Correlation of Anti Mullerian Hormone and Antral Follicular Count in Ovarian Reserve Testing
Article Information
Anuradha K1*, Partha Majumder S2, Shiffin R3
1Assistant Professor, Department of Obstetrics and Gynae, Enam Medical College Hospital, Savar, Bangladesh 2Assistant Professor, Department of Pediatric Surgery, Dhaka Medical College Hospital, Dhaka, Bangladesh 3Assistant Professor, Department of Obstetrics and Gynae, Anwer Khan Modern Medical College Hospital, Dhaka, Bangladesh
*Corresponding author: Anuradha K, Assistant Professor, Department of Obstetrics and Gynae, Enam Medical College Hospital, Savar, Bangladesh
Received: 20 July 2022; Accepted: 26 July 2022; Published: 12 August 2022
Citation: Anuradha K, Partha Majumder S, Shiffin R. Correlation of Anti Mullerian Hormone and Antral Follicular Count in Ovarian Reserve Testing. Obstetrics and Gynecology Research 5 (2022): 170-174.
Share at FacebookAbstract
Background: Ovarian reserve defines a woman’s reproductive ability and the number and quality of oocytes she possesses. It is a complex clinical state dependent on age, genetics, and environmental issues. It can reflect women’s endocrine function and fertility which may gradually decrease with increasing age.
Methods: This study was a cross-sectional study conducted at the out-patient department of Obstetrics and Gynae, in Enam Medical College Hospital, Savar and a local private hospital at Savar. The study was conducted during the period of July 2019-December 2019. The sample size for this study was 120.
Result: The most respondent 44 (36.7%) were in between 35-40 years. The mean ± SD of BMI was 26.61 ± 1.96 and followed by duration of infertility (years) was 3.75 ± 1.64, total ovarian volume (ml) was 7.66 ± 1.32. Tubal factor was found in 27 (21.7%) cases and followed by male factor was in 24 (20%), PCOS was in 20 (16.7%), endometriosis was in 6 (5%), unexplained infertility was in 22 (18.3%). In low group AFC (mean ± SD) was 07.15 ± 4.82 where AMH (mean ± SD) was 6.66 ± 5.34 and followed by normal was 09.38 ± 3.59 and 9.48 ± 3.91 and high was 15.45 ± 5.46 and 16.08 ± 5.23. There was no significance correlation found in these two predictors.
Conclusion: AMH is considered as most reliable investigation for ovarian reserve testing. Serum AMH level has strong correlation with comparatively low cost Antral follicular count. Antral follicular count can be done in poor patients for ovarian reserve test.
Keywords
Anti Mullerian Hormone (AMH), Antral Follicular Count (AFC), Ovarian Reserve Testing
Anti Mullerian Hormone articles Anti Mullerian Hormone Research articles Anti Mullerian Hormone review articles Anti Mullerian Hormone PubMed articles Anti Mullerian Hormone PubMed Central articles Anti Mullerian Hormone 2023 articles Anti Mullerian Hormone 2024 articles Anti Mullerian Hormone Scopus articles Anti Mullerian Hormone impact factor journals Anti Mullerian Hormone Scopus journals Anti Mullerian Hormone PubMed journals Anti Mullerian Hormone medical journals Anti Mullerian Hormone free journals Anti Mullerian Hormone best journals Anti Mullerian Hormone top journals Anti Mullerian Hormone free medical journals Anti Mullerian Hormone famous journals Anti Mullerian Hormone Google Scholar indexed journals Antral Follicular Count articles Antral Follicular Count Research articles Antral Follicular Count review articles Antral Follicular Count PubMed articles Antral Follicular Count PubMed Central articles Antral Follicular Count 2023 articles Antral Follicular Count 2024 articles Antral Follicular Count Scopus articles Antral Follicular Count impact factor journals Antral Follicular Count Scopus journals Antral Follicular Count PubMed journals Antral Follicular Count medical journals Antral Follicular Count free journals Antral Follicular Count best journals Antral Follicular Count top journals Antral Follicular Count free medical journals Antral Follicular Count famous journals Antral Follicular Count Google Scholar indexed journals Ovarian Reserve Testing articles Ovarian Reserve Testing Research articles Ovarian Reserve Testing review articles Ovarian Reserve Testing PubMed articles Ovarian Reserve Testing PubMed Central articles Ovarian Reserve Testing 2023 articles Ovarian Reserve Testing 2024 articles Ovarian Reserve Testing Scopus articles Ovarian Reserve Testing impact factor journals Ovarian Reserve Testing Scopus journals Ovarian Reserve Testing PubMed journals Ovarian Reserve Testing medical journals Ovarian Reserve Testing free journals Ovarian Reserve Testing best journals Ovarian Reserve Testing top journals Ovarian Reserve Testing free medical journals Ovarian Reserve Testing famous journals Ovarian Reserve Testing Google Scholar indexed journals woman’s reproductive ability articles woman’s reproductive ability Research articles woman’s reproductive ability review articles woman’s reproductive ability PubMed articles woman’s reproductive ability PubMed Central articles woman’s reproductive ability 2023 articles woman’s reproductive ability 2024 articles woman’s reproductive ability Scopus articles woman’s reproductive ability impact factor journals woman’s reproductive ability Scopus journals woman’s reproductive ability PubMed journals woman’s reproductive ability medical journals woman’s reproductive ability free journals woman’s reproductive ability best journals woman’s reproductive ability top journals woman’s reproductive ability free medical journals woman’s reproductive ability famous journals woman’s reproductive ability Google Scholar indexed journals ovarian response articles ovarian response Research articles ovarian response review articles ovarian response PubMed articles ovarian response PubMed Central articles ovarian response 2023 articles ovarian response 2024 articles ovarian response Scopus articles ovarian response impact factor journals ovarian response Scopus journals ovarian response PubMed journals ovarian response medical journals ovarian response free journals ovarian response best journals ovarian response top journals ovarian response free medical journals ovarian response famous journals ovarian response Google Scholar indexed journals infertility articles infertility Research articles infertility review articles infertility PubMed articles infertility PubMed Central articles infertility 2023 articles infertility 2024 articles infertility Scopus articles infertility impact factor journals infertility Scopus journals infertility PubMed journals infertility medical journals infertility free journals infertility best journals infertility top journals infertility free medical journals infertility famous journals infertility Google Scholar indexed journals sonographic articles sonographic Research articles sonographic review articles sonographic PubMed articles sonographic PubMed Central articles sonographic 2023 articles sonographic 2024 articles sonographic Scopus articles sonographic impact factor journals sonographic Scopus journals sonographic PubMed journals sonographic medical journals sonographic free journals sonographic best journals sonographic top journals sonographic free medical journals sonographic famous journals sonographic Google Scholar indexed journals menstrual cycle articles menstrual cycle Research articles menstrual cycle review articles menstrual cycle PubMed articles menstrual cycle PubMed Central articles menstrual cycle 2023 articles menstrual cycle 2024 articles menstrual cycle Scopus articles menstrual cycle impact factor journals menstrual cycle Scopus journals menstrual cycle PubMed journals menstrual cycle medical journals menstrual cycle free journals menstrual cycle best journals menstrual cycle top journals menstrual cycle free medical journals menstrual cycle famous journals menstrual cycle Google Scholar indexed journals oocytes articles oocytes Research articles oocytes review articles oocytes PubMed articles oocytes PubMed Central articles oocytes 2023 articles oocytes 2024 articles oocytes Scopus articles oocytes impact factor journals oocytes Scopus journals oocytes PubMed journals oocytes medical journals oocytes free journals oocytes best journals oocytes top journals oocytes free medical journals oocytes famous journals oocytes Google Scholar indexed journals endometriosis articles endometriosis Research articles endometriosis review articles endometriosis PubMed articles endometriosis PubMed Central articles endometriosis 2023 articles endometriosis 2024 articles endometriosis Scopus articles endometriosis impact factor journals endometriosis Scopus journals endometriosis PubMed journals endometriosis medical journals endometriosis free journals endometriosis best journals endometriosis top journals endometriosis free medical journals endometriosis famous journals endometriosis Google Scholar indexed journals
Article Details
1. Introduction
Ovarian reserve defines a woman’s reproductive ability and the number and quality of oocytes she possesses [1]. It is a complex clinical state dependent on age, genetics, and environmental issues [2]. It can reflect women’s endocrine function and fertility which may gradually decrease with increasing age [3- 5]. This decline is unavoidable but the rate of primordial follicles lose varies significantly along with variation on the onset of barrenness and time of menopausal transition [2]. Ovarian reserve tests (ORT) helps in distinguishing and treating infertility and in evaluating prior to in vitro fertilization [6]. ORT needs to be easy to perform and followed up and reliable for making decision [7]. There are two best ovarian reserve markers to forecast ovarian response to FSH are mean antral follicle count (AFC) and anti-Mullerian hormone (AMH) [8, 9]. Both these markers considered to be accurate in predicting response to control ovarian stimulation in the in-vitro fertilization setting and have higher reliable predictive value for poor ovarian response (POR) comparing to other indicators [10-14]. However, AMH and AFC may show varied results, especially in where AMH and AFC level could be at odds with each other [8, 15]. AMH is formed by the granulosa cells of pre-antral and small antral follicles, and the menstrual cycle don’t affect its level or exogenous hormonal supplementation [16, 17]. Hence, AMH levels can better represent the number of primordial follicles and reflect ovarian reserve function. The AFC denotes to the number of follicles with diameters of 2 mm to 9 mm and these follicles tends to grow after enrollment in the luteal phase of the previous cycle and mostly reflect the number of follicles that will continue to mature at the time of ovulation treatment cycle [18]. Some studies had emphasis that AMH can reflect both the number of antral follicles and the quality of oocytes [19, 20]. Usually, it is thought that AMH is maintained throughout the menstrual cycle and it is stable as well [21-23]. Hence, AMH is measured to be the best indicator to assess ovarian reserve. The objective of this study was to find out the correlation between the anti-Mullerian hormone (AMH) and antral follicular count (AFC) in ovarian reserve testing.
1.1 Objective of the study
The objective of this study was to find out the correlation of anti-Mullerian hormone (AMH) and antral follicular count (AFC) in ovarian reserve testing.
2. Materials and Methodology
This study was a cross-sectional study conducted at the out-patient department of Obstetrics and Gynae, in Enam Medical College Hospital, Savar and a local private hospital at Savar. The study was conducted during the period of July 2019-December 2019. The sample size for this study was 120.
2.1 Inclusion criteria
- The adult patients who were aged more than 20 years were included in this
- The patients who came for infertility
- The patients having the normal sonographic texture of
- Patients with no signs of hyper-
- The patients who were willing to give their consent after knowing the study
2.2 Exclusion criteria
- The patients who had the history of ovarian surgery, ovarian cyst, or endocrine disease.
- The patients who were not willing to give their consent after knowing the study
The AMH and AFC measurements were done on the second or third day of the menstrual cycle and this was done consistently. The clinical history of all the respondents was recorded with due consents from the hospital authority. Besides, all the respondents were given a consent from where they agreed to give their consent after knowing the study purpose. All participants were assured of high confidentiality. The baseline and demographic data of all the study patients was also recorded which further used in this study. For statistical analysis, the SPSS version 21 was used as the statistical tool.
3. Result
Figure 1, shows the age distribution of the respondents. A few of the respondents 10 (8.3%) were aged between 20-24 years and followed by 37 (30.8%) were 25-29 years, 29 (24.2%) were 30-34 years and the most 44 (36.7%) were 35-40 years. Table 1 represents the baseline characteristics of the respondents where the mean ± SD of BMI was 26.61 ± 1.96 and followed by duration of infertility (years) was 3.75 ± 1.64, total ovarian volume (ml) was 7.66 ± 1.32, number of oocytes was 6.72 ± 3.68 and Number of embryos was 5.36 ± 2.40. Figure 2 shows the etiological factors of the respondents where tubal factor was found in 27 (21.7%) cases and followed by male factor was in 24 (20%), PCOS was in 20 (16.7%), endometriosis was in 6 (5%), unexplained infertility was in 22 (18.3%) and more than 1 factor was in 22 (18.3%) cases. Table 2 represents the distribution of the study patients according to serum AMH level. Serum AMH level <1.0 (Low) was seen in 2 (8.7%) cases of 25-29 years, 8 (27.6%) cases of 30-34 years and 21 (47.7%) cases of 35-40 years and followed by level 1.0-3.5 (Normal) was in 9 (90%),35 (91.3%), 20 (69%) and 23 (52.3%) and level >3.5 (High) was seen in 1 (10%) and 1 (3.4%) of these age groups. The Mean ± SD of Serum AMH of these age groups was 2.67 ± 0.80, 2.24 ± 0.77, 1.57 ± 1.10,1.17 ± 1.06 where the range (min-max) was in between (1.50-3.50), (0.46-3.50), (0.18-3.30) and (0.02-3.48).
Table 3 shows Distribution of the study patients according to total AFC level. AFC level <5 (Low) was seen in 2 (8.7%) cases of 25-29 years, 2 (6.9%) cases of 30-34 years and 10 (22.7%) cases of 35-40 years and followed by level 5-15 (Normal) was in 10 (100%), 31 (83.8%), 27 (93.1%) and 33 (75%) and level >15 (High) was seen in 4 (10.8%) and 1 (2.3%). The Mean ± SD of AFC level of these age groups was 12.5 ± 1.7, 12.3 ± 3.2, 8.8 ± 3.1, 7.5 ± 3.3 where the range (min-max) was in between (11.0-15.0), (4.0-18.0), (4.0-14.0) and (4.0-16.0). Table 4 explains the comparison of average follicle number among AMH and AFC groups. In low group AFC (mean ± SD) was 07.15 ± 4.82 where AMH (mean ± SD) was 6.66 ± 5.34 and followed by normal was 09.38 ± 3.59 and 9.48 ± 3.91 and high was 15.45 ± 5.46 and 16.08 ± 5.23. There was no significance correlation found in these two predictors. Table 5 shows the cost of ovarian reserve testing. The Mullerian Hormone test costs Tk 7000 where Antral Follicular test around Tk 1500.
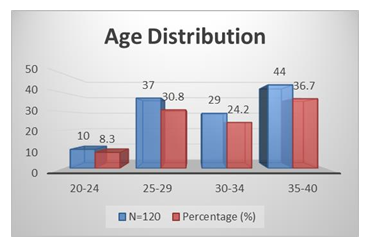
Figure 1: Age Distribution of the Respondents.
|
Baseline Characteristics |
Mean ±SD |
P value |
|
BMI |
26.61 ± 1.96 |
0.724 (NS) |
|
Duration of infertility, years |
3.75 ± 1.64 |
0.672 (NS) |
|
Total ovarian volume (ml) |
7.66 ± 1.32 |
0.067 (NS) |
|
Number of oocytes |
6.72 ± 3.68 |
0.000 (HS) |
|
Number of embryos |
5.36 ± 2.40 |
0.000 (HS) |
Table 1: Baseline Characteristics of the Respondents.
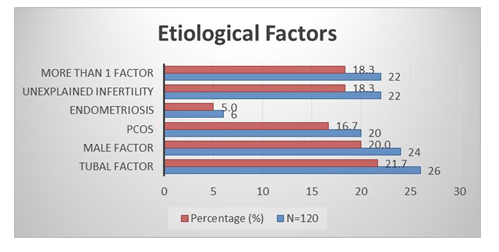
Figure 2: Etiological Factors.
|
Serum AMH (ng/ml) |
20-24 years |
25-29 years |
30-34 years |
35-40 years |
P-value |
||||
|
N=10 |
(%) |
N=37 |
(%) |
N=29 |
(%) |
N=44 |
(%) |
||
|
<1.0 (Low) |
0 |
0.0 |
2 |
8.7 |
8 |
27.6 |
21 |
47.7 |
|
|
1.0-3.5 (Normal) |
9 |
90.0 |
35 |
91.3 |
20 |
69.0 |
23 |
52.3 |
|
|
>3.5 (High) |
1 |
10.0 |
0 |
0 |
1 |
3.4 |
0 |
0.0 |
|
|
Mean ±SD |
2.67 ± 0.80 |
2.24 ± 0.77 |
1.57 ± 1.10 |
1.17 ± 1.06 |
0.001 |
||||
|
Range (min-max) |
(1.50-3.50) |
(0.46-3.50) |
(0.18-3.30) |
(0.02-3.48) |
|||||
Table 2: Distribution of the study patients according to Serum AMH Level.
|
Total AFC (Number) |
20-24 years |
25-29 years |
30-34 years |
35-40 years |
P-value |
||||
|
N=10 |
(%) |
N=37 |
(%) |
N=29 |
(%) |
N=44 |
(%) |
||
|
<5 (Low) |
0 |
0 |
2 |
8.7 |
2 |
6.9 |
10 |
22.7 |
|
|
5-15 (Normal) |
10 |
100 |
31 |
83.8 |
27 |
93.1 |
33 |
75.0 |
|
|
>15 (High) |
0 |
0 |
4 |
10.8 |
0 |
0.0 |
1 |
2.3 |
|
|
Mean ±SD |
12.5 ± 1.7 |
12.3 ± 3.2 |
8.8 ± 3.1 |
7.5 ± 3.3 |
0.001 |
||||
|
Range (min-max) |
(11.0-15.0) |
(4.0-18.0) |
(4.0-14.0) |
(4.0-16.0) |
|||||
Table 3: Distribution of the study patients according to total AFC Level.
|
Sub group |
AFC (mean ± SD) |
AMH (mean ± SD) |
p value |
|
Low |
07.15 ± 4.82 |
6.66 ± 5.34 |
0.54 |
|
Normal |
09.38 ± 3.59 |
9.48 ± 3.91 |
0.99 |
|
High |
15.45 ± 5.46 |
16.08 ± 5.23 |
0.76 |
Table 4: Comparison of average follicle number among AMH and AFC groups.
|
Name of Test |
Cost in Tk |
|
Mullerian Hormone |
7000 |
|
Antral Follicular |
1500 |
Table 5: Cost of Ovarian Reserve Testing.
4. Discussion
A few of the respondents 8.3% were aged between 20-24 years and followed by 30.8% were 25-29 years, 24.2% were 30-34 years and the most 36.7% were 35-40 years [Figure 1]. Juthi Bhowmik et al. in their study showed a few of the respondents 8.1% were aged between 21-25 years and followed by 31.1% were 26-30 years, 24.3% were 301-35 years and the most 36.5% were 36-40 years [24]. The mean ± SD of BMI was 25.61 ± 1.96 and followed by duration of infertility (years) was 3.65 ± 1.64, total ovarian volume (ml) was 7.56 ± 1.32, number of oocytes was 6.62 ± 3.68 and Number of embryos was 5.26 ± 2.40. [table I] Shahinaz H. El-Shorbagy found the mean ± SD of BMI was 26.61 ± 1.96 and followed by duration of infertility (years) was 3.75 ± 1.64, total ovarian volume (ml) was 7.66 ± 1.32, number of oocytes was 6.72 ± 3.68 and Number of embryos was 5.36 ± 2.40 [25]. Tubal factor was found in 21.7% cases and followed by male factor was in 20%, PCOS was in 16.7%, endometriosis was in 5%, unexplained infertility was in 18.3% and more than 1 factor was in 18.3% cases [Figure 2].
Shembekar CA et al in their study found the tubal factor was present in 22% cases and followed by male factor was in 20%, PCOS was in 17%, endometriosis was in 5%, unexplained infertility was in 18% and more than 1 factor was in 18% cases [26]. Serum AMH level <1.0 (Low) was seen in 8.7% cases of 25-29 years, 27.6% cases of 30-34 years and 47.7% cases of 35-40 years and followed by level 1.0-3.5 (Normal) was in 90%, 91.3%, 69% and 52.3% and level >3.5 (High) was seen in 10% and 3.4% of these age groups. The Mean ±SD of serum AMH of these age groups was 2.67 ± 0.80, 2.24 ± 0.77, 1.57 ± 1.10, 1.17 ± 1.06 where the range (min-max) was in between (1.50-3.50), (0.46-3.50), (0.18-3.30) and (0.02-3.48) [Table 2]. In a related study, the serum AMH level <1.0 (Low) was seen in 4.3% cases of 26-30 years, 27.8% cases of 31-35 years and 48.1% cases of 36-40 years and followed by level 1.0-3.5 (Normal) was in 100%, 95.7%, 72.2% and 51.9% and the Mean ±SD of serum AMH of these age groups was 2.87 ±0.80, 2.44 ± 0.77, 1.77 ± 1.10,1.37 ± 1.06 where the range (min-max) was in between (1.50-3.50), (0.46-3.50), (0.18-3.30) and(0.02-3.48).24 AFC level <5 (Low) was seen in 8.7% cases of 25-29 years, 6.9% cases of 30-34 years and 22.7% cases of 35-40 years and followed by level 5- 15 (Normal) was in 100%, 83.8%, 93.1% and 75% and level >15 (High) was seen in 10.8% and 2.3%. The Mean ±SD of AFC level of these age groups was 12.5 ± 1.7, 12.3 ± 3.2, 8.8 ± 3.1, 7.5 ± 3.3 where the range (min-max) was in between (11.0-15.0), (4.0- 18.0), (4.0-14.0) and (4.0-16.0) [Table 3]. Juthi Bhowmik et al. in their study found the AFC level <5 (Low) was in 4.3% cases of 26-30 years, 5.6% cases of 31-35 years and 22.2% cases of 36-40 years and followed by level 5-15 (Normal) was in 100%, 82.6%, 94.4% and 74.1% and level >15 (High) was seen in 13% and 3.7%. The Mean ± SD of AFC level of these age groups was 13.5±1.7, 13.3±3.2, 9.8±3.1, 8.5±3.3 where the range (min-max) was in between (11.0-15.0), (4.0-18.0), (4.0-14.0) and (4.0-16.0) [24]. In low group AFC (mean ± SD) was 07.15 ± 4.82 where AMH (mean ± SD) was 6.66 ± 5.34 and followed by normal was 09.38 ± 3.59 and 9.48 ± 3.91 and high was 15.45 ± 5.46 and 16.08 ± 5.23. There was no significance correlation found in these two predictors [Table 4]. In the study of Parvathy T et al, the low group AFC (mean ± SD) was seen 07.25 ± 4.82 where AMH (mean ± SD) was 6.76 ± 5.34 and followed by normal was 09.48 ± 3.59 and 9.58 ± 3.91 and high was 15.55 ±5.46 and 16.08 ± 5.23. There was no significance correlation found in these two predictors of ovarian reserve [27]. The Mullerian Hormone test costs Tk 7000 where Antral Follicular test around Tk 1500 [Table 5].
5. Conclusion
Although there is a lack of data to accomplish which of the two markers served better to predict ovarian reserve, but most of the studies claimed for the two best ovarian reserve markers to forecast ovarian response to FSH are mean antral follicle count (AFC) and anti-Mullerian hormone (AMH). Although, AMH is considered to be more effective in predicting the ovarian response but many authors thought that AMH and AFC are having the same level of accuracy and clinical value in prediction of ovarian response. So, those authors emphasis that AFC can be considered as a substitute of expensive AMH estimation in predicting the ovarian response. However, better understanding of patients AMH and AFC level, the physician can make a better treatment plan which will bring better treatment outcome.
References
- Practice Committee of the American Society for Reproductive Testing and interpreting measures of ovarian reserve: A committee opinion. Fertil Steril 103 (2015): 9-17.
- Tal R, Seifer DB. Ovarian reserve testing: A user’s guide. American Journal of Obstetrics Gynaecology 217 (2017): 129-140.
- Fabregues F, Iraola A, Casals G, et Evaluation of Two Doses of Recombinant Human Luteinizing Hormone Supplementation in Down-Regulated Women of Advanced Reproductive Age Undergoing Follicular Stimulation for IVF: A Randomized Clinical Study. Eur J Obstet Gynecol Reprod Biol 158 (2011): 56-61.
- Azhar E, Seifer DB, Melzer K, et Knowledge of Ovarian Reserve and Reproductive Choices. J Assist Reprod Genet 32 (2015): 409-415.
- Kirshenbaum M, Orvieto Premature Ovarian Insufficiency (POI) and Autoimmunity-An Update Appraisal. J Assist Reprod Genet 36 (2019): 2207-2215.
- Scott RT, Hofmann Prognostic assessment of ovarian reserve. Fertil Steril 63 (1995): 1-11.
- Kwee J, Elting ME, Schats R, et al. Ovarian volume and Antral follicle count for the prediction of low and hyper-responders with in vitro fertilization. Reprod Biol Endocrinol 5 (2007):
- Zhang Y, Xu Y, Xue Q, et al. Discordance between antral follicle counts and anti- Müllerian hormone levels in women undergoing in vitro Reprod Biol Endocrinol 17 (2019): 51.
- Klenov V, Jungheim AntiMullerian hormone (AMH) is a better predictor of response to controlled ovarian hyperstimulation (COH) than antral follicle count (AFC) in women with discordant markers of ovarian reserve. Fertil Steril 106 (2016): e123-e124.
- Depmann M, Broer SL, van der Schouw YT, et Can we predict age at natural menopause using ovarian reserve tests or mother's age at menopause? A systematic literature review. Menopause 23 (2016): 224-232.
- Medicine Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril 103 (2015): e9-e17
- Broer SL, Dolleman M, Opmeer BC, et al. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update 17 (2011): 46-54.
- Vural B, Cakiroglu Y, Vural F, et Hormonal and functional biomarkers in ovarian response. Arch Gynecol Obstet 289 (2014): 1355-1361.
- Broer SL, Mol BW, Hendriks D, et al. The role of anti-müllerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril 91 (2009): 705-714.
- Tal R, Seifer DB. Ovarian reserve testing: a user’s Am J Obstet Gynecol 217 (2017): 129-140.
- Cook CL, Siow Y, Taylor S, et al. Serum müllerian -inhibiting substance levels during normal menstrual Fertil Steril 73 (2000): 859-861.
- Kelsey TW, Wright P, Nelson SM, et al. A validated model of serum anti-müllerian hormone from conception to PLoS One 6 (2011): e22024.
- Rajpert-De Meyts E, Jorgensen N, Graem N, et al. Expression of anti-mullerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab 84 (1999): 3836-
- Iliodromiti S, Kelsey TW, Wu O, et al. The predictive accuracy of anti-Müllerian hormone for live birth after assisted conception: a systematic review and meta- analysis of the Hum Reprod Update 20 (2014): 560-570.
- Pilsgaard F, Grynnerup AG, Løssl K, et al. The use of antiMullerian hormone for controlled ovarian stimulation in assisted reproductive technology, fertility assessment and -counseling. Acta Obstet Gynecol Scand 97 (2018): 1105-1113.
- La Marca A, Stabile G, Artenisio AC, et al. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod 21 (2006): 3103-3107.
- Massarotti C, La Pica V, Sozzi F, et Influence of age on response to controlled ovarian stimulation in women with low levels of serum anti-Müllerian hormone. Gynecol Endocrinol 9 (2020): 1-5.
- Younis JS, Iskander R, Fauser BCJM, et al. Does an association exist between menstrual cycle length within the normal range and ovarian reserve biomarkers during the reproductive years? A systematic review and Hum Reprod Update 26 (2020): 904-928.
- Juthi Bhowmik, Parveen Fatima, Jesmine Banu, et Correlation Between Follicle Stimulating Hormone, Anti-Müllerian Hormone and Antral Follicle Count with Different Age Groups in Infertile Women, Chattogram Maa-O-Shishu Hospital Medical College Journal (2021).
- El-Shorbagy Comparison of the Predictive Value of Antral Follicle Count, Anti-Müllerian Hormone and Follicle- Stimulating Hormone in Women Following GnRH-Antagonist Protocol for Intracytoplasmic Sperm Injection. Open Journal of Obstetrics and Gynecology 7 (2017): 432-446.
- Shembekar CA, Upadhye JJ, Shembekar MC, et al. Anti-Mullerian hormone (AMH) as predictor of ovarian reserve. Int J Reprod Contracept Obstet Gynecol 6 (2017): 4006-
- Parvathy T, Fessy Louis T, Ramesh P, et al. Ante Mullerian Hormone versus Antral Follicle Count as a predictor of ovarian response to controlled ovarian hyper stimulation in Assisted Reproductive Technique- A prospective study (2009).
