Defective Interfering Particles and Their Role in Disease Progression and Persistence
Article Information
Alemayehu Reta
Department of Medical Laboratory Science, College of Health Sciences, Debre Markos University, PO Box 269, Debre Markos, Ethiopia
*Corresponding Author: Alemayehu Reta, Department of Medical Laboratory Science, College of Health Sciences, Debre Markos University, PO Box 269, Debre Markos, Ethiopia
Received: 10 June 2017; Accepted: 23 June 2017; Published: 27 June 2017
Share at FacebookAbstract
Defective interfering particles (DIPs) are products of inner deletion mutants of viruses that reproduce at the disbursement of the parent virus. This review article aimed at reviewing current science on DIPs of their molecular and immunological features, role in disease progression and persistence, impact on vaccine production and viral vectors, and future directions. Defective interfering particles are very important to the field of biotechnology and genetic engineering due to their nature of stimulating the immune system and attenuating some of the live viruses during live-attenuated vaccine production, however, they have a devastating effect like interfering with vaccine production, that is decreasing the viral titer, and facilitate pathogenesis and persistence of some viral infections.
Keywords
Defective Interfering Particles; Persistence; Virus
Article Details
1. Introduction
Viruses are DNA or RNA fragments covered by protein that are incapable to reproduce independently rather they replicate within cells by using the genetic machinery of those cells. During 1947 Von Magnus discovered incomplete particles after he performs consecutive unmixed passages of influenza viruses [1]. Nayak supports Von Magnus experiment by preparing chicken embryo cells and making it to be infected with equine influenza virus, in the first 10hr he obtained the whole RNA component of infectious virus, but after 20 to 30 hours the virus released was Von Magnus type and contained the RNAs of incomplete virus. These defective particles represent smaller units of the standard genome and defective in replication. They can interfere with replication of homologous helper viruses [2]. Holland and his colleagues discover DIPs of laboratory setting [3, 4]. In early 1970s, Huang and Baltimore introduced the term defective interfering particles to describe Von Magnus incomplete particles more accurately and to distinguish DIPs from other noninfectious non-interfering particles [5]. The following definitions of DIPs were proposed: (A) they contain the same structural proteins as the standard virus that they are derived from and are antigenically identical; (B) DIPs contain only a part of the viral genome which makes them defective, since they cannot produce all viral proteins on their own; (C) DIPs can be propagated during coinfections when a complete standard virus, also called helper virus, provides the missing protein(s); and (D) during coinfections, DIPs interferes specifically with the reproduction of their helper virus leading to the release of mainly DI progeny [6].
2. General features
2.1 Molecular feature
Defective viral genomes (DVGs) are shortened forms of the viral genome that are produced during virus reproduction at high titers. DVGs can not replicate without helper virus due to lack essential genes. Deletion DVGs are shortened forms of the parental virus genome that habitually share the 3’ and 5’ ends with the original virus. They are produced when the viral polymerase falls off the parental template strand and reattaches further downstream, causing in a genomic deletion. Copy-back DVGs, and the associated snap-back DVGs, comprise of a piece of the viral genome lined by reverse complementary versions of its 5’ end. Copy-back DVGs occurs when the viral polymerase detaches from the template and reattaches to the newly synthesizing strand, copying back the 5’ end of the genome [7, 8]. DVGs were primarily described as the genomes of incomplete viral particles able to obstruct with regular virus replication, therefore receiving the name of defective interfering (DI) particles [9]. An experimental molecular study on influenza virus reveals that the genome of influenza virus comprises of eight negative-sense RNA segments (viral RNA (vRNA)) that encode 10 to 12 proteins, based on the subtype.
DI RNAs are produced through an abnormal replication events and during high-multiplicity passage of the original virus. Internal sequences of the original vRNA of Defective interfering RNA segments are deleted, whereas holding certain 5’ and 3’ end-specific sequences of the progenitor vRNA. Identical terminal sequences where found in all DI RNAs of influenza A virus resulting from the first 13 nucleotides of the 5’ end (AGUAGAAACAAGG) and the last 12 nucleotides of the 3’ end (-CCUGCUUUCGCU-OH) of the segments and typically contain the critical packaging signals, which are within the terminal coding sequences of each gene segment [10, 11]. In general, DI genomes represent smaller units of the standard genome, which can be replicated and packaged into virus particles they served as a tool to identify sequence elements such as origins of replication, promoters and packaging signals.
2.2 Immunological feature
Viruses like parainfluenza virus, measles, and the respiratory syncytial virus (RSV) interfere with the host natural immune arm allowing the virus to replicate to high titers before being controlled by the immune system [12, 13]. During peak replication of those viruses they produce incomplete viral genomes that contain large genomic deletions and are incapable to replicate in the absence of helper virus [14, 15]. Since 2006 Jacob and his associates perform an experiment on Sendai virus and they demonstrate that DIPs present in Sendai virus -Cantell stocks are required for its robust dendritic cell (DC) maturation ability.
dsRNA replication intermediaries were formed from the enhancement in virus-induced maturation of dendritic cells delivered by incomplete viral particles. This distinctive ability of DI particles cannot be simulated by simply growing the dose of standard virus [16]. DC maturation undergone through two important mechanisms, which are toll like receptor (TLR)-dependent and TLR-independent. TLR-dependent maturation begins with the recognition of viral machineries by TLRs confined on the cell membrane and primes to the expression of type I interferons (IFNs) as well as the induction into genes involved in DC maturation [17]. TLR ? independent maturation and type I IFN signaling are based on the intracellular recognition of a viral component and requires viral replication. This TLR-independent signaling mechanism is adequate for the proficient maturation of DCs and the successive initiation of immunity [18]. Finally, Jacob and his colleagues conclude that, Sendai virus strain Cantell has a particularly strong ability to mature DCs independently of type I IFNs and TLR signaling due to a novel role of DI particles, which are produced in higher level in this viral strain, as enhancer of TLR-independent pathway for DC maturation in addition to their reported effect in improving type I IFN production in infected cells. Once DCs are matured they start to voyage to the lymph nodes, present foreign antigens to T-cells, and initiate the acquired/adaptive immune system [19, 20].
A recent study on naturally occurring immunostimulatory defective viral genomes (iDVGs) reveals that, they are generated during RSV replication, are strong inducers of the innate/natural antiviral immune response to RSV in mice and humans. Infection of explanted human lung tissue from different donors shown that the most humans can respond to RSV iDVGs and that the rate of accumulation of iDVGs during infection directly allies with the quality of the antiviral response comprising the production of proinflammatory cytokines, chemokines, antiviral genes, and cell surface molecules [21].
A great testimony to sustain virus-host coexistence is the late generation of DVGs in the virus infection cycle, together with the effective detection of DVGs by host cellular proteins and the stimulation of a potent antiviral response [22].
In general DIP take part in stimulation of both natural and acquired immune arms in order to defend against some of medically important viral agents.
2.3 Role in disease progression and chronicity
Huang and Baltimore proposes that defective particles may influence the development and course of certain viral diseases [5]. Different literatures show that infection with viruses like reovirus, vesicular stomatitis virus, human immunodeficiency virus (HIV) and hepatitis viruses become persistent due to the involvement of defective interfering particles [23-26].
Most of the viruses produced during early stage of HIV infection are defective virus, that is noninfectious due to error-prone process of reverse transcription. The defective HIV particles pouring pathogenesis through activating CD4 T cells, rendering them permissive for productive HIV replication, and by giving a large pool of continuously changing HIV peptides that are presented on major histocompatibility complex (MHC) class II molecules to unremittingly stimulate resting CD4 T cells of different antigen specificities [23]. This process leads to activation of lots of CD4 T cells and result in excess production of HIV virion that can contribute to disease progression as long as HIV disease is a disease of immune activation. Seldom, activated infected cells survive and become long-lived memory cells with integrated, latent provirus. Reactivation of these cells also makes them permissive to HIV replication [23].
Internal deletion of human hepatitis B virus core antigen (HBcAg) is often found in HBV infections across the globe. During characterization of the mutants found in such infection reveals the presence of DIPs. The existence of this DIP leads virus ? virus interactions between the group of wild type and mutants which provide a way of quantitative variation of immune targets in virus ? host interactions in pathogenesis and persistence of HBV infection [27]. Miller and his colleagues also try to demonstrate by analyzing the sequence of the genome of HBV in different infected individuals and they obtained that most of the chronic carriers have mutated or defective virus. They conclude that defective viruses play a crucial role in the formation of persistent hepadnavirus infection [25].
During late 1970s Spandidos and Graham try to perform an experiment and write their investigation by saying defective virions of VSV and reovirus induce chronic infection in animal systems, especially in murine models [24].
A figure developed by Benito and Ocampo (Figure 1) clearly showed that Viral RNA-dependent RNA polymerase (RdRP) and cellular Retrotransposon Retro transcriptase (RRT) through Dicer-2 are intricate in the generation of defective viral genomes which are able to challenge for viral and cellular factors required for translation of the original virus. The viral cDNAs produced by the RRT could inhibit viral replication through Dicer-2 and the RNAi pathway [28].
2.4 Impact on vaccines and viral vectors
Those Viruses which have weak DC maturation abilities can be changed into potent DC stimulators with the addition of incomplete viral particles, supporting a potential application for DI particles as a novel natural adjuvant for viral immunizations [16].
Nowadays, viruses are not only propagated to generate vaccines against viral disease, but also used as vectors to express recombinant proteins in target cells or to stably integrate genes into target cell genomes. In any of the biotechnological production processes of viruses, DIPs can affect the viral yield and in some cases, such as live-attenuated vaccines or viral vectors for gene therapy, they can also influence the biological activity of the product [6]. Different scholars try to study the effect of DIPs on vaccine production and finally it was confirmed that DIPs decrease the virus yield in Madin-Darby canine kidney (MDCK) cells commonly used for influenza vaccine manufacturing. By using two seed virus preparations of the same influenza strain which differed remarkably with the amount of DIPs, it has been confirmed that DIPs in addition to interfering with viral RNA synthesis, they also induce a strong cellular response by enhancing IFN and inducing apoptosis [29]. Thus, the amount of DIPs should be kept low in working seed virus preparations used for vaccine production.
During production of live attenuated vaccine through the process of serial passaging, some of the DIPs contribute to attenuation, example: yellow fever vaccine production [30].
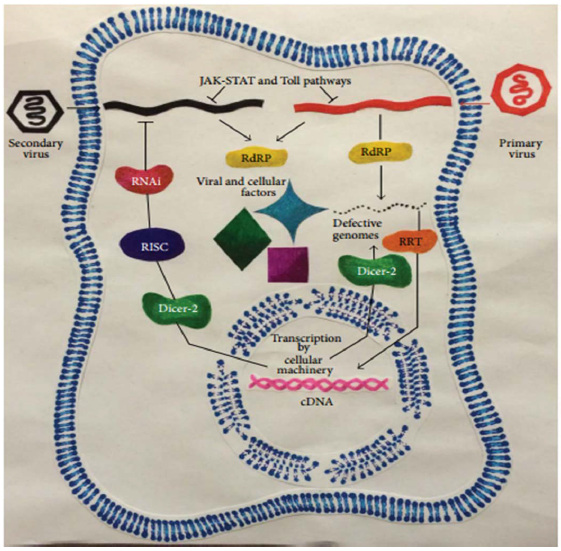
Figure 1: Mechanisms involved in viral interference/persistence. The Janus kinase-signal transducer and activator of transcription (JAK-STAT) and Toll pathways represent the innate immune system. RISC - RNA-induced silencing complex [28].
2.5 Contributions to the field and future direction
Even though, DIPs interfere with viral vaccine production and disease progression, they might become valuable biotechnological product like being an adjuvant as long as they have immunostimulatory activity. To obtain such benefits and to understand in a comprehensive manner researchers should perform quantitative real-time PCR, next generation sequencing and reverse genetics in combination with mathematical modeling. A recent demonstration states that DIPs accumulation during infections in vivo stimulates a potent antiviral response [31] proves that these universal by-products of virus replication play a vital role in natural virus-host interactions for therapeutic intervention.
The cellular pathways and secondary RNA motifs involved in the efficient recognition and potent response to DIPs in the presence of functional virus antagonists remain poorly understood, and it is likely that novel circuits that modulate the function of viral intracellular pattern recognition receptors like RLRs (RIG-I-like receptors) are involved. Prominently, the role of DIPs in determining virus pathogenesis remains to be studied. Vigorous research on the mechanisms and impact upon immunostimulatory DIPs in various viral infections will fill these gaps between knowledge.
3. Conclusion
Defective interfering particles contain only a part of the viral genome which makes them defective, since they cannot produce all viral proteins on their own. They have their own specific molecular and immunological characteristics. Defective interfering particles play a pivotal role in the field of biotechnology due to their nature of stimulating the immune system and attenuating some of the live viruses during live-attenuated vaccine production, however, they have a devastating effect like interfering with vaccine production and facilitate pathogenesis and persistence of some viral infections.
Acknowledgement
I would like to acknowledge Professor Thomas Seebeck for his immense contribution by sending relevant articles which cannot be accessed in developing countries for the development of this review article.
Author Contributions
Concepts, design, definition of intellectual contents, literature search, data acquisition, manuscript preparation, manuscript editing, manuscript review and guarantor.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Von Magnus. Studies on interference in experimental influenza: Biological observations. Ark Kemi mineral Geol 24 (1947): 1.
- Nayak DP. Defective virus RNA synthesis and production of incomplete influenza virus in chick embryo cells. J gen Virol 14 (1972): 63-67.
- Holland JJ. Defective interfering rhabdoviruses: In the rhabdoviruses. (R. Wagner, ed.), p. 297-360, plenum publishing Co., New York, 1987.
- Dimmock NJ. Antiviral activity of defective interfering influenza virus in vivo: In viral and other infections of the human respiratory tract. (S.Myint and D. Taylor-Robinson, eds.), chapter 22, p. 421-445, 1996.
- Huang AS, Baltimore D. Defective viral particles and viral disease processes. Nature 226 (1970): 325-327.
- Frensing T. Defective interfering viruses and their impact on vaccines and viral vectors. Biotechnol J 10 (2015): 681-689.
- Dimmock NJ and Easton AJ. Defective interfering influenza virus RNAs: time to reevaluate their clinical potential as broad spectrum antivirals? J Virol 88 (2014): 5217-5227.
- Lazzarini RA, Keene JD, Schubert M. The origins of defective interfering particles of the negative-strand RNA viruses. Cell 26 (1981): 145-154.
- Pathak KB, Nagy PD. Defective interfering RNAs: foes of viruses and friends of virologists. Viruses 1 (2009): 895-919.
- Davis AR, Nayak DP. Sequence relationships among defective interfering influenza viral RNAs. Proc Natl Acad Sci 76 (1979): 3092-3096.
- Davis AR, Hiti AL, Nayak DP. Influenza defective interfering viral RNA is formed by internal deletion of genomic RNA. Proc Natl Acad Sci 77 (1980): 215-219.
- Guo Z, Chen LM, Zeng H, Gomez JA, Plowden J, et al. NS1 protein of influenza A virus inhibits the function of intracytoplasmic pathogen sensor, RIG-I. Am J Respir Cell Mol Biol 36 (2007)): 263-269.
- Versteeg GA, Garcia SA. Viral tricks to grid-lock the type I interferon system. Curr Opin Microbiol 13 (2010): 508-516.
- Valdovinos MR, Gomez B. Establishment of respiratory syncytial virus persistence in cell lines: association with defective interfering particles. Intervirology 46 (2003): 190-198.
- Shingai M, Ebihara T, Begum NA, Kato A, Honma T, et al. Differential type I IFN inducing abilities of wild-type versus vaccine strains of measles virus. J Immunol 179 (2007): 6123-6133.
- Yount JS, Kraus TA, Horvath CM, Moran TM and Lo´pez CB. A Novel Role for Viral-Defective Interfering Particles in Enhancing Dendritic Cell Maturation. The Journal of Immunology 177 (2006): 4503-4513.
- Mazzoni A and DM Segal. Controlling the Toll road to dendritic cell polarization. J Leukocyte Biol 75 (2004): 721-730.
- Lopez CB, Moltedo B, Alexopoulou L, Bonifaz L, Flavell RA, et al. TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J Immunol 173 (2004): 6882- 6889.
- Mellman I and RM Steinman. Dendritic cells: specialized and regulated antigen processing machines. Cell 106 (2001): 255-258.
- Banchereau J and RM Steinman. Dendritic cells and the control of immunity. Nature 392 (1998): 245-252.
- Sun Y, Jain D, Koziol-White CJ, Genoyer E, Gilbert M, et al. Immunostimulatory defective viral genomes from respiratory syncytial virus promote a strong innate antiviral response during infection in mice and humans. PLoS Pathog 11 (2015): e1005122.
- Lopez CB. Defective viral genomes: Critical danger signals of viral infections. Journal of Virology 88 (2014): 8720-8723.
- Finzi D, Plaeger SF, and Dieffenbach CW. Defective virus drives human immunodeficiency virus infection, persistence and pathogenesis. Clinical and Vaccine Immunology 13 (2006): 715-721.
- Spandidos DA and Graham AF. Generation of defective virus after infection of newborn rats with reovirus. Journal of Virology 20 (1976): 234-247.
- Miller RH, Girones R, Coteo PJ, Hornbuckle WE, Chestnut T, et al. Evidence against a requisite role for defective virus in the establishment of persistent hepadnavirus infections. Proc Natl Acad Sci 87 (1990): 9329-9332.
- Sultanik P and Pol S. Hepatitis Delta Virus: Epidemiology, natural course and treatment. J Infect Dis Ther 4 (2016): 271.
- Wakita T, Kakumu S, Shibata M, Yoshioka K, Ito Y, et al. Detection of pre-C and core region mutants of hepatitis B virus in chronic hepatitis B virus carriers. J Clin Invest 88 (1991): 1793-1801.
- Salas-Benito JS and De Nova-Ocampo M. Viral interference and persistence in Mosquito borne Flaviviruses. Journal of Immunology Research 2015.
- Frensing T, Pflugmacher A, Bachmann M, Peschel B, Reichl U. Impact of defective interfering particles on virus replication and antiviral host response in cell culture-based influenza vaccine production. Appl Microbiol Biotechnol 2014.
- Barrett AD, Monath TP, Cropp CB, Adkins JA, Ledger TN, et al. Attenuation of wild-type yellow fever virus by passage in HeLa cells. J Gen Virol 71 (1990): 2301-2306.
- Tapia K, Kim WK, Sun Y, Mercado LX, Dunay E, et al. Defective viral genomes arising in vivo provide critical danger signals for the triggering of lung antiviral immunity. PLoS Pathog 9 (2013): e1003703.
