC, AC, and FL. Additionally, we used correlation coefficients to ascertain the association between each parameter evaluated by ultrasonography and gestational age. Nomograms were created by obtaining the 5th, 50th, and 95th percentile values from normal pregnancies. In cases of intrauterine growth restriction, routine ultrasound measurements were found to be below the 5th percentile for the GA, as compared to nomograms based on pregnancies that were considered normal. The analysis was conducted using Fischer's exact test. Initially, we conducted a normality test to determine whether our data follows the normality test (Kolmogorov, Smirnov, and Wilk test). If they do, we proceed to a parametric test. The T-test was employed to determine the significance of differences in means in our study, while an ANOVA was used when there were more than two groups. We resorted to non-parametric testing in the event that our data did not conform to the normality test. Statistical tests such as the Mann-Whitney U test and the Friedman test, or the Chi-square test, were employed to analyse categorical variables in independent groups. A standard measure of significance was selected, and it was determined to be P = 0.05. When determining statistical significance, any result that was calculated to be equal to or lower than the value of 0.05 was considered to be significant. In order to diagnose IUGR, a sensitivity, specificity, PPV, and NPV of TCD/AC, HC/AC, and FL/AC ratios were determined.
Results
A total of 524 antenatal women, with GA ranging from 18 to 40 weeks, were referred to Department of Radiology for ultrasound examination. 24 antenatal women were lost during follow up and not delivered in our hospital, thus they were omitted from the research. Remaining 500 antenatal women were included for statistical analysis. The antenatal women included in the investigation were aged between 18 and 35, with a mean age of 25.78 ± 4.53 years. Among a sample of 500 women, 231 of them, accounting for 46.2% of the total, fell between the age range of 21 and 25 years (Table 1).
|
Age (in years) |
Frequency |
Percentage( %) |
|
18 - 20 |
39 |
7.8 |
|
21 - 25 |
231 |
46.2 |
|
26 - 30 |
194 |
38.8 |
|
31- 35 |
36 |
7.2 |
|
Total |
500 |
100 |
Table 1: Age distribution in antenatal women
Out of 500 women, 346 (69.2%) were primi gravidae. Appropriate for gestational age was observed in 410 (82%) out of 500 expectant mothers, and IUGR was observed in 90 (18%) out of 500 expectant mothers (Figure 2).
FGR: Fetal Growth Restriction, AGA: Appropriate for Gestational Age
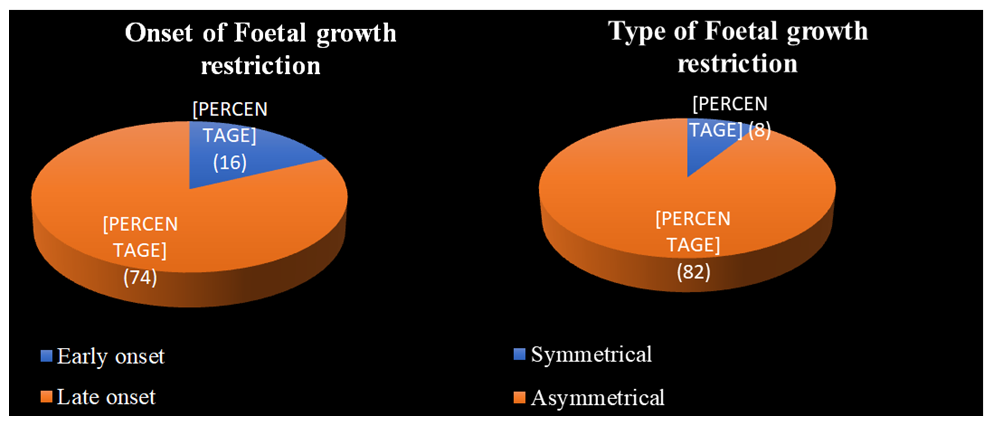
Early-onset IUGR was seen in 16 (17.7%) out of 90 antenatal women, and late-onset IUGR was observed in 74 (82.2%) out of 90 antenatal women. Asymmetrical IUGR was observed in 82 (91.1%) out of 90 antenatal women, and symmetrical IUGR was observed in 8 (8.8%) out of 90 antenatal women (Figure 3).
The current study revealed a substantial positive proportional correlation among TCD and GA (r = 0.9412; p = 0.0001), a substantial positive relationship among AC and GA (r = 0.9132; p = 0.0001), a strong positive correlation among TCD and AC (r = 0.9124; p = 0.0001), a positive correlation among HC and GA (r = 0.9231; p = 0.0001), and a positive correlation among FL and GA (r = 0.9251; p = 0.0001) (Table 2).
|
Correlation between different foetal parameters and Gestational age |
r value |
p value |
|
TCD and Gestational age |
0.9412 |
0.0001 |
|
AC and Gestational age |
0.9132 |
0.0001 |
|
HC and Gestational age |
0.9231 |
0.0001 |
|
FL and Gestational age |
0.9251 |
0.0001 |
|
AC and TCD |
0.9124 |
0.0001 |
Table 2: Correlation between different foetal variables and Gestational age
The present research revealed no correlation among TCD/AC ratio and GA (r2 = 0.1412; p > 0.05), the HC/AC ratio and GA (r2 = 0.1314; p > 0.05), and FL/AC ratio and GA (r2 = 0.1298; p > 0.05) beyond 18 weeks of pregnancy (Table 3).
|
Correlation between different foetal parameters and Gestational age |
r2 value |
p value |
|
TCD/AC and Gestational age |
0.1412 |
> 0.05 |
|
HC/AC and Gestational age |
0.1314 |
> 0.05 |
|
FL/AC and Gestational age |
0.1298 |
> 0.05 |
Table 3: Correlation between different foetal variables and Gestational age
In the current study, a majority of the normal foetuses had a TCD/AC ratio that was below 15, while it was above 16 in the majority of IUGR foetuses. The average TCD/AC ratio was 14.12 ± 0.89. In the majority of normal foetuses, HC/AC ratio was usually less than 1.1. However, in IUGR foetuses, the HC/AC ratio was often above 1.1. The average HC/AC ratio was 1.05 ± 0.04. In the majority of foetuses with normal growth, the FL/AC ratio was below 22. Conversely, in the majority of IUGR foetuses, the FL/AC ratio was greater than 23. The average FL/AC ratio was 22.04 ± 0.98. The cutoff value (mean±2SD) of the TDC/AC ratio, HC/AC ratio and FL/AC ratio for diagnosing IUGR was 15.9, 1.13 and 24.0 respectively (Table 4).
|
Parameters |
Mean |
Standard deviation (SD) |
Cut off value (mean±2SD) |
|
TCD/AC |
14.12 |
0.89 |
15.9 |
|
HC/AC |
1.05 |
0.04 |
1.13 |
|
FL/AC |
22.04 |
0.98 |
24 |
Table 4: Diagnostic values of the current study
In the present study, TCD/AC ratio showed a sensitivity of 87.5%, a specificity of 93.6%, a PPV of 91.7%, and a NPV of 89.3% in predicting intrauterine growth restriction; for the HC/AC ratio, these values were 81.2%, 89.3%, 86.4%, and 81.5%, respectively; and for the FL/AC ratio, they were 52.4%, 88.3%, 43.6%, and 89.2%, respectively (Table 5).
|
Parameter |
Sensitivity |
Specificity |
PPV |
NPV |
|
TCD/AC ratio |
87.5% |
93.6% |
91.7% |
89.3% |
|
HC/AC ratio |
81.2% |
89.3% |
86.4% |
81.5% |
|
FL/AC ratio |
52.4% |
88.3% |
43.6% |
89.2% |
Table 5: Diagnostic values in present study
Discussion:
Early diagnosis of intrauterine growth restriction is essential for proper obstetric management to decrease the occurrence of late foetal complications, perinatal morbidity and mortality [1-3]. While it has been hypothesised that foetal growth restriction does not have an effect on TCD and foetal AC gets affected early in IUGR. As a result, TCD/AC ratio is increased in IUGR, which is rather consistent throughout the normal pregnancy [5-8]. HC is another measure that is only marginally influenced by external pressure effects and growth alterations. FL is minimally affected in IUGR. Both HC/AC and FL/AC ratios are gestational age-independent measures that can be used to predict IUGR [8-11]. Ultrasonographic measurements of standard parameters were given in Figure 1.
The present study found that the majority of the pregnant women, 231 (46.2%) out of 500, were between the ages of 21 and 25 years old (Table 1); 346 (69.2%) out of 500 pregnant women were primi gravida; out of 500 pregnant women, 410 (82%) were appropriate for gestational age; 90 (18%) were found to have a foetal growth restriction (Figure 2); and out of 90 antenatal women who had a foetal growth restriction, 82 (91.1%) were asymmetrical, and 8 (8.8%) were symmetrical (Figure 3). The present study found that the frequency of FGR was 18%, which is comparable to the 24% reported by Srividya et al. [3] and higher than the 3-7% reported by Romo A et al. [12].
In the present study most of the antenatal women with FGR, 74 (82.2%) out of 90 presented after 32-34 weeks of gestation (Figure 3). Similar incidence was reported by Malhotra A et al. in their study with 80% cases of foetal growth restriction presenting after 32 weeks of pregnancy [13].
The current research found a substantial proportional relation among TCD and GA (r = 0.9412; p = 0.0001). Furthermore, a good relation was found among abdominal circumference and gestational age (r = 0.9132; p = 0.0001), as well as among TCD and abdominal circumference (r = 0.9124; p = 0.0001). Investigation performed by Meyer WJ et al. [14] demonstrated a substantial correlation among TCD and gestational age (r2 = 0.9464), AC and gestational age (r2 = 0.9685), and the TCD and abdominal circumference (r2 = 0.9561). Aforementioned findings are comparable to those found in the current investigation. Sharma G and Ghode R conducted research in the Wardha district of Maharashtra and found a strong proportional correlation among the TCD and gestational age, as well as a strong correlation among AC and GA. These results correspond to the current study [15].
The present research demonstrated a proportional association among between the TCD and advancing gestational age in both AGA and FGR foetuses. Studies by Bansal M et al. and Chavez MR et al. showed similar observations [16,17]. This can be explained by the fact that the transcerebellar diameter is only minimally influenced by foetal growth restriction [16,17].
According to the present study, after 18 weeks of gestation, there was an insignificant association (r2 = 0.1412; p > 0.05) among TCD/AC ratio and GA (Table 3). Therefore, despite a slight raise in TCD/AC ratio with advancing GA, this difference is not significant, suggesting that the ratio remains consistent irrespective of GA. An investigation done by Bhimarao et al. discovered a statistically insignificant relation (r2 = 0.13, p > 0.05) among the TCD/AC and GA beyond 20-weeks of gestation, which is compaable with the present study [8].
In the current investigation, majority of the normal foetuses had a TCD/AC ratio that was below 15, while it was above 16 in the majority of IUGR foetuses. Average TCD/AC ratio is 14.12 ± 0.89 (Table 4). An investigation done by Bhimarao et al reported a mean TCD/AC ratio of 13.63±1.2, which is close to the current study [8]. Another research carried out by Meyer WJ et al. reported a mean TCD/AC ratio of 13.68±0.96, which is consistent with the present study as well [14].
In the present investigation, a threshold value of TDC/AC ratio to detect intrauterine growth restriction was determined to be 15.9 (mean ± 2SD) (Table 4). It exhibits a resemblance to an investigation done by Meyer WJ et al., in which they established 15.9 as the threshold value for detecting IUGR [14]. In a different research by Tongsong et al., the threshold value was determined to be 15.4, which aligns with the observations of our study [18].
According to the present research, TCD/AC ratio accurately detected 74% of symmetric FGR cases. In an investigation done by Meyer WJ et al., TCD/AC ratio precisely predicted 71% of cases of symmetric FGR, which aligns with the observations of our study [14].
In the present investigation, TCD/AC ratio showed a sensitivity of 87.5%, a specificity of 93.6%, a PPV of 91.7%, and a NPV of 89.3% in diagnosing IUGR. In the studies performed by Bhimarao et al., Devi et al., Meyer et al., and Roy J. et al., the researchers reported outcomes that were comparable to the current research [8,11,14,19] (Table 6).
|
Diagnostic values of TCD/AC ratio in various studies |
Sensitivity |
Specificity |
PPV |
NPV |
|
Bhimrao et al., [8] |
88.00 |
93.50 |
77.10 |
96.30 |
|
Devi KL et al., [11] |
89.50 |
83.00 |
98.00 |
62.40 |
|
Meyer WJ et al., [14] |
83.90 |
96.20 |
94.50 |
88.20 |
|
Roy J et al., [19] |
100 |
80.90 |
87.80 |
100 |
|
Present study |
87.50 |
93.60 |
91.70 |
89.30 |
Table 6: Comparison of TCD/AC ratio of the present study with various studies
The present study found a strong correlation among HC and gestational age (r = 0.9231; p = 0.0001), among FL and gestational age (r = 0.9251; p = 0.0001) (Table 2), and an insignificant association among HC/AC ratio and GA (r2 = 0.1314; p > 0.05) and among FL/AC ratio and GA (r2 = 0.1298; p > 0.05) beyond 18 weeks of pregnancy (Table 3). The results of the studies performed by Bhimarao et al, Campbell et al, Hadlock et al, and Devi KL et al. are similar to our research [8-11].
In the current research, in the majority of normal foetuses, the HC/AC ratio is usually below 1.1. However, in IUGR foetuses, HC/AC ratio was often above 1.1. Average HC/AC ratio was 1.05 ± 0.04. A threshold value of the HC/AC ratio to detect intrauterine growth restriction was determined to be 1.13 (mean ± 2SD) (Table 4). A research performed by Gangadhar PY et al., reported a mean HC/AC ratio of 1.04±0.05 (mean±SD) and a threshold value of 1.14, which is close to our research [20].
In this present investigation, HC/AC ratio could diagnose IUGR with a sensitivity of 81.2%, a specificity of 89.3%, a PPV of 78.4%, and an NPV of 81.5%. . Similar conclusions were noted in the investigations performed by Bhimarao et al., and Benson et al. [8,21] (Table 7).
|
Diagnostic values of HC/AC ratio in various studies |
Sensitivity |
Specificity |
PPV |
NPV |
|
Bhimarao et al., [8] |
84.00 |
92.0 |
72.4 |
95.8 |
|
Meyer WJ et al. [14] |
49.3 |
87.6 |
75.6 |
69.0 |
|
Gangadhar PY et al. [20] |
61.1 |
93.9 |
68.7 |
91.6 |
|
Benson et al. [21] |
82.0 |
94.0 |
62.0 |
98 |
|
Present study |
81.2 |
89.3 |
78.4 |
81.5 |
Table 7: Comparison of the HC/AC ratio of the present study with various studies
In this present investigation, in majority of normal foetuses, FL/AC ratio was usually below 22. However, in IUGR foetuses, FL/AC ratio was often above 23. Average FL/AC ratio was 22.04±0.98. The cutoff value of the FL/AC ratio to detect intrauterine growth restriction was determined to be 24.0 (mean ± 2SD) (Table 4). A study performed by Hadlock FP et al. reported a mean FL/AC ratio of 22.00±1.00 (mean±SD), which is comparable to our study [10]. Another study conducted by Gangadhar PY et al. reported a mean FL/AC ratio of 22.10±1.13 (mean±SD), which is consistent with the present study as well [20].
In this present investigation, FL/AC ratio could detect IUGR with sensitivity of 52.4%, specificity of 88.3%, PPV of 43.6%, and NPV of 89.2%. Similar conclusions were reported in an investigation performed by Hadlock FP et al. [10] (Table 8).
|
Diagnostic values of FL/AC ratio in various studies |
Sensitivity |
Specificity |
PPV |
NPV |
|
Hadlock et al., [10] |
60.0 |
90.0 |
25.0 |
98.0 |
|
Devi et al. [11] |
69.5 |
61.0 |
89.0 |
- |
|
Gangadhar PY et al. [20] |
77.7 |
95.1 |
77.0 |
95.1 |
|
Shalev et al. [22] |
29.9 |
91.2 |
13.6 |
96.3 |
|
Present study |
52.4% |
88.3% |
43.6% |
89.2% |
Table 8: Comparison of the FL/AC ratio of the present study with various studies
It was noted that certain expectant mothers reported pain when moving the ultrasonography probe over the head of the foetus in order to get the sub-occipito-bregmatic view needed to measure the TCD in the late third trimester. Furthermore, in a few expectant mothers, we could not see the borders of the foetal cerebellum clearly due to shadows cast by the foetal skull. The foetus in a few of the patients was very active, and we encountered certain difficulties when we attempted to measure the TCD using an ultrasound machine. These expectant mothers were among the handful with whom we had difficulty. In spite of the fact that the percentage was lower than 2%, we explicitly admit that the transcerebellar diameter might have been inadequate in the situations described above.
It is essential to point out that the current research does, in fact, fall under the category of having specific limitations inherent in it. We selected a limited number of expectant mothers from one healthcare facility to conduct the current research. This research was performed over a comparatively short period of time. Expectant mothers with an uncertain LMP, foetal anomalies, multiple gestations, polyhydramnios, a gestational age less than 18 weeks, or who did not deliver in our hospital were excluded from the study. Nevertheless, conducting extensive research endeavours with a greater number of individuals participating can lead to a more precise depiction of the topic being examined.
Conclusion:
The present study established a substantial correlation among TCD and AC during pregnancy, with a stable TCD/AC ratio throughout the gestation. A cutoff value of 15.9 may be utilised as a reliable measure to detect IUGR. TCD/AC, HC/AC, and FL/AC ratios are independent of gestational age and can be used to detect intrauterine growth restriction, especially in cases where the gestational age is uncertain. These ratios had a high level of diagnostic accuracy for diagnosing IUGR. Compared to HC/AC and FL/AC ratios, TCD/AC ratio showed greater sensitivity, specificity, PPV, and NPV in diagnosing intrauterine growth restriction.
Conflict of Interest
None
References
- Sharma D, Shastri S, Sharma P. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clin Med Insights Pediatr 10 (2016): 67-83.
- L.J. Salomon, Z. Alfirevic, F. Da Silva Costa et-al. ISUOG Practice Guidelines: ultrasound assessment of fetal biometry and growth. Ultrasound in Obstetrics & Gynecology 53 (2019): 715.
- Srividya R, Himavarshini K, Pathri M. Evaluation of transverse cerebellar diameter/abdominal circumference ratio-in assessing fetal growth restriction. Int J Acad Med Pharm 4 (2022): 634-639.
- Leitner, Y., Fattal-Valevski, A., Geva, R., et al. Neurodevelopmental outcome of children with intrauterine growth retardation: a longitudinal, 10-year prospective study. Journal of child neurology 22 (2007): 580-587.
- Nardozza LM, Caetano AC, Zamarian AC, et al. Arch Gynecol Obstet 295 (2017): 1061-1077.
- Meyer, W. J., Gauthier, D. W., Goldenberg, B., et al. The fetal transverse cerebellar diameter/abdominal circumference ratio: a gestational age-independent method of assessing fetal size. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine 12 (1993): 379-382.
- Manning F. A. The use of sonography in the evaluation of the high-risk pregnancy. Radiologic clinics of North America, 28 (1990): 205-216.
- Bhimarao, Nagaraju RM, Bhat V, et al. Efficacy of Transcerebellar Diameter/Abdominal Circumference Versus Head Circumference/Abdominal Circumference in Predicting Asymmetric Intrauterine Growth Retardation. J Clin Diagn Res 9 (2015): TC01-5.
- Campbell S, Thoms A. Ultrasound measurement of the fetal head to abdomen circumference ratio in the assessment of growth retardation. Br J Obstet Gynaecol 84 (1977): 165-174.
- Hadlock FP, Deter RL, Harrist RB, et al. A date-independent predictor of intrauterine growth retardation: femur length/abdominal circumference ratio. AJR Am J Roentgenol 141 (1983): 979-84.
- Devi KL, Durga DVK, Shivaparvathi D et al. Study of transcerebellar diameter to abdominal circumference ratio in prediction of fetal growth restriction in high risk pregnancies. International Journal of Health Sciences 6 (2022): 5017-5025.
- Romo A, Carceller R, Tobajas J. Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatr Endocrinol Rev 6 (2009): 332-336.
- Malhotra A, Allison BJ, Castillo-Melendez M, et al. Neonatal Morbidities of Fetal Growth Restriction: Pathophysiology and Impact. Front Endocrinol (Lausanne) 10 (2019): 55.
- Meyer WJ, Gauthier D, Ramakrishnan V, et al. Ultrasonographic detection of abnormal fetal growth with the gestational age-independent, transverse cerebellar diameter/abdominal circumference ratio. Am J Obstet Gynecol 171 (1994): 1057-1063.
- Sharma G, Ghode R. Fetal transcerebellar diameter and transcerebellar diameter--abdominal circumference ratio as a menstrual age independent parameter for gestational age estimation with grading of cerebellar maturity. IJRCOG 4 (2015): 2036-2041.
- Bansal M, Bansal A, Jain S, et al. A study of Correlation of Transverse Cerebellar Diameter with Gestational Age in the Normal & Growth Restricted Fetuses in Western Uttar Pradesh. PJSR 7 (2014): 16-21.
- Chavez, M. R., Ananth, C. V., Smulian, J. C., et al. Fetal transcerebellar diameter measurement for prediction of gestational age at the extremes of fetal growth. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine 26 (2007): 1167-1174.
- Tongsong T, Wanapirak C, Thongpadungroj T. Sonographic diagnosis of intrauterine growth restriction (IUGR) by fetal transverse cerebellar diameter (TCD)/abdominal circumference (AC) ratio. Int J Gynaecol Obstet 66 (1999):1-5.
- Roy J, Sanyal P, Saboo V, et al. Role of the ratio of trans cerebellar diameter and abdominal circumference in detecting asymmetrical intra uterine growth restriction. J. Evolution Med. Dent. Sci 8 (2019): 3858-3862
- Gangadhar PY, Pillai NS. Comparison of ultrasound parameters for diagnosis of IUGR. Int J Reprod Contracept Obstet Gynecol 7 (2018): 596-601.
- Benson, C. B., Belville, J. S., Lentini, J. F., et al. Intrauterine growth retardation: diagnosis based on multiple parameters--a prospective study. Radiology 177 (1990): 499-502.
- Shalev E, Romano S, Weiner E, et al. Predictive value of the femur length to abdominal circumference ratio in the diagnosis of intrauterine growth retardation. Isr J Med Sci 27 (1991): 131-133.