Correlation of Different Grades of Intravesical Prostatic Protrusion with Uroflowmetry Parameters in Patients with Symptomatic Benign Enlargement of Prostate
Article Information
Dr. Md. Abed Billah1*, Prof. Dr. Mohammed Monowar-UL-Haque2, Dr. Tahmina Akter3, Dr. Md. Motiur Rahman4, Dr. Mohammad Mamunur Rashid5, Dr. Md. Mazedur Rahman6, Dr. Md. Mostofa Kamal7
1Medical Officer, Department of Urology, Shaheed Ziaur Rahman Medical College Hospital Bogura, Bangladesh
2Professor and Head, Department of Urology, Chittagong Medical College, Chattogram, Bangladesh
3Registrar, (Burn and Plastic Surgery), Shaheed Ziaur Rahman Medical College Hospital Bogura, Bangladesh
4Assistant Registrar, Department of Urology, Shaheed Ziaur Rahman Medical College Hospital Boura, Bangladesh
5Medical Officer, Department of Urology, Shaheed Ziaur Rahman Medical College Hospital Bogura, Bangladesh
6Registrar, Department of Urology, Shaheed Ziaur Rahman Medical College Hospital Bogura, Bangladesh
7Medical Officer, Department of Urology, Khulna Medical College Hospital, Khulna, Bangladesh
*Corresponding Author: Dr. Md. Abed Billah, Medical Officer, Department of Urology, Shaheed Ziaur Rahman Medical College Hospital, Bogura, Bangladesh.
Received: 20 December 2023; Accepted: 05 February 2024; Published: 16 February 2024
Citation:
Abed Billah, Mohammed Monowar- UL-Haque, Tahmina Akter, Motiur Rahman, Mohammad Mamunur Rashid, Mazedur Rahman, Mostofa Kamal. Correlation of Different Grades of Intravesical Prostatic Protrusion with Uroflowmetry Parameters in Patients with Symptomatic Benign Enlargement of Prostate. Archives of Nephrology and Urology. 7 (2024): 01-08.
Share at FacebookAbstract
Background: Enlargement of the prostate is a common healthcare problem in aging men around the world. The enlargement occurs due to the proliferation of smooth muscles and epithelial cells within the prostatic tissue. Intravesical prostatic protrusion is a phenomenon in which the prostate adenoma enlarges into the bladder along the plane of least resistance. Uroflowmetry is simple and non-invasive investigation of various lower urinary tract diseases by calculating the rate of urine expulsion against the time unit in second.
Aim: To determine the Correlation of different grades of Intravesical Prostatic Protrusion with Uroflowmetry parameters in patients with Symptomatic Benign Enlargement of Prostate.
Methods: This cross-sectional study was carried out in the Department of Urology of Chittagong Medical College Hospital. Study period was November, 2020-October 2021. A total of 42 patients attending with intravesical prostatic protrusion in symptomatic benign enlargement of prostate on abdominal Ultrasonography and age belonged to 45-75 years were enrolled in this study. They were divided into three groups according to grade of Intravesical Prostatic Protrusion. Patients having Intravesical Prostatic Protrusion less than 5mm were considered as Grade I, those with 5-10 mm were considered as grade II and more than 10 mm were considered as Grade III. Uroflowmetry parameters are recorded. Statistical analyses of the results were done by SPSS version 22.0.
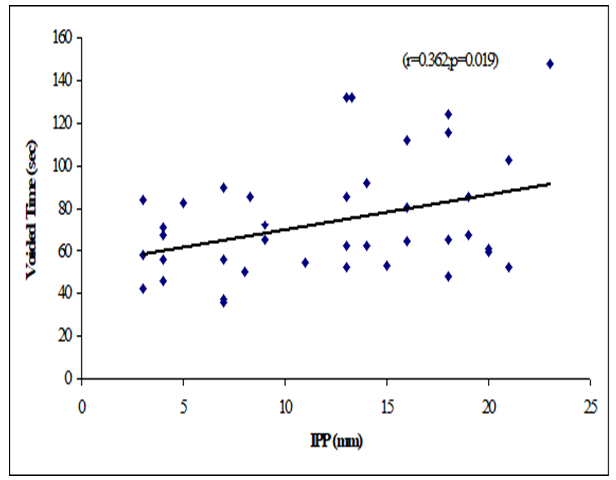
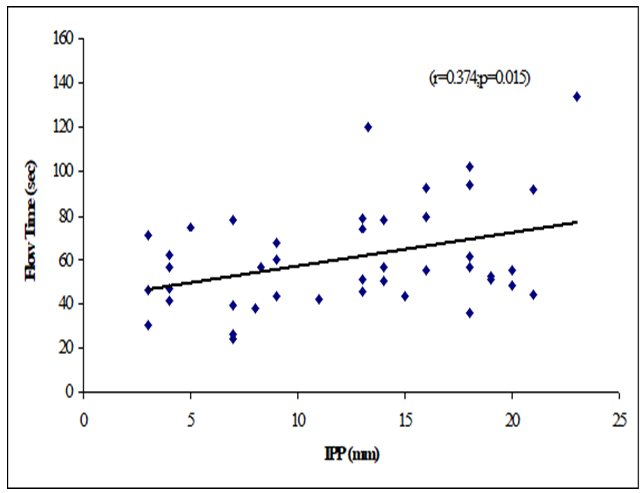
Results: The mean age was 62 ± 7.94 years in grade I, 66.9 ± 7.02 years in grade II and 66.48 ± 6.23 years in grade III. The mean prostate volume was 48.86 ± 29.75 (grams) in grade I, 49.9 ± 19.41 (grams) in grade II and 66.84 ± 40.51 (grams) in grade III. The difference was statistically not significant (p>0.05) among three groups. The mean IPP was 3.56 ± 0.55 (mm) in grade I, 7.63 ± 1.28 (mm) in grade II and 16.57 ± 3.18 (mm) in grade III. The difference was statistically significant (p<0.05) among three groups. The mean Qmax was 11.21 ± 6.05 (mL/s) in grade I, 11.71 ± 3.36 (mL/s) in grade II and 8 ± 2.67 (mL/s) in grade III. The difference was statistically significant (p<0.05) among three groups. The mean Qmean was 5.16 ± 2.61 (mL/s) in grade I, 4.3 ± 2.28 (mL/s) in grade II and 3.75 ± 1.26 (mL/s) in grade III. The difference was statistically not significant (p>0.05) among three groups. The mean voided time was 60.57 ± 14.63 (sec) in grade I, 64 ± 19.32 (sec) in grade II and 81.52 ± 30.02 (sec) in grade III. The difference was statistically not significant (p>0.05) among three groups. The mean flow time was 50.43 ± 13.7 (sec) in grade I, 50.6 ± 19.43 (sec) in grade II and 67.64 ± 25.94 (sec) in grade III. The difference was statistically not significant (p>0.05) among three groups. There were a negative significant Pearson’s correlations (r=-0.450, p=0.003) between IPP and Qmax. Positive significant Pearson’s correlation between IPP with voided time (r=0.362, p=0.019) and flow time (r=0.374, p=0.015). Prostate volume was not significantly correlated with IPP.
Conclusion: Most of the patients were in 7th decade in all three groups. The mean flow time and voided time were significantly higher in grade III, however Qmax was significantly less in grade iii. There was a significant negative correlation between IPP with Qmax, significant positive correlations with voided time and flow time. This study concludes that uroflowmetry is a very useful tool to detect severity of bladder outlet obstruction (BOO) in symptomatic BEP patients.
Keywords
Prostate; Benign enlargement; Intravesical; Uroflowmetry; Qmax,. Bladder outlet obstruction (BOO)
Article Details
1. Introduction
Enlargement of the prostate is common among aging men. It is a progressive condition, prostate enlargement accompanied by lower urinary tract symptoms that can result in long-term complications [1]. The prevalence increases with age. Enlargement of the prostate generally leads to bladder outlet obstruction (BOO) and it causes a variety of bothersome lower urinary tract symptoms (LUTS) [2]. The prostate enlargement is also manifested by the development of intravesical prostatic protrusion (IPP), as a morphological change by which the prostate protrudes into the bladder [3]. Enlarged prostate is characterized by obstructive and/or irritative symptoms and varying degrees of bladder outlet obstruction (BOO). An evaluation of LUTS due to enlarged prostate has been conventionally done by most urologists via a clinical appraisal of LUTS like International Prostate Symptom Score (IPSS), DRE, Prostate specific antigen (PSA) and a transabdominal ultrasonographic evaluation. The IPSS is one of the ideal instruments which can be used to grade baseline symptom severity. The IPSS is based on the answers to seven questions (Irritative and voiding) which concern urinary symptoms. Each question is assigned points from 0 to 5 which indicate increasing severity of the particular symptom and a total score which ranges from 0 to 35 [2]. Clinically significant BOO is urodynamically characterized by increased detrusor pressure and a decreased urinary flow rate. The pressure-flow study (PFS) is the reference standard for diagnosing bladder outlet obstruction (BOO) caused by benign prostatic enlargement (BPE). The extent of BOO is calculated using the BOO index according to the formula BOOI=PdetQmax-2xQmax (where Pdet is detrusor pressure at the peak flow rate and Qmax, is the peak flow rate). BOOI of >40 indicates definite obstruction, 20-40 is equivocal and <20 indicates no obstruction [4]. However, PFS is an invasive procedure followed by side effects including hematuria, urinary tract infection, and difficulty in urination, which make it difficult to perform in all cases [5]. Invasive urodynamic testing with detrusor pressure is not routinely done in all patients with enlarged prostate. Non-invasive methods to diagnose BOO include symptom evaluation (an IPSS), ultrasound derived parameters like prostate-volume, Intravesical Prostatic Protrusion (IPP), and Post Voidal Residue (PVR). Among these, ultrasound estimation of the prostate size and PVR with Uroflowmetry have been routinely used by most urologists worldwide to determine the presence of BOO [6]. Patients with prostatic enlargement suffer from negative changes in their quality of life and restriction of their daily activities due to the disease symptoms [7]. There is a reverse relationship exists between the intensity of urinary symptoms and quality of life [8,9]. The intravesical prostatic protrusion (IPP) is a morphological change due to overgrowth of prostatic median and/or lateral lobes into the bladder and may lead to dyskinetic movement of bladder during voiding [5]. Intravesical prostatic protrusion (IPP) produces bladder outlet obstruction and related storage and voiding symptoms [10]. Intravesical prostatic protrusion seems to support infravesical obstruction, which may trigger a “ball-valve” type of obstruction, disrupting laminar flow at the bladder neck and distorting the funneling effect of the normal prostatic-urethral angle [11]. Intravesical prostatic protrusion (IPP) is graded into three grades with Transabdominal Ultrasound with the bladder volume of 150-200 mL [12], by measuring the vertical distance from the tip of the protrusion to the circumference of the bladder at the base of the prostate gland in millimeters [13]. IPP is graded into three grades by USG of KUB region. Grade I is <5 mm; Grade II is 5-10 mm; Grade III is >10 mm of intravesical protrusion. The grade of IPP is strongly correlated to the clinical progression of BEP. Uroflowmetry is an investigation tool of various urinary tract diseases. Von Garrelts introduced uroflowmeter in 1957. It calculates the rate of urine expulsion against the time unit in second. Uroflowmetry is simple and non-invasive diagnostic method [14]. Uroflowmetry is indicated in patients who have signs and symptoms, which are suggestive of bladder outlet obstruction. A Qmax of <15 mL/s has been interpreted to be suggestive of Djavan [15]. Parameters derived from uroflowmetry are considered to be clinically related only if voided volume > 150 ml. [16]. Uroflowmetry is useful to diagnose the symptomatic BEP supported by Ultrasonography of prostate. However, diagnosis of detrusor muscle over activity and neurogenic bladder require further more invasive urodynamic study. A hospital based prospective study is carried out by Garg et al. [2] has recommended that to judge the severity of the disease due to BEP uroflowmetry and IPSS should be considered. Among the parameters, which are obtained by uroflowmetry, maximum flow rate is the most representative of the symptom severity of the patient. Parameters like time to peak flow, flow time, voiding time, voided volume has no correlation with the symptoms of the patient. Average flow rate also correlates positively with the symptoms of the patient, and it can be considered as useful as maximum flow rate in the assessment of symptom severity. Considering the burden of BEP among the geriatric male population the aim of this study is to determine the correlation between intravesical prostatic protrusion and uroflowmetry parameters in symptomatic enlarged prostate patients.
2. Objectives
2.1 General objective:
To observe the Correlation of different grades of Intravesical Prostatic Protrusion with Uroflowmetry parameters in patients with Symptomatic Benign Enlargement of Prostate.
2.2 Specific Objectives:
- To evaluate the different grades of intravesical prostatic protrusion (IPP) among the symptomatic BEP patients by abdominal Ultrasonography
- To determine maximum (Qmax) flow rate of the patients by uroflowmetry and correlation with different grades of IPP
- To determine average flow rate (Qmean) of the patients by uroflowmetry and correlation with different grades of IPP
- To find out the Voided time & flow time of the respondents and correlation with different grades of IPP
3. Materials and Method
This was a Cross sectional study. The patients were selected purposively. A total of 42 patients were included in this study. The study was conducted in the department urology OPD and Urology, Chittagong Medical College Hospital from November, 2020 to 31st October, 2021.
3.1 Inclusion criteria
The patients with following characteristics were included-
- Symptomatic BEP patients with intravesical prostatic protrusion on abdominal Ultrasonography
- Age between 45-75 years
3.2 Exclusion criteria
The patients with following characteristics were excluded-
- Urinary bladder stone
- Stricture urethra
- Urinary bladder neoplasm
- Previous history of per urethral surgery
- Spine trauma/spine surgery or any neurological deficit (Excluded by focal neurological examination)
3.3 Study procedure
This Cross-sectional study was conducted in the Department of Urology, Chittagong Medical College Hospital (CMCH). All adult male age between 45-75 years presented with lower urinary tract symptoms (LUTS) suggesting of BEP attending to urology OPD were recruited. Initial evaluation by International Prostate Symptom Score (IPSS) and Quality of Life index (QOL) were done by supplying a Bengali questionnaire form. Then history taking and physical examination including Digital Rectal Examination (DRE) and focal neurological examination were done to exclude Ca prostate or any neurological deficit and neurologically related bladder dysfunction. Patients with raised PSA underwent prostate biopsy to exclude malignancy. Then ultrasonography of KUB region was done to detect Intravesical Prostatic Protrusion (IPP) with grading. Aims, objectives, procedures, risks and benefits of this study were explained to the patients with IPP. They were also assured about the secrecy of information and records. 42 patients were selected according to inclusion and exclusion criteria by purposive sampling. Informed written consent was taken from agreed patients. They were encouraged for voluntary participation. Then uroflowmetry were done and maximum flow rate (Qmax), Voided time, flow time, average flow rate was recorded.
3.4 Data analysis
Data were processed and analyzed by using computer-based software SPSS-22 (Statistical package for Social Science). Different statistical method applied for data analysis. For presentation of quantitative data, mean ± SD and for qualitative data frequency and percentage used. Difference among the groups was analyzed by Student ANOVA test in case of numerical variables. Using Pearson’s correlation coefficient, uroflowmetry parameters were correlated with the IPP. P value was considered as statistically significant when it is less than 0.05 and confidence interval set at 95% level.
3.5 Ethical clearance
Ethical clearance of study was taken from the Ethical Review Committee of Chittagong Medical College. Written consent was taken from the participant of the study.
4. Results
|
Grading |
Age ( In Years) |
||
|
Frequency |
Mean ± SD |
p value |
|
|
Grade I |
7 |
62 ± 7.94 |
0.261ns |
|
Grade II |
10 |
66.9 ± 7.02 |
|
|
Grade III |
25 |
66.48 ± 6.23 |
|
Table 1: Distribution of the study patients by age (N=42).
Table 1 showed the mean age was 62 ± 7.94 years in grade I, 66.9 ± 7.02 years in grade II and 66.48 ± 6.23 years in grade III. The difference was statistically not significant (p>0.05) among three groups.
|
Grading |
Prostate volume (grams) |
||
|
Frequency |
Mean ± SD |
p value |
|
|
Grade I |
7 |
48.86 ± 29.75 |
0.301ns |
|
Grade II |
10 |
49.9 ± 19.41 |
|
|
Grade III |
25 |
66.84 ± 40.51 |
|
Table 2: Distribution of the study patients by prostate volume (N=42).
Table 2 showed the mean prostate volume was 48.86 ± 29.75 (grams) in grade I, 49.9 ± 19.41 (grams) in grade II and 66.84 ± 40.51 (grams) in grade III. The difference was statistically not significant (p>0.05) among three groups.
|
Grading |
IPP (mm) |
||
|
Frequency |
Mean ± SD |
p value |
|
|
Grade I |
7 |
3.57 ± 0.53 |
0.001s |
|
Grade II |
10 |
7.63 ± 1.28 |
|
|
Grade III |
25 |
16.57 ± 3.18 |
|
Table 3: Distribution of the study patients by IPP (N=42).
Table 3 showed the mean IPP was 3.56 ± 0.55 (mm) in grade I, 7.63 ± 1.28 (mm) in grade II and 16.57 ± 3.18 (mm) in grade III. The mean IPP was significantly (p<0.05) higher in grade III.
|
Grading |
Qmax (mL/s) |
||
|
Frequency |
Mean ± SD |
P value |
|
|
Grade I |
7 |
11.21 ± 6.05 |
0.012s |
|
Grade II |
10 |
11.7 ± 3.36 |
|
|
Grade III |
25 |
8 ± 2.67 |
|
Table 4: Distribution of the study patients by Qmax (N=42).
Table 4 showed the mean Qmax was 11.21 ± 6.05 (mL/s) in grade I, 11.71 ± 3.36 (mL/s) in grade II and 8 ± 2.67 (mL/s) in grade III. The mean Qmax was significantly (p<0.05) reduced in grade III.
|
Grading |
Q-mean (mL/s) |
||
|
Frequency |
Mean ± SD |
p value |
|
|
Grade I |
7 |
5.16 ± 2.61 |
0.187ns |
|
Grade II |
10 |
4.3 ± 2.28 |
|
|
Grade III |
25 |
3.75 ± 1.26 |
|
Table 5: Distribution of the study patients by Qmean (N=42).
Table 5 showed the mean Qmean was 5.16 ± 2.61 (mL/s) in grade I, 4.3 ± 2.28 (mL/s) in grade II and 3.75 ± 1.26 (mL/s) in grade III. The mean Qmean was higher in grade I followed by grade II and grade III, but not statistically significant among three groups.
|
Grading |
Voided Time (sec) |
||
|
Frequency |
Mean ± SD |
p value |
|
|
Grade I |
7 |
60.57 ± 14.63 |
0.078ns |
|
Grade II |
10 |
64 ± 19.32 |
|
|
Grade III |
25 |
81.52 ± 30.02 |
|
Table 6: Distribution of the study patients by voided time (N=42).
Table 6 showed the mean voided time was 60.57 ± 14.63 (sec) in grade I, 64 ± 19.32 (sec) in grade II and 81.52 ± 30.02 (sec) in grade III. The mean voided time almost similar (p>0.05) among three groups, no statistically significant difference was observed among groups.
|
Grading |
Flow Time (sec) |
||
|
Frequency |
Mean ± SD |
p value |
|
|
Grade I |
7 |
50.43 ± 13.7 |
0.073ns |
|
Grade II |
10 |
50.6 ± 19.43 |
|
|
Grade III |
25 |
67.64 ± 25.94 |
|
Table 7: Distribution of the study patients by flow time (N=42).
Table 7 showed the mean flow time was 50.43 ± 13.7 (sec) in grade I, 50.6 ± 19.43 (sec) in grade II and 67.64 ± 25.94 (sec) in grade III. The mean flow time (sec) was statistically not significant (p<0.05) higher in grade III followed by grade II and grade I.
|
Variables |
r-value |
p-value |
|
Age |
0.068 |
0.667ns |
|
Prostate volume |
0.256 |
0.102 ns |
|
Voided Volume |
0.103 |
0.518ns |
|
Q-max |
-0.45 |
0.003s |
|
Q-mean |
-0.255 |
0.103ns |
|
Voided Time |
0.362 |
0.019s |
|
Flow Time |
0.374 |
0.015s |
Table 8: The Pearson’s correlations of IPP with Age, Prostate volume, Qmax, Q-mean, Voided Time and Flow Time (N=42).
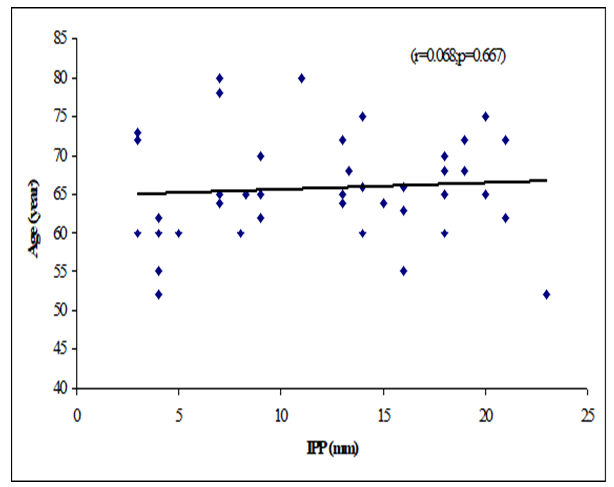
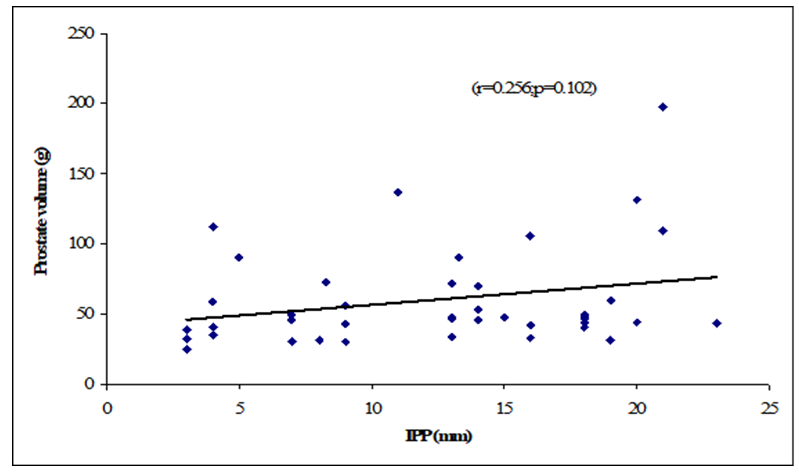
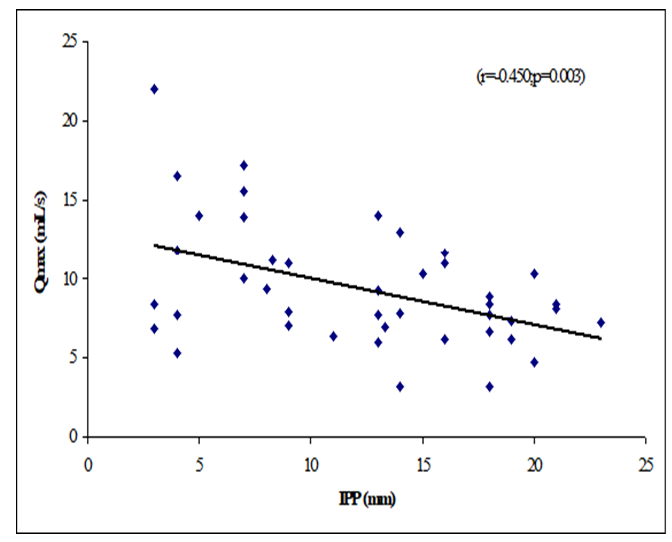
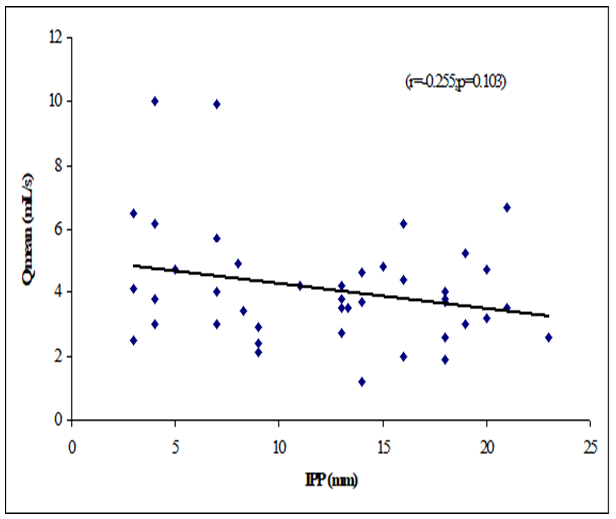
Table 8 showed the Pearson’s correlations of IPP with Age, Prostate volume, Qmax, Qmean, Voided Time and Flow Time. It was observed that IPP was positively significant correlated with voided Time (r=0.362, p=0.019), flow time (r=0.374, p=0.015) and positively not significant correlated with age (r=0.068; p=0.667), prostate volume (r=0.256, p=0.102) (Figure 1-6). IPP was negatively significant correlated with Qmax (r=-0.450, p=0.003) and not significant correlated with Qmean (r=-0.255, p=0.103).
5. Discussions
This cross-sectional study was carried out with an aim to determine the correlation of different grades of intravesical prostatic protrusion with uroflowmetry parameters. Where maximum flow rate (Qmax), average flow rate (Qmean), the Voided time and flow time of the respondents were determined by uroflowmetry and the grades of intravesical prostatic protrusion (IPP) among the symptomatic group by abdominal Ultrasonography. A total of 42 patients attending with symptomatic benign enlargement of prostate in Department of Urology of Chittagong Medical College Hospital, from 1st November, 2020 to 31st July, 2021 were included in this study. They were divided in to three groups according to intravesical Prostatic Protrusion. Patients having intravesical prostatic protrusion less than 5 mm was considered as Grade I, 5-10 mm considered as grade II and more than 10 mm considered as Grade III. Patients with intravesical prostatic protrusion in symptomatic benign enlargement of prostate on abdominal Ultrasonography and age belonged to 45-75 years were enrolled in this study. Diagnosed case of Ca prostate, urinary bladder stone, stricture urethra, urinary bladder neoplasm, previous history of per urethral surgery and spine trauma/spine surgery or any neurological deficit were excluded from the study. The present study findings were discussed and compared with previously published relevant studies. In this present study, it was observed that the mean age was 62 ± 7.94 years in grade I, 66.9 ± 7.02 years in grade II and 66.48 ± 6.23 years in grade III. The mean age was almost alike (p>0.05) among three groups, no statistically significant difference was observed among three groups. Hamza et al. [17] study found the mean age was 61.65 ± 7.5 years in grade I, 65 ± 9.3 year in grade II and 65 ± 9.6 years in grade III. Most of the patients in this study presented in the seventh decade, which is consistent with the current study. The mean age is similar to the findings of Udeh et al. [18] in north central Nigeria who reported a mean age of 65.6 ± 9.84 years, and in southwestern Nigeria who reported a mean age of 64.4 ± 8.88 years [19,20]. Similar findings have also been reported by other studies [21]. The peak age of presentation is similar to the findings of Udeh et al. [18]. In another study Agbo et al. [5] observed that most of the patients presented in the seventh decade of life with mean age of 65.4 years. Similar observations also observed by Hurmuz et al. [22]. In this current study, it was observed that the mean prostate volume was 48.86 ± 29.75 (grams) in grade I, 49.9 ± 19.41 (grams) in grade II and 66.84 ± 40.51 (grams) in grade III. The mean prostate volume was almost alike (p>0.05) among three groups, but also higher in grade III followed by grade II and grade I. Hamza et al. [17] study observed that the mean prostate volume was 46.9 ± 21.4 (g) in grade I, 64.1 ± 41.3 (g) in grade II and 72.2 ± 37.2 (g) in grade III, which was also higher in grade III. The mean prostate volume in their study was 40 ml. The geographical location and smaller mean prostate volume may explain why there was preponderance of grade I IPP, which is comparable with the current study. Thapa and Agrawal [23] study found the mean prostatic size was 39.95 cc varied from 22-87. Vesely et al. [24] showed the average prostate volume was found to be 40.1 cc in, where the study was conducted on 354 patients. Dicuio et al. [25] found average prostate volume to be 41 cc, in a study conducted on 25 men. In another study conducted by Chalise and Agrawal [26] the average volume of prostate was 46.1cc. The above findings regarding the prostatic volume are comparable with the current study. Patients with grade III IPP can be identified earlier as such patients are more likely to require surgical intervention [27]. In this present study, it was observed that the mean IPP was 3.56 ± 0.55 (mm) in grade I, 7.63 ± 1.28 (mm) in grade II and 16.57 ± 3.18 (mm) in grade III. The mean IPP was significantly (p<0.05) higher in grade III followed by grade II and grade I. Similarly, Hamza et al. [17] study also found that the mean IPP was significantly (p<0.05) elevated in grade III followed by grade II and grade I, where they found the mean IPP was 2.1 ± 1.9 mm in grade I, 7.8 ± 1.0 mm in grade II and 18.7 ± 4 mm in grade II. Similar findings also observed by Agbo et al. [5] and Sidgel et al. [28] where they reported that the mean of IPP were 12.9 mm and 14.6 mm, respectively. Similar findings regarding the mean IPP were also observed by Hurmuz et al. [5], Agbo et al. [11] and Foo [17]. However other studies have found preponderance of IPP grade I and II [3,29]. The relative smaller prostate volume in those studies is likely the reason for the reported lower IPP grades in this present study, it was observed that the mean Qmax was 11.21 ± 6.05 (mL/s) in grade I, 11.71 ± 3.36 (mL/s) in grade II and 8 ± 2.67 (mL/s) in grade III. The mean Qmax was significantly (p<0.05) reduced grade III. Hamza et al. [17] study found the mean Q max was 13.4 ± 5.2 (mL/s) in grade I, 13.8 ± 6.0 (mL/s) in grade II and 9.7 ± 4.8 (mL/s) in grade III. Most of the patients with grade III IPP had Qmax < 10 ml/s. Gohil and Vyas [14] study had average Qmax was 9.09 ml/sec which is comparable with the present study. On the other hand, Lee et al. [4] study found the mean Qmax was 14.7 varied from 12.5-20.0 in grade I, 14.5 varied from 13.2-20.0 in grade II and 4.5 varied from 13.5-17.7 in grade III, which is higher with the present study. The higher mean value of Qmax was also found by Thapa and Agrawal [23], which may be due to large sample size in their respective studies, geographical variations, racial and genetic causes. In this current study, it was observed that the mean Qmean was 5.16 ± 2.61 (mL/s) in grade I, 4.3 ± 2.28 (mL/s) in grade II and 3.75 ± 1.26 (mL/s) in grade III. The mean Q- mean was higher in grade I followed by grade II and grade III, but not statistically significant among three groups. The mean Qmean in Thapa and Agrawal [23] study was found to be 6.242 mL/s, which varied from 0.6-17 mL/which supports this current study. In this present study, it was observed that the mean voided time was 60.57 ± 14.63 (sec) in grade I, 64 ± 19.32 (sec) in grade II and 81.52 ± 30.02 (sec) in grade III. The mean voided time almost similar (p>0.05) among three groups, no statistically significant difference was observed among groups. In this current study, it was observed that the mean flow time was 50.43 ± 13.7 (sec) in grade I, 50.6 ± 19.43 (sec) in grade II and 67.64 ± 25.94 (sec) in grade III. The mean flow time (sec) was significantly (p<0.05) higher in grade III followed by grade II and grade I. In this current study, it was observed that there was a positive not significant Pearson’s correlations (r=0.068; p=0.667) was found between IPP and age. Agbo et al. [5] study found there was statistically not significant correlation between IPP and age (r=0.07; p>0.05). Turk and Un [7], which support with the present study. In this present study, it was observed that there was a positive not significant Pearson’s correlations (r=0.256, p=0.102) was found between IPP and Prostate volume. In a prospective study of 114 men with BOO secondary to BPH [4], found a positive correlation between IPP and prostate volume. IPP had a better correlation with BOO than prostate volume. Hamza et al. [17] and Gyawali et al. [30] study found that IPP had no correlation with PV, which support with the present study. On the other hand, Han et al. (2010) also reported significant positive correlation between IPP with prostate volume (r=0.534, p<0.05). In the analysis of correlation between IPP and prostate volume Agbo et al. [5] study found a positive correlation. In this present study it was observed that there were a significant negative Pearson’s correlations (r=-0.471; p=0.001) was found between IPP and Qmax. Hamza et al. [17] study found there was a significant negative correlation between the IPP and Qmax. Similarly, Han et al. [31] found out that the degree of IPP was negatively correlated with the Qmax (r=− 0.364, p<0.05. Agbo et al. [5] study showed a strong negative correlation between IPP and Qmax (r = −0.519, p = 0.000), implicating that the higher the grade of IPP, the lower the Qmax. Shin et al. [32] found a strong negative correlation between IPP and Qmax (r= −0.551, p<0.05) and concluded that an IPP exceeding 5.5 mm was significantly associated with BOO [33]. The finding of a negative correlation between IPP and Qmax was seen in many studies [28]. In this study it was observed that there was a negative not significant Pearson’s correlations (r=-0.255, p=0.103) between IPP and Qmean. Thapa and Agrawal [23] study showed there was no significant correlation was found between IPP and Qmean. In this present series it was observed that there were a positive significant Pearson’s correlations (r=0.362, p=0.019) was found between IPP and voided time. Turk and Un [7] also found statistically significant correlations with IPP and voided time. In this present study it was observed that there were a positive significant Pearson’s correlations (r=0.374, p=0.015) was found between IPP (mm) and flow time (sec).
Limitation of the study
- Intravesical prostatic protrusion is more accurately measured by trans rectal ultrasonography which was not done here
- The present study was conducted at a very short period of time
- Small sample size was also a limitation of the present Therefore, in future further study may be undertaken with large sample size
6. Conclusion and Recommendation
This study found that there is a strong correlation between different grades of intravesical prostatic protrusion (IPP) and uroflowmetry parameters which is a very useful tool to detect severity of bladder outlet obstruction (BOO) in symptomatic BEP patients. Further studies can be undertaken by including large number of patients.
References
- Naslund MJ, Miner M. A review of the clinical efficacy and safety of 5α- reductase inhibitors for the enlarged prostate. Clinical Therapeutics 29 (2007): 17-25.
- Garg R, Singla S, Singla A, et al. Experience with uroflowmetry in evaluation of lower urinary tract symptoms in patients with benign prostatic Journal of clinical and diagnostic research 8 (2014): NC01- NC03.
- Lee LS, Sim HG, Lim KB, et al. Intravesical prostatic protrusion predicts clinical progression of benign prostatic enlargement in patients receiving medical treatment. International Journal of Urology 17 (2010): 69-74.
- Lee A, Lee HJ, Lim KB, et al. Can intravesical prostatic protrusion predict bladder outlet obstruction even in men with good flow? Asian Journal of Urology 3 (2016): 39-43.
- Agbo CA, Ramyil VM, Dakum NK, et al. The value of intravesical prostatic protrusion in evaluation of bladder outlet obstruction from benign prostatic enlargement in Nigeria. African Journal of Urology 24 (2018): 342-346.
- Singh I, Kumar S. Does Intravesical Prostatic Protrusion Predict Bladder Outlet Obstruction in Patients with Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia? UroToday Int J 6 (2013): 40.
- Turk H, Un S. Correlation between lower urinary tract symptoms and objective measures of uroflowmetry. Archivio Italiano di Urologia e Andrologia 89 (2017): 130-133.
- McVary KT. BPH: epidemiology and comorbidities. The American Journal of Managed Care, [online] 12(2006): S122-128.
- Franciosi M, Koff WJ, Rhoden EL. Correlation between the total volume, transitional zone volume of the prostate, transitional prostate zone index and lower urinary tract symptoms (LUTS). International Urology and Nephrology 39 (2007): 871-877.
- Gandhi J, Weissbart SJ, Kim AN, et al. Clinical Considerations for Intravesical Prostatic Protrusion in the Evaluation and Management of Bladder Outlet Obstruction Secondary to Benign Prostatic Hyperplasia. Current Urology 12 (2018): 6-12.
- Foo KT. Solving the benign prostatic hyperplasia puzzle. Asian Journal of Urology 3 (2016): 6-9.
- Kang M, Kim M, Choo MS, et al. Urodynamic Features and Significant Predictors of Bladder Outlet Obstruction in Patients with Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia and Small Prostate Volume. Urology 89 (2016): 96-102.
- Franco G, De Nunzio C, Leonardo C, et al. Ultrasound Assessment of Intravesical Prostatic Protrusion and Detrusor Wall Thickness-New Standards for Noninvasive Bladder Outlet Obstruction Diagnosis? Journal of Urology 183 (2010): 2270-2274.
- Gohil VB, Vyas AV, Kinjal A prospective study of uroflowmetry in 100 patients with lower urinary tract symptoms. International Surgery Journal 6 (2019): 3762.
- Djavan B, Margreiter M, Dianat SS. An algorithm for medical management in male lower urinary tract Current Opinion in Urology 21 (2011): 5-12.
- Yoo S, Lee Y, Park J, et al. Voided volume < 150 mL on initial uroflowmetry in men with storage symptoms: Is it an unreliable test result or a sign of severe storage symptoms? PLoS ONE 14 (2019): e0207208.
- Hamza BK, Ahmed M, Bello A, et al. Correlation of intravesical prostatic protrusion with severity of lower urinary symptoms among patients with benign prostatic hyperplasia. African Journal of Urology 27 (2021): 1-7.
- Udeh EI, Ozoemena OFN, Ogwuche E. The relationship between prostate volume and international prostate symptom score in Africans with benign prostatic hyperplasia. Nigerian Journal of Medicine, 21 (2012): 290-295.
- Badmus T, Asaleye C, Badmus S, et al. Benign prostate hyperplasia: average volume in southwestern Nigerians and correlation with anthropometrics (2013).
- Reis LO, Barreiro GC, Baracat J, et al. Intravesical protrusion of the prostate as a predictive method of bladder outlet obstruction. International Braz J Urol 34 (2008): 627-637.
- Mohammed AZ, Alhassan SU, Edino ST, et al. Histopathological review of prostatic diseases in Kano, Nigeria. The Nigerian Postgraduate Medical Journal 10 (2003): 1-5.
- HURMUZ P. Does Intravesical Prostatic Protrusion Affect Oncological Outcomes and Toxicity in Prostate Cancer Patients Receiving Definitive Radiotherapy? International Journal of Hematology and Oncology 31 (2021): 1-7.
- Thapa Dr N, Agrawal DCS. Correlation of Uroflowmetry with Lower Urinary Tract Symptoms in Patients with Symptomatic Benign Prostatic Hyperplasia at Eastern Part of Nepal: A Prospective Study. IOSR Journal of Dental and Medical Sciences 16 (2017): 86-91.
- Vesely S, Knutson T, Damber JE, et al. Relationship between age, prostate volume, prostate-specific antigen, symptom score 37 (2003): 322-328.
- Dicuio M, Pomara G, Vesely S, et al. The use of prostatic intravesical protrusion correlated with uroflowmetry: a new method to measure obstruction in patients with LUTS due to BOO without using P/F studies. Arch Ital Urol Androl 77 (2005): 50-53.
- Chalise PR, Agrawal CS. Change in urinary symptoms and quality of life in men with benign prostatic hyperplasia after transurethral resection of prostate. Nepal Med Coll J 9 (2007): 255-258.
- Tan YH, Foo KT. Intravesical prostatic protrusion predicts the outcome of a trial without catheter following acute urine The Journal of Urology 170 (2003): 2339-2341.
- Sigdel G, Belokar WK. Clinical significance of intravesical prostatic protrusion in patients with benign prostatic hyperplasia. Journal of Universal College of Medical Sciences 3 (2015): 6-10.
- Kuei CH, Liao CH, Chiang BJ. Significant intravesical prostatic protrusion and prostatic calcification predict unfavorable outcomes of medical treatment for male lower urinary tract Urological Science 27 (2016): 13-16.
- Gyawali PR, Shrestha GK, Joshi BR, et al. Intravesical prostatic protrusion is better than prostate volume in predicting symptom severity in benign prostatic hyperplasia: A prospective clinical study. Post-Graduate Medical Journal of NAMS 10 (2010): 24-28.
- Han WK, Shan GZ, Jin J. Correlation of intravesical prostatic protrusion with clinical evaluation parameters in BPH patients. Zhonghua nan ke xue National Journal of Andrology 16 (2010): 254-257.
- Shin SH, Kim JW, Kim JW, et al. Defining the degree of intravesical prostatic protrusion in association with bladder outlet obstruction. Korean Journal of Urology 54 (2013): 369-372.
- Aganovic D, Prcic A, Hadziosmanovic O. Does the combination of intravesical prostatic protrusion and bladder outlet obstruction number increase test accuracy according to benign prostatic obstruction at the individual level? Acta Informatica Medica 20 (2012): 160.