Covid-19, Diagnostic History and Mortality from Medicare 1999-2021, In an All-Cause Mortality Approach
Article Information
Nick Williams1*
1The Lister Hill National Center for Biomedical Communications, National Library of Medicine, National Institutes of Health
*Corresponding Author: Nick Williams, Ph.D, The Lister Hill National Center for Biomedical Communications, National Library of Medicine, National Institutes of Health 8600 Rockville Pike, Bethesda, MD 20894.
Received: 26 September 2023; Accepted: 09 October 2023; Published: 30 October 2023
Citation: Nick Williams, Ph.D. Covid-19, Diagnostic History and Mortality from Medicare 1999-2021, In an All-Cause Mortality Approach. Archives of Internal Medicine Research. 6 (2023): 74-85.
Share at FacebookAbstract
Introduction:
SARS-CoV-2 infections co-occurred with other diverse pre-existing clinical conditions in mortality cases. We use encounter level health data to evaluate the impact of non-Covid-19 diagnostic events on allcause mortality observed among Covid-19 positive cases billing Medicare. We further investigate prior diagnostic codes which occur in pre-pandemic study years among cases presenting to Medicare clinically with Covid-19 and cases with Covid-19 who experience all-cause mortality to inform patient population management.
Methods:
We aggregated encounter level records sourced from all Medicare beneficiaries from 1999-2021. Odds ratios were constructed using diagnostic history, age decile, study year and survival status. We used Generalized Linear Model (GLM) to predict the Decedent Observation Odds Ratio (DOOR) from study year, case observation odds ratio, age decile, non-covid conditions within counts of distinct covid-ever cases and their decedents. Odds ratios are relative to covid-never cases, or cases who did not present with Covid-19 clinically.
Results:
High explanatory DOOR measures are observed for diagnostic codes commonly associated with inpatient Covid-19 mortality. High DOOR measures are also observed for individuals living with specific kinds of cancers, experiencing cardiac arrest or acute tubular necrosis.
Conclusion:
Covid-ever mortality is influenced by primary infection itself and exacerbations of pre-existing conditions. Consequences of primary infection are observable in GLM, as well as meaningful prior clinical risk factors such as cancer, diabetes, cardiac and respiratory disease. Longcovid conditions require surviving Covid-19 clinical presentation and are predictable from GLM models.
Keywords
Covid-19; Mortality; Generalized Linear Model (GLM)
Article Details
Introduction
Sars-Cov-2 (Covid-19) is a highly infectious pathogen with pandemic reach and high mortality [1,2]. Perhaps half of all residents of the United States (US) have experienced Covid-19 infection (at least once) as of this writing [3-5]. Reported attributable mortality for Covid-19 reached over one million dead in the US alone [6,7]. Despite a wealth of data, a robust analysis using real world data to understand the impact of clinical co-factors on pandemic mortality among patients with pre-existing conditions remains poorly characterized.
Further, the course of Covid-19 illness in pathophysiological terms and populations affected remain incompletely described. This is perhaps because of the diversity of the infected individuals, as well as the severity of preexisting conditions in the US [8-12]. In turn, parsing and segmenting Covid-19 infection and mortality effects from clinical experiences in the general population is natively difficult. Preexisting conditions can have high mortalities, further complicating the evaluation of the impact of Covid-19 [13]. It is difficult to say if a patient who experiences Covid-19 infection dies because of Covid-19 or their underlying morbidity or perhaps the interactions of both. Lastly, individuals who present with Covid-19 clinically may have documented prior conditions. The impact of these prior conditions to inform downstream mortality is under-considered when evaluating the impact of Covid-19 [14-18]. However, current data shows the association of high mortality rates among patients with pre-existing conditions.
To address the need for population level risk assessment, pathophysiology and to inform further and identify subjects for future research, this paper reviews the clinical diagnostic events of the Medicare population and considers the impact of their retrospective care from years 1999 to 2021. We compare the odds of dying from Covid-19 among Medicare beneficiaries to those who never contract Covid-19. This study uses machine learning methods to discover which non-covid conditions are statistically associated with variations in mortality within observed, historic non-covid clinical presentations. Study results may be useful to better identify indexes of concern for pandemic Covid-19 mortality and potentially inform care for individuals with preexisting conditions.
Methods
Data collection
We collected all identifiable claims records from Medicare from 1999 through 2021. Any claim which contained a diagnostic code ICD9-CM or ICD10-CM was considered. Data was acquired through the Virtual Research Data Center’s Chronic Conditions Warehouse. Cases that billed for diagnostic code U07.1, (Emergency use of U07.1 | COVID-19) was considered Covid-19 positive.
Data transformation
We first mapped claim level diagnostic codes to SNOMED-CT. Study records are not aggregated within ICD10-CM or ICD9-CM but within the controlled diagnostic vocabulary of SNOMED-CT. To support interoperability the Observational Medical Outcomes Partnership’s (OMOP) Athena vocabulary was used to transform ICD9-CM and ICD10-CM to SNOMED-CT.
Study index
The study dataset is an aggregated index of distinct case volumes. Case volumes are disaggregated by year of observation, SNOMED-CT (diagnostic) code and 10-year age group membership at code utilization. This age-year-diagnostic unit, or AYD forms the basis of the study’s count data models. The AYD units were disaggregated by study ever-survival status and covid-ever status. Cases were counted as ever-dead if they died (failed to survive) in the study period. Because the covid-ever population gains qualification in 2020, retrospective death should be understood as a public health opportunity. The final study dataset describes covid-ever and Medicare baseline (all) case volumes who died or survived from 1999 through 2021 by their age at diagnostic code utilization within a study year. This study produced 1,703,246 AYD units for the covid-ever Medicare population. Case AYD aggregates with fewer than 10 individuals were dropped from the study to preserve privacy and prevent individual care from conflating this population level analysis.
Data analysis
Four relative rates (risk panels) were used to calculate two odds ratios used in this study.
- Baseline Observation Rate
The baseline observation rate was calculated as any Medicare case within AYD unit divided by any Medicare case within AY. This expresses the risk of being observed with an AYD unit for the baseline population with emphasis on inequality within diagnosis.
- Covid-Ever Observation Rate
The covid-ever observation rate was calculated as any covid-ever case within AYD divided by any covid-ever case within AY. This expresses the risk of a covid-ever case being observed within an AYD unit with emphasis on inequality within diagnosis.
- Case Observation Odds Ratio (COOR)
The case observation odds ratio was calculated by dividing baseline observation rate (1) by covid ever observation rate (2). The COOR describes the relative odds of a beneficiary contained within an AYD ever presenting clinically with Covid-19 versus beneficiaries never presenting clinically with Covid-19 within an AYD unit.
- Baseline Survival Rate
The baseline survival rate was calculated as any Medicare case that did not survive the study within AYD unit divided by any Medicare case that did not survive the study within AY. This expresses the risk of observing a decedent within an AYD unit for the baseline population with emphasis on inequality within diagnosis.
- Covid-Ever Survival Rate
The covid-ever survival rate was calculated as any covid-ever case that did not survive the study within AYD unit divided by any covid-ever case that did not survive the study within AY. This expresses the risk of observing a covid-ever decedent within an AYD unit for the covid-ever population with emphasis on inequality within diagnosis.
- Decedent Observation Odds Ratio (DOOR)
The decedent observation odds ratio was calculated by dividing baseline survival rate by covid-ever survival rate. The DOOR expresses the relative odds of an AYD containing a beneficiary that did not survive the study in the covid- ever group versus the covid-never group.
The above calculations return the risk of observing a covid-ever case or decedent, retrospectively, relative to a covid-never case or decedent. Note survival is ‘study survival status’ being attributed retrospectively. In this way cases can die in 2021 or 1999 and have their retrospective care qualified in the survived or decedent pool (within AYD). Decedent cases who die in 2020 would have their retrospective care classified as ‘decedent’ which would then be compared across covid-ever/covid-never groups and survived/ever-died.
Generalized Linear Model
This study evaluates the variance of the AYD odds ratios described above, and the attributable explanation of specific AYDs to specific ORs observed in the Medicare population. This variance is ranked as a coefficient above or below the intercept within H2o.AI models. The model ranked AYDs for their ability to explain variance in DOOR. In the model AYD was expressed as three features, not a combined term. The result of interest is the GLM covariate term for each diagnosis. Due to hardware constraints the model only considers AYD units that contained at least 500 cases. We do not report model summary statistics, because the goal of the model is not predictive; nor should it inform information technology products. Rather the model intends to segment DOOR from itself and order the results set.
Human Subjects Protections
This study was exempted from traditional Internal Review Board (IRB) review under exemption category four subsection two: ”Exemption category four applies to secondary research of identifiable private information or identifiable biospecimens, if at least one of the following criteria is met: (1) When the identifiable materials are publicly available or (2) when the data is recorded by the investigator in a de-identified manner (analysis dataset), ie. no identifiers are accessible to the research once the analysis begins. For example, the researcher conducts a retrospective medical chart review and records the necessary data in a datasheet for future analysis without any personal identifiers nor a code which would allow the investigator to link back to subjects.”
The analysis dataset does not contain identifiable information. Because the study itself does not consider identifiable records we are exempt from review. Creating an aggregated dataset within year of birth with a large population and without race, gender or place identifiers may be a candidate method for making conclusions found in at-scale identifiable data available to researchers without compromising privacy or supporting reidentification.
|
1999-2020 |
Cases |
Deaths |
Rate |
|
Medicare Beneficiaries Never-Covid |
11,40,82,395 |
4,88,66,264 |
42.83% |
|
Medicare Beneficiaries Ever-Covid |
42,34,351 |
7,58,105 |
17.90% |
Table 1: Cases, decedents and covid-ever cases
Table 1 describes distinct individuals over study time. Covid-ever cases survived into 2020, unlike never-covid cases who did not present with Covid-19 clinically. Note: only 32 distinct individuals presented with Covid-19 clinically in 2019. In turn the death rates should be interpreted with care, as the Medicare beneficiaries with clinical covid died within the years of 2020 and 2021, while Medicare beneficiaries who never experienced covid could die at any time (1999-2021).
|
20-29 |
30-39 |
40-49 |
50-59 |
60-69 |
70-79 |
80-89 |
90-99 |
100-109 |
|
|
1999 |
16 |
40 |
39 |
187 |
167 |
||||
|
2000 |
16 |
47 |
52 |
214 |
222 |
3 |
|||
|
2001 |
16 |
53 |
60 |
252 |
275 |
10 |
|||
|
2002 |
21 |
56 |
76 |
299 |
324 |
22 |
|||
|
2003 |
20 |
68 |
86 |
338 |
376 |
40 |
|||
|
2004 |
1 |
23 |
71 |
106 |
360 |
425 |
65 |
||
|
2005 |
1 |
24 |
79 |
137 |
387 |
475 |
102 |
||
|
2006 |
1 |
23 |
83 |
148 |
425 |
524 |
143 |
||
|
2007 |
1 |
30 |
87 |
162 |
463 |
566 |
192 |
||
|
2008 |
1 |
32 |
97 |
183 |
503 |
606 |
236 |
||
|
2009 |
1 |
35 |
106 |
205 |
538 |
649 |
294 |
2 |
|
|
2010 |
2 |
42 |
121 |
230 |
581 |
692 |
360 |
4 |
|
|
2011 |
5 |
47 |
139 |
258 |
631 |
757 |
432 |
18 |
|
|
2012 |
6 |
54 |
145 |
283 |
677 |
808 |
507 |
33 |
|
|
2013 |
6 |
57 |
158 |
308 |
724 |
877 |
574 |
60 |
|
|
2014 |
6 |
61 |
161 |
333 |
791 |
936 |
641 |
108 |
|
|
2015 |
5 |
65 |
174 |
405 |
961 |
1,170 |
812 |
163 |
|
|
2016 |
6 |
61 |
173 |
416 |
960 |
1,171 |
825 |
210 |
|
|
2017 |
7 |
64 |
188 |
443 |
1,051 |
1,296 |
920 |
304 |
|
|
2018 |
8 |
71 |
199 |
478 |
1,129 |
1,419 |
1,024 |
408 |
|
|
2019 |
9 |
78 |
220 |
507 |
1,223 |
1,548 |
1,145 |
519 |
4 |
|
2020 |
12 |
90 |
232 |
549 |
1,324 |
1,662 |
1,268 |
649 |
17 |
|
2021 |
12 |
92 |
227 |
526 |
1,312 |
1,693 |
1,262 |
617 |
22 |
Table 2: Distinct diagnostic code utilization within age group and year for covid-ever cases, 1999-2021
Table 2 shows the retrospective diagnostic breadth of covid-ever cases. Distinct diagnostic codes, (without patient volume) appear stable within age group over time for the covid-ever group.
By 2016, CMS transitioned to ICD10-CM codes, which are more verbose than ICD9-CM, with perhaps 10 times the volume of distinct codes available. While increases in distinct code volume are observed they are perhaps due to cases surviving to present with covid clinically (in 2019 and beyond). Because the sample is retrospective and case qualifying conditions occur in the end of the study period, diagnostic volumes should decrease retrospectively, as not all cases present in 2020 are enrolled in 1999. Diagnostic breadth decreases in some age groups from 2021 to 2020, perhaps because of mortality among the covid-ever cases.
Towards clinical demography, cases who survive past two standard deviations of median survival within birth cohort tend to avoid presenting with high mortality chronic diseases because they avoid cancer, heart disease, diabetes and exposure deaths (HIV, opioids, tobacco, homicide). In turn, very old adults (90+) may have smaller diagnostic breadths.
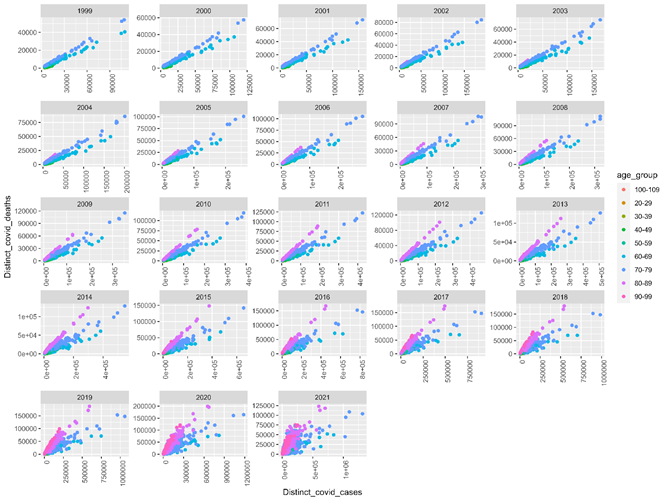
Fig 1 displays distinct covid-ever cases (x axis) by deaths if observed (y axis) by retrospective diagnostic code (points) and age group (color). Retrospective variation by decedent volume and case volume are detected when axis variation is considered. Note that AYD units should have larger volumes towards the end of the study, which contains the terms of enrollment (covid-ever). Cases did not need to die in a given study year to be observed and counted as retrospectively deceased.
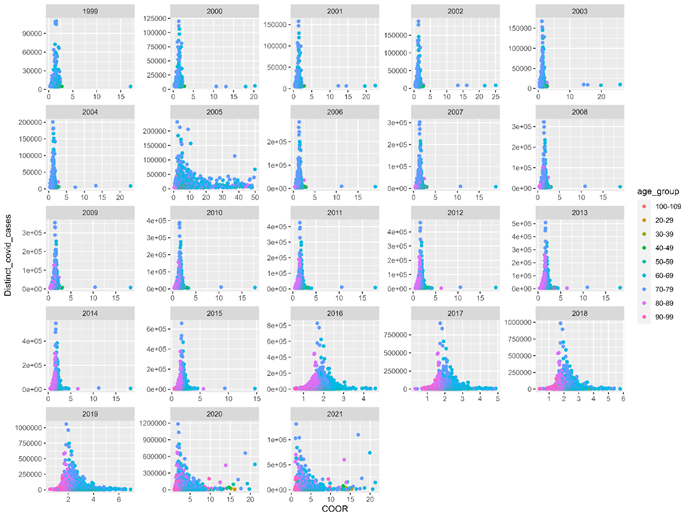
Fig 2 shows distinct covid-ever cases within AYD units by their COOR. This figure caps COOR at 50, to avoid outliers distorting the distribution. Said outliers are not counterfactual, they simply represent AYD units where AYD members had extreme shares of covid-ever versus covid-never cases. Study year 2005 presents with high retrospective COOR, suggesting acute events within past years may provide positive predictive value of individuals presenting with Covid-19 clinically. Prior influenza seasons and outbreaks of infectious diseases in nursing homes are candidate explanations. AYD units above a COOR of 1, or no difference are detected in all study years. Study years 2020 and 2021 show AYD units that have high COOR and population counts; this is perhaps because those AYD units are related to Covid-19 clinical episodes.
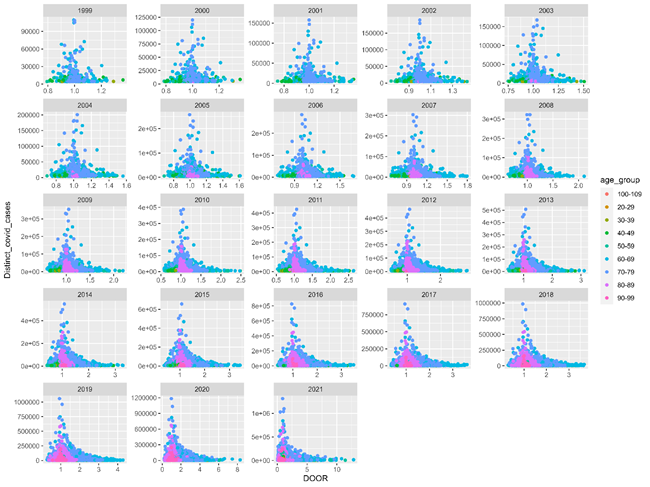
Fig 3 describes DOOR by distinct covid cases within AYD units. DOOR is relatively small, ranging from 0 to 2 until 2013. 2020 and 2021, covid pandemic years see DOOR for specific AYD units expand to 8 and 10, respectively. DOOR can parse AYD units to highlight which AYD are overrepresented among mortality cases with a given segmentation, in this case covid-ever versus. covid-never retrospective study.
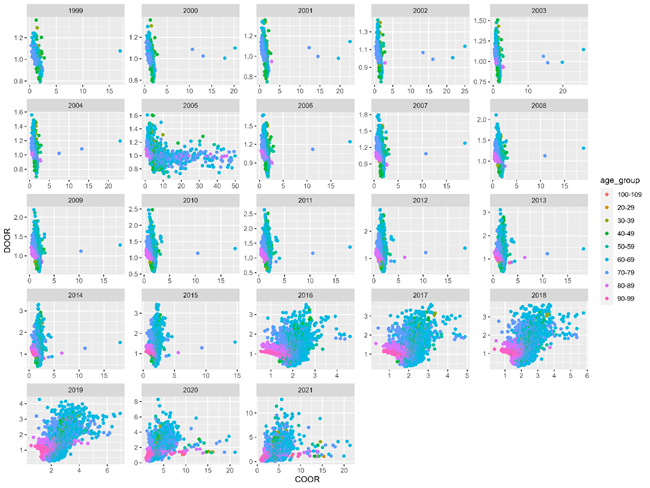
Fig 4 shows the relationships between DOOR and COOR within AYD units. As above, COOR is capped at 50. DOOR ranges from 0-5 until 2020, where even higher values are observed. COOR decreases in 2016, perhaps because of the added specificity of ICD10-CM code utilization. Study years 2016 through 2019 show retrospective predictive value of all-cause mortality relative to patients who did not present with Covid-19. Thin bands observed prior to study year 2016 indicate that although cases were not radically more likely to present with Covid-19 within diagnostic and age group, they were more likely to die if ever described within AYD relative to patients who did not present with Covid-19. AYD units depart from the trend in 2005, and outliers are observed in all years prior to 2016.
|
OR Rank |
Diagnosis |
Year |
Age Group |
COOR |
DOOR |
Covid Cases |
Covid Dead |
|
DOOR 1 |
Cardiac arrest |
2021 |
60-69 |
5.621116 |
12.7996 |
14,517 |
11,728 |
|
DOOR 2 |
Cardiac arrest |
2021 |
50-59 |
4.443997 |
11.4116 |
4,532 |
3,514 |
|
DOOR 3 |
Palliative care |
2021 |
60-69 |
5.572379 |
10.787 |
35,764 |
24,350 |
|
DOOR 4 |
Mediastinal emphysema |
2021 |
60-69 |
9.570231 |
9.8766 |
5,037 |
3,140 |
|
DOOR 5 |
Not for resuscitation |
2021 |
60-69 |
6.34527 |
9.86593 |
41,817 |
26,040 |
|
DOOR 6 |
Septic shock |
2021 |
60-69 |
8.073529 |
9.80267 |
27,114 |
16,776 |
|
DOOR 7 |
Cardiac arrest |
2021 |
70-79 |
4.511979 |
9.54759 |
22,335 |
18,689 |
|
DOOR 8 |
Shock |
2021 |
60-69 |
6.894183 |
9.49667 |
13,143 |
7,878 |
|
DOOR 9 |
Dependence on respirator |
2021 |
60-69 |
8.126317 |
9.26419 |
17,384 |
10,165 |
|
DOOR 10 |
Anoxic encephalopathy |
2021 |
60-69 |
6.383796 |
9.16421 |
4,412 |
2,552 |
|
DOOR 11 |
Palliative care |
2021 |
50-59 |
4.20244 |
9.00703 |
8,255 |
5,052 |
|
DOOR 12 |
Cardiogenic shock |
2021 |
60-69 |
4.710182 |
8.92264 |
4,812 |
2,710 |
|
DOOR 13 |
Acute respiratory distress syndrome |
2021 |
60-69 |
14.46835 |
8.85802 |
21,964 |
12,280 |
|
DOOR 14 |
Not for resuscitation |
2021 |
50-59 |
4.831182 |
8.8497 |
8,751 |
5,262 |
|
DOOR 15 |
Mechanical failure of instrument or apparatus during procedure |
2021 |
60-69 |
7.818232 |
8.82151 |
7,008 |
3,902 |
|
DOOR 16 |
Mixed acid-base balance disorder |
2021 |
60-69 |
7.818232 |
8.82151 |
7,008 |
3,902 |
|
DOOR 17 |
Cardiac arrest |
2020 |
60-69 |
3.750514 |
8.28268 |
10,680 |
8,380 |
|
DOOR 18 |
Palliative care |
2021 |
70-79 |
4.679915 |
8.0542 |
75,737 |
53,461 |
|
DOOR 19 |
Acute tubular necrosis |
2021 |
60-69 |
6.880515 |
7.91728 |
17,818 |
8,904 |
|
DOOR 20 |
Pneumothorax |
2021 |
60-69 |
5.792531 |
7.91375 |
9,934 |
4,962 |
|
COOR 1 |
Infection due to Group A Shigella |
2011 |
70-79 |
12199.88 |
1.25434 |
5,744 |
1,950 |
|
COOR 2 |
Infection due to Group A Shigella |
2012 |
60-69 |
5024.259 |
1.54 |
4,494 |
1,073 |
|
COOR 3 |
Late effects of central nervous system tuberculosis |
2003 |
70-79 |
3250.723 |
1.03057 |
7,973 |
3,533 |
|
COOR 4 |
Late effects of central nervous system tuberculosis |
2001 |
70-79 |
1969.732 |
1.05506 |
6,139 |
3,010 |
|
COOR 5 |
Late effects of central nervous system tuberculosis |
1999 |
70-79 |
1866.427 |
1.00972 |
4,303 |
2,165 |
|
COOR 6 |
Amebic ulcer of skin |
2006 |
60-69 |
1731.046 |
1.2367 |
4,651 |
1,367 |
|
COOR 7 |
Amebic ulcer of skin |
2006 |
70-79 |
1515.433 |
1.14381 |
5,670 |
2,410 |
|
COOR 8 |
Late effects of central nervous system tuberculosis |
2002 |
70-79 |
1475.157 |
1.02186 |
7,168 |
3,276 |
|
COOR 9 |
Late effects of central nervous system tuberculosis |
2000 |
70-79 |
1445.705 |
1.01437 |
5,095 |
2,485 |
|
COOR 10 |
Amebic ulcer of skin |
2008 |
70-79 |
1181.716 |
1.15818 |
5,367 |
2,049 |
|
COOR 11 |
Amebic ulcer of skin |
2007 |
70-79 |
1149.49 |
1.14039 |
5,114 |
2,045 |
|
COOR 12 |
Blepharoconjunctivitis |
2005 |
60-69 |
1093.759 |
1.20108 |
7,348 |
2,249 |
|
COOR 13 |
Amebic ulcer of skin |
2009 |
70-79 |
844.551 |
1.1305 |
5,045 |
1,765 |
|
COOR 14 |
Amebic ulcer of skin |
2011 |
70-79 |
749.9762 |
1.18576 |
5,104 |
1,638 |
|
COOR 15 |
Amebic ulcer of skin |
2010 |
70-79 |
711.1185 |
1.18202 |
5,109 |
1,750 |
|
COOR 16 |
Blepharoconjunctivitis |
2005 |
70-79 |
619.4802 |
1.10596 |
8,662 |
3,758 |
|
COOR 17 |
Amebic ulcer of skin |
2014 |
70-79 |
546.5532 |
1.35909 |
4,720 |
1,391 |
|
COOR 18 |
Somatic dysfunction of lumbar region |
2005 |
70-79 |
538.5339 |
0.90879 |
32,791 |
11,690 |
|
COOR 19 |
Cervical somatic dysfunction |
2005 |
70-79 |
535.2009 |
0.8902 |
24,610 |
8,594 |
|
COOR 20 |
Somatic dysfunction of lumbar region |
2005 |
60-69 |
514.2503 |
0.83224 |
27,999 |
5,938 |
Table 3: Highest ranked COOR and DOOR values by AYD with ever-cases and ever-decedents
Table 3 shows AYD units with high odds ratios. Highest DOOR AYD units are, given the study years perhaps Covid-19 cases experiencing Covid-19 related mortality. Highly ranked COOR values indicate conditions common in old age, institutionalized populations (nursing homes with infectious disease outbreaks prior to Covid-19 pandemic era). Conditions common to adults already old (70+) in 2005 provide predictive value, where 15 years later they succumb to respiratory disease (at 85+). Very high COOR and DOOR values indicate that covid-ever cases are experiencing observation and mortality risks above the AYD Medicare baselines.
|
COEF Rank |
Diagnosis |
COEF |
Standard Error |
Z Value |
P Value |
|
1 |
Anoxic encephalopathy |
6.747676 |
0.23155 |
29.14134 |
2.65E-185 |
|
2 |
Cardiac arrest |
5.717084 |
0.127645 |
44.78893 |
0 |
|
3 |
Mediastinal emphysema |
5.578207 |
0.192414 |
28.99065 |
1.99E-183 |
|
4 |
Cardiogenic shock |
4.37418 |
0.169499 |
25.8065 |
5.41E-146 |
|
5 |
Secondary malignant neoplasm of liver |
4.082191 |
0.192413 |
21.21578 |
1.69E-99 |
|
6 |
Shock |
4.07086 |
0.142956 |
28.47627 |
4.29E-177 |
|
7 |
Mechanical failure of instrument or apparatus during procedure |
3.765781 |
0.14295 |
26.34327 |
5.23E-152 |
|
8 |
Mixed acid-base balance disorder |
3.765781 |
0.14295 |
26.34327 |
5.23E-152 |
|
9 |
Dependence on respirator |
3.627979 |
0.117473 |
30.88341 |
1.13E-207 |
|
10 |
Pressure ulcer of hip |
3.594287 |
0.321513 |
11.1793 |
5.53E-29 |
|
11 |
Acute respiratory distress syndrome |
3.526631 |
0.122105 |
28.88199 |
4.43E-182 |
|
12 |
Sepsis due to methicillin resistant Staphylococcus aureus |
3.407956 |
0.169496 |
20.10641 |
1.35E-89 |
|
13 |
Hepatic failure |
3.229828 |
0.1344 |
24.03138 |
5.79E-127 |
|
14 |
Tracheostomy present |
3.15557 |
0.169496 |
18.61739 |
3.98E-77 |
|
15 |
Acute thrombosis of superficial vein of upper extremity |
3.097249 |
0.321513 |
9.633369 |
6.01E-22 |
|
16 |
Finding of urine output |
3.040841 |
0.231536 |
13.13337 |
2.43E-39 |
|
17 |
Pneumonia due to Gram negative bacteria |
3.029736 |
0.231535 |
13.08542 |
4.55E-39 |
|
18 |
Hypovolemic shock |
3.016566 |
0.231536 |
13.02852 |
9.59E-39 |
|
19 |
Pain due to neoplastic disease |
2.859936 |
0.154118 |
18.55683 |
1.22E-76 |
|
20 |
Secondary malignant neoplasm of lung |
2.780388 |
0.231535 |
12.00848 |
3.52E-33 |
Table 4: Highest ranked coefficients when predicting DOOR from AYD values
Table 4 shows the highest ranked DOOR diagnoses; which are ranked by their ability to explain variance in DOOR learned from AYD units. While conditions associated with Covid-19 mortality are present, liver cancer, lung cancer, bacterial pneumonia, upper body thrombosis and MERSA sepsis (perhaps due to being hospitalized with Covid-19) all feature in the top 20 conditions.
|
COEF Rank |
Diagnosis |
COEF |
Standard Error |
Z Value |
P Value |
|
2169 |
Snapping thumb syndrome |
-1.1783 |
0.16949 |
-6.9523 |
3.63E-12 |
|
2170 |
Chalazion of lower eyelid |
-1.1875 |
0.19241 |
-6.1718 |
6.80E-10 |
|
2171 |
Lateral epicondylitis |
-1.1898 |
0.16949 |
-7.0202 |
2.24E-12 |
|
2172 |
Contact dermatitis due to plants, except food |
-1.1994 |
0.1344 |
-8.9241 |
4.63E-19 |
|
2173 |
Acute disease |
-1.1994 |
0.19242 |
-6.2331 |
4.61E-10 |
|
2174 |
Iliotibial band friction syndrome |
-1.2013 |
0.23153 |
-5.1885 |
2.13E-07 |
|
2175 |
Disorder of knee |
-1.2104 |
0.32151 |
-3.7646 |
1.67E-04 |
|
2176 |
Keratoconjunctivitis sicca, in gren's syndrome |
-1.2296 |
0.32151 |
-3.8244 |
1.31E-04 |
|
2177 |
Lichen sclerosus et atrophicus |
-1.2436 |
0.19241 |
-6.4635 |
1.03E-10 |
|
2178 |
Human papilloma virus screening |
-1.2507 |
0.13439 |
-9.3068 |
1.36E-20 |
|
2179 |
Loss of sense of smell |
-1.2867 |
0.19241 |
-6.6873 |
2.29E-11 |
|
2180 |
Chronic cough |
-1.2879 |
0.19242 |
-6.6928 |
2.21E-11 |
|
2181 |
Horseshoe retinal tear without detachment |
-1.3215 |
0.32151 |
-4.1104 |
3.96E-05 |
|
2182 |
Telogen effluvium |
-1.4681 |
0.32151 |
-4.5664 |
4.97E-06 |
|
2183 |
Vertebrogenic pain syndrome |
-1.5276 |
0.23155 |
-6.5976 |
4.22E-11 |
Table 5: Lowest ranked coefficients when predicting DOOR from AYD variances
Table 5 shows the least explanatory AYD units when considering DOOR. Though counter intuitive, clinical diagnosis that was not explanatory of DOOR variance can yield clinically meaningful results. To experience long-covid, cases must have Covid-19 and survive to present with long-covid. These individuals would have a low DOOR score and their AYD units would not explain variance in DOOR. Said presentations are observed with clinical diagnosis, ‘loss of sense of smell’ and ‘chronic cough’. They populate at the bottom of the model features when ordered by DOOR coefficients at row 2179 out of 2183 rows.
Limitations
This method, though robust, should not be used for the evaluation of nested sub-populations unless they are specifically controlled for in the baseline extract. This model should only be used to evaluate covid-ever cases versus their AYD baselines. This study only considered covid-ever case status within the subset. Interactions within the subset should not be assumed or assigned greater meaning other than ‘more or less likely than baseline’. Coinfection cases (for example, HIV and Covid-19) could be evaluated if the subset model and baseline model considered coinfection negative cases. This generalist model is useful, however for identifying candidates for further research both at the benchtop and bedside.
This model only considered Medicare claims data. While robust and spanning multiple study years and treatment sites, this model should not be used to interpret outcomes from other patient populations. Note the Medicare population here is any individual who billed Medicare from 1999 through 2021. This population include recipients of Social Security Disability Insurance, individuals over the age of 65, patients experiencing end stage renal disease, organ transplant recipients and spouse survivors of Medicare beneficiaries and may include undocumented individuals living in the United States. Hospice, nursing home, long term care and Part-B, Part-C and Part-D beneficiaries were not excluded or disambiguated. The findings presented here are true of the Medicare population in all its complexity.
Discussion
Machine vision for clinical research is traditionally thought of in machine learning for image analysis [19-21]. Here, machine vision for human pathology via machine learning is attempted using tabulated real-world data. The goal of the model is perhaps unusual, depending on the level of mathematics training of the audience [22,23]. The model is not attempting to predict the future, but rather segment AYD units and patient volumes to learn which AYD units are related to covid-ever and survival status over study time [17].
A method for classifying retrospective care to inform the specificity of a clinical condition is sorely lacking. Here, we demonstrate that such a method is well within reach (generalized linear models are not new) and can achieve both known-knowns (ventilators) all too familiar to providers caring for dying Covid-19 patients and perhaps some known-unknowns, like higher mortality in Covid-19 cancer and cardiac arrest cases which warrants further investigation [14,24-27]. Cardiac arrest often results from serious conditions (acute or pre-existing) that may be responsible for this event. How Covid-19 impacts patients whose life courses are already intersecting with environmental exposures to causal agents of chronic conditions can be informed by the study dataset. Further, how Covid-19 recourses through human communities (especially nursing homes) as one of many infectious agents should demonstrate the need to better understand the environments which produce Covid-19 exposure, including clinical settings [28]. Many of these patients are immunocompromised which may explain the dire consequences seen in this patient population.
Covid-19 is unlikely to be the last emerging infectious disease to impact the Medicare population. In turn, the lessons that can be learned and deployed are high value to limit mortality and improve quality of care. Table 1 demonstrates that the Medicare population is ‘high mortality’ and is perhaps not ‘just another health insurance program’. Rather, Medicare insures individuals at the end of their lives, regardless of how old they are when their lives end. Table 2 demonstrates the diagnostic breath within the covid-ever population. While some conditions may be synonyms for each other, this study used SNOMED-CT mappings and counted distinct cases within AYD units to improve clinical accounting. The covid-ever population is diagnostically diverse, when retrospective years are considered. Care might be improved by taking a broader, retrospective patient history (decades) instead of a ‘chief complaint’ as this study does [29-31].
Figure 1 demonstrates the shifting burden of care within the covid-ever population; which is to be expected as Figure 1 is uncontrolled for enrollment or ageing. Prior events (prior to Covid-19 diagnosis) appear robust, as expected in this large population study. Figures two and three demonstrate that retrospective case events are determining, in some cases, down stream risk of being observed with Covid-19 clinically or dying in the study period. In this study, positive predictive value is detected, though not assessed; as the goal of this study is to understand statistically attributable explanations of AYD variance of the DOOR statistic. Figure 4 highlights the study risk panels over time, and indicates that prior risk (especially in 2005) impacts the risk of presenting with Covid-19 clinically and dying in the study period.
Table 3 highlights high COOR and DOOR AYD units. Prior to model segmentation, AYD rankings for COOR and DOOR can be informative. Table 4 details the highest ranked DOOR covariates from the GLM model. While inpatient mortality diagnoses associated with covid-ever cases are observed, cardiac arrest, cancer and diabetes related diagnosis is predominant. This means that the variance of DOOR across AYD units is best explained by these diagnoses. There is substantial clinical research indicating that cardiac, cancer and diabetes outcomes are influenced by Covid-19 infection, and vise-versa [24,26,27,32]. Table 5 suggests that cases that survive Covid-19 are eligible for long-covid syndromes [33-35]. While long covid syndromes are still being described, Medicare cases may provide case evidence to support benchtop or bedside conclusions.
Conclusion
Machine vision for pathology segmentation is readily available with at-scale real world data. The Medicare population has taken the brunt of Covid-19 and presents a natural experiment to understand the interrelatedness of pathology emergency and vulnerability. Prior clinical conditions may impact Covid-19 mortality and inform caregivers of patients presenting clinically with Covid-19.
Acknowledgement
This work was supported by the Lister Hill National Center for Biomedical Communications of the National Library of Medicine (NLM), National Institutes of Health. Special thanks to Craig Mayer and Paul Fontelo for editing the manuscript.
References
- Hiscott J, Alexandridi M, Muscolini M, et al. The global impact of the coronavirus pandemic. Cytokine Growth Factor Rev 53 (2020): 1-9.
- Noor AU, Maqbool F, Bhatti ZA, et al. Epidemiology of CoViD-19 Pandemic: Recovery and mortality ratio around the globe. Pak J Med Sci 36 (2020): S79–84.
- Clarke KEN. Seroprevalence of Infection-Induced SARS-CoV-2 Antibodies — United States, September 2021–February 2022. MMWR Morb Mortal Wkly Rep [Internet]. 2022 [cited 2023 Mar 7]; 71.
- Ahmad FB. Provisional Mortality Data — United States, 2021. MMWR Morb Mortal Wkly Rep [Internet]. 2022 [cited 2023 Mar 7]; 71.
- Akinbami LJ. SARS-CoV-2 Serology and Self-Reported Infection Among Adults — National Health and Nutrition Examination Survey, United States, August 2021–May 2022. MMWR Morb Mortal Wkly Rep [Internet]. 2022 [cited 2023 Mar 7]; 71.
- COVID-19 Provisional Counts - Weekly Updates by Select Demographic and Geographic Characteristics [Internet]. 2023 [cited 2023 Mar 7].
- Excess Deaths Associated with COVID-19 [Internet]. 2023 [cited 2023 Mar 7].
- Djaharuddin I, Munawwarah S, Nurulita A, et al. Comorbidities and mortality in COVID-19 patients. Gac Sanit 35 (2021): S530-532.
- Greenwald SD, Chamoun NG, Manberg PJ, et al. Covid-19 and excess mortality in medicare beneficiaries. PloS One 17 (2022): e0262264.
- Tarazi WW, Finegold K, Sheingold SH, et al. COVID-19-Related Deaths And Excess Deaths Among Medicare Fee-For-Service Beneficiaries. Health Aff Proj Hope 40 (2021): 879-885.
- Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis 21 (2021): 855.
- Alimohamadi Y, Tola HH, Abbasi-Ghahramanloo A, et al. Case fatality rate of COVID-19: a systematic review and meta-analysis. J Prev Med Hyg 62 (2021): E311-320.
- McKenna MT, Zohrabian A. U.S. burden of disease--past, present and future. Ann Epidemiol. 2009 Mar;19(3) :212-219.
- Zhang JJ, Dong X, Liu GH, et al. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin Rev Allergy Immunol 64 (2023): 90-107.
- Rea IM, Alexander HD. Triple jeopardy in ageing: COVID-19, co-morbidities and inflamm-ageing. Ageing Res Rev 73 (2022): 101494.
- Gallo Marin B, Aghagoli G, Lavine K, et al. Predictors of COVID-19 severity: A literature review. Rev Med Virol 31(2021): 1-10.
- Gasmi A, Peana M, Pivina L, et al. Interrelations between COVID-19 and other disorders. Clin Immunol Orlando Fla 224 (2021): 108651.
- Ejaz H, Alsrhani A, Zafar A, et al. COVID-19 and comorbidities: Deleterious impact on infected patients. J Infect Public Health 13 (2020): 1833-1839.
- Madabhushi A, Lee G. Image analysis and machine learning in digital pathology: Challenges and opportunities. Med Image Anal 33 (2016): 170-175.
- McAlpine ED, Michelow P, Celik T. The Utility of Unsupervised Machine Learning in Anatomic Pathology. Am J Clin Pathol 157 (2022): 5-14.
- Roohi A, Faust K, Djuric U, et al. Unsupervised Machine Learning in Pathology: The Next Frontier. Surg Pathol Clin 13 (2020): 349-358.
- Kushner RF, Sorensen KW. Lifestyle medicine: the future of chronic disease management. Curr Opin Endocrinol Diabetes Obes 20 (2013): 389-395.
- Saleem F, Al-Ghamdi ASAM, Alassafi MO, et al. Machine Learning, Deep Learning, and Mathematical Models to Analyze Forecasting and Epidemiology of COVID-19: A Systematic Literature Review. Int J Environ Res Public Health 19 (2022): 5099.
- Williams N. Prehospital Cardiac Arrest should be considered when evaluating Covid-19 mortality in the United States. Methods Inf Med (2023).
- Smulowitz PB, O’Malley AJ, Khidir H, et al. National Trends In ED Visits, Hospital Admissions, And Mortality For Medicare Patients During The COVID-19 Pandemic. Health Aff Proj Hope 40 (2021): 1457-1464.
- Langerbeins P, Hallek M. COVID-19 in patients with hematologic malignancy Blood 140 (2022): 236-252.
- Marjot T, Webb GJ, Barritt AS, et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol 18 (2021): 348-364.
- Carnahan JL, Lieb KM, Albert L, et al. COVID-19 disease trajectories among nursing home residents. J Am Geriatr Soc 69 (2021): 2412-2418.
- Alwin DF. Integrating Varieties of Life Course Concepts. J Gerontol B Psychol Sci Soc Sci 67 (2012): 206–220.
- Jones NL, Gilman SE, Cheng TL, et al. Life Course Approaches to the Causes of Health Disparities. Am J Public Health 109 (2019): S48–55.
- Settersten RA, Bernardi L, Härkönen J, et al. Understanding the effects of Covid-19 through a life course lens. Curr Perspect Aging Life Cycle 45 (2020): 100360.
- Chang MH, Moonesinghe R, Truman BI. COVID-19 Hospitalization by Race and Ethnicity: Association with Chronic Conditions Among Medicare Beneficiaries, J Racial Ethn Health Disparities 9 (2020): 325-334.
- Salerno S, Messana JM, Gremel GW, et al. COVID-19 Risk Factors and Mortality Outcomes Among Medicare Patients Receiving Long-term Dialysis. JAMA Netw Open 4 (2021): e2135379.
- Kamal M, Abo Omirah M, Hussein A, et al. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract 75 (2021): e13746.
- Duggal P, Penson T, Manley HN, et al. Post-sequelae symptoms and comorbidities after COVID-19. J Med Virol 94 (2022): 2060–2066.