Conservation Genomics and Species Distribution Models Motivate Proactive and Collaborative Conservation in an Era of Rapid Change
Article Information
Maria Sagatelova1, Rosa A Rodriguez-Pena 1, Thomas J Rodhouse2*, Jeffrey Lonneker3, Kirk R Sherrill4, Andrea D Wolfe1
1Department of Evolution, Ecology, and Organismal Biology, The Ohio State University, 318 W. 12th Avenue, Columbus, OH, USA
2National Park Service, Oregon State University-Cascades, 1500 SW Chandler Ave. Bend, OR 97702
3National Park Service, 105 E. 2nd St. Moscow, ID 83843
4National Park Service, 1201 Oakridge Drive, Ft. Collins, CO 80525, USA
*Corresponding author: Thomas J Rodhouse. National Park Service, Oregon State University-Cascades, 1500 SW Chandler Ave. Bend, OR 97702, USA
Received: 22 August 2023; Accepted: 30 August 2023; Published: 22 September 2023
Citation:
Maria Sagatelova, Rosa A Rodriguez- Pena, Thomas J Rodhouse, Jeffrey Lonneker, Kirk R Sherrill, Andrea D Wolfe. Conservation Genomics and Species Distribution Models Motivate Proactive and Collaborative Conservation in an Era of Rapid Change. Journal of Bioinformatics and Systems Biology. 6 (2023): 263-285.
Share at FacebookAbstract
Small, fragmented plant populations with low genetic diversity are susceptible to deterministic and stochastic events that can affect long-term persistence of species. Penstemon lemhiensis Keck (Plantaginaceae) is a rare endemic with small, scattered populations across Idaho and Montana threatened by cumulative impacts of biological invasion, drought, and altered fire regimes. When contextualized by an understanding of rangewide distributions under different environmental change scenarios, conservation genetics can be leveraged to motivate proactive conservation action among collaborating stakeholder groups. We applied a genotypingby- sequencing (GBS) approach across eight populations and 93 individuals of P. lemhiensis. Genetic differentiation among populations followed an isolation-by-distance pattern and ranged from low to moderate (FST = 0.095-0.280). Values of inbreeding were low, and often negative (FIS = -0.039-0.032), indicating outbreeding within populations. Population structure analyses identified six ancestral populations and admixture across all individuals. We contextualized these findings by fitting bioclimatic niche models to past, present, and future climate regime scenarios. Habitat connectivity peaked mid-Holocene and nearly disappeared in the future scenario. Genetic analyses and species distribution models indicated that the species may experience drastic range contraction and accelerated isolation and inbreeding in future. We identified a core area in the Upper Big Hole Valley, Montana most likely to persist as suitable habitat. The National Park Service, Bureau of Land Management, and US Forest Service were identified as key stakeholders in that valley. We outline a proactive collaborative conservation strategy that aim to maintain wild P. lemhiensis populations.
Keywords
Climate change, Conservation genetics, Conservation policy, Penstemon, Protected areas
Climate change articles; Conservation genetics articles; Conservation policy articles; Penstemon articles; Protected areas articles
Article Details
1. Introduction
Earth is experiencing an unprecedented level of biodiversity loss as a consequence of the ongoing Holocene extinction, a series of anthropogenically-driven global extinction events that are threatening > 1 million animal and plant species [1,2]. The extinction risk to plant species, which form much of the ecological bases for life, is of particular concern. The greatest threats to plant species include changes in land use [3,4], invasive species encroachment [5], climate related range shifts [6], habitat degradation and fragmentation [7], and genetic erosion [8]. In recent years, plant extinction rates have skyrocketed to 500 times the historic background rate [9], a value that is likely underestimated due to a lack of comprehensive global assessment of plant species. Proactive and multi-jurisdictional collaborative conservation initiatives and natural resource management strategies that anticipate accelerating changes are required to counteract these drastic losses [10].
Plant conservation efforts receive far less public attention than those of charismatic animal species, despite the essential roles of plants in many ecosystem services [11]. Increased plant diversity and species richness promote overall ecosystem productivity [12,13], and plants act as ecosystem engineers within many local environments [14]. While a greater proportion of plant species are listed under the federal Endangered Species Act (ESA) than any other taxonomic group, plants receive significantly less conservation funding per species [15], which directly impacts our ability to help them recover [16]. The quality of a species recovery plan is also associated with species recovery potential [17]. Taxonomic bias present in the language of ESA has seeped into aspects of recovery such as plan implementation and revision, critical habitat designation, and more [18-21].
Plant species recovery plans and conservation efforts seldom incorporate or consider genetic factors [22] despite the importance of understanding and preserving genetic diversity [23]. The omission of genetic data within conservation initiatives directly reflects the deficit of genetic studies on vulnerable species. Genetic data can be remarkably insightful in elucidating the variation within individual populations [24] and can be leveraged to understand where state and federal agencies should place conservation priorities [25]. Modern genomic approaches allow for discovery of minute patterns of genetic variation within and among populations at low cost. Genotyping-by-sequencing (GBS) is a common genomics approach that has been applied readily over the last decade to understand variation within high diversity groups [26,27]. GBS offers an advantage over other commonly used techniques, such as single sequence repeats (SSRs) and neutral markers, in that it assesses more markers and improves the precision of diversity measurements [28,29,30]. GBS has been an essential tool in many population genetics studies [29] and has proven effective in evaluating the genetic diversity of multiple populations of plant species [31]. Further, in comparison to other PCR or array-based techniques, little prior knowledge of the genome is needed for GBS studies [32], so it can be readily used in nonmodel species such as those in the genus Penstemon.
Penstemon Schmidel, ca. 300 species, (Plantaginaceae) is the largest plant genus endemic to North America [33]. Many species within the genus are rare and restricted to narrow geographic ranges, making them vulnerable to abiotic and biotic threats [33]. The vulnerability within Penstemon is well established; 52 species have previously been reviewed for federal listing under the ESA [34], with only P. haydenii, P. debilis, and P. penlandii [35,36,37] officially listed. Previous genetic and demographic assessments of P. debilis, P. albomarginatus, P. caryi, and P. scariosus have highlighted high inbreeding coefficients and decreased genetic diversity within isolated populations, increasing their vulnerability to stochastic events (Stone et al. 2019; Rodríguez-Peña et al. 2018; Wolfe et al. 2014; [38]. Given the observed vulnerability in genetically assessed species within Penstemon, it is clear that further insights on other rare Penstemon are warranted.
Penstemon lemhiensis Keck is presently one of many Penstemon species of major conservation concern. The largest population resides within Big Hole National Monument, a National Park Service managed protected area in Montana with an estimated 2,500 individuals [39, 40]. Outside of this area, only three known populations with more than 300 individuals are known [41]. Despite its initial proposal for listing in 1993, the species is not currently listed under ESA, despite initial remarks of declining populations. Penstemon lemhiensis is, however, listed as sensitive by both the Bureau of Land Management and the US Forest Service. Penstemon lemhiensis does not have sufficient data to infer vulnerability in accordance with the US Fish and Wildlife Service [34]. However, the species is thought to be substantially threatened by interruptions to fire regimes [42], encroachment by the invasive spotted knapweed (Centaurea stoebe) [39], as well as anthropogenic actions, and increase of drought frequency [43].
Given that P. lemhiensis occurs on lands managed by numerous federal, state, private, and non-governmental entities, conservation measures have achieved varied success. To date, several monitoring studies have provided valuable insight into ecological threats to P. lemhiensis including competition from invasive weeds and, in the absence of fire or other disturbances, competition from native steppe vegetation [44-47]. The knowledge gained from this work has led to the testing of conservation management practices in Big Hole National Monument including prescribed fire and use of herbicides to suppress invasive weeds [39, 40]. However, no comprehensive range wide conservation strategy currently exists for P. lemhiensis. Additionally, to date, no study has focused on a species-wide assessment of genetic diversity for P. lemhiensis. An understanding of the genetic variation within and among populations is essential to evaluating the ability of individual populations to respond to long-term genetic threats such as inbreeding depression (Edwards et al. 2008) [8] and create comprehensive conservation strategies. Given the complexity of conservation management that occurs across a variety of ownership and jurisdictions, the uncertain climate future, and the lack of genetic data about the species, concrete strategies must consider underlying genetic conditions, future scenarios of range dynamics, and address cross-jurisdictional collaboration.
We used the combination of GBS and species distribution modeling (SDM) results to develop a robust conservation management path, identifying a core area within the species’ range and stakeholders that can be leveraged for proactive collaborative conservation. Because this study utilizes a GBS approach to infer genetic structure among different populations and subpopulations of P. lemhiensis, findings from this study will clarify whether isolation among populations has yet had an impact on genetic diversity. The integration with the SDM analysis provides the context required to then recommend which populations to prioritize for immediate conservation and what a possible future conservation stakeholder network might look like.
2. Methods
2.1 Study Species
Penstemon lemhiensis is a rare, regional endemic perennial subshrub. that has deep blue-purple flowers with no visible nectar guides [48] and is pollinated primarily by Pseudomasaris vespoides wasps [49,42]. Penstemon lemhiensis flowers are protandrous and thus the species is a near-obligate outcrosser [49, 41]. Penstemon lemhiensis is distributed across subranges of the Rocky Mountains in four Montana counties, Beaverhead, Deer Lodge, Ravalli, and Silver Bow, and in Lemhi County, Idaho [50].
2.2 Specimen Sampling and DNA Extraction
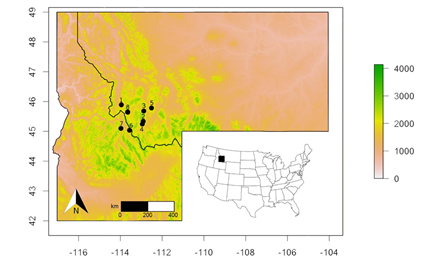
Eight sites of P. lemhiensis, which we consider as populations within this study, were sampled across Montana and Idaho, along with three subpopulations within Big Hole National Monument in Montana, the most densely sampled area (Figure 1, Table 1). Leaf tissue was collected in-situ and immediately placed in silica gel. DNA was extracted from dried leaf tissue following the CTAB (cetyl trimethylammonium bromide) protocol [51] with modifications [52]. In total, 95 individuals were selected for sequencing. This value is due to both budgetary constraints and low DNA concentration after Qubit DNA quantification assay.
Eight individuals per population are sufficient to accurately estimate population genetic differentiation and genetic diversity parameters [53]. This directly influenced our sequencing protocol. Our sampling strategy aimed to maximize the number of populations at the expense of more individuals because this would allow us to determine changes in genetic differentiation across a larger geographic scale without compromising the demographic estimates [54] and the number of individuals at the expense of depth of genomic coverage [55] as this will yield more accurate estimate of population genetic parameters. We therefore sampled across ten total populations, seven populations and three assumed subpopulations, with 8-13 individual each and with an average genomic coverage of 20X per individual. Meaning that, on average, each genetic base or genomic region is represented 20 times.
Table 1: Demographic Information for Penstemon Lemhiensis populations
|
Population |
Latitude |
Longitude |
Elevation (m) |
Estimated |
|
Population Size |
||||
|
POP1 |
45.8894 |
-113.95 |
1396 |
20-30 |
|
POP2 |
45.3315 |
-112.91 |
2162 |
1300 |
|
POP3 |
45.684 |
-112.88 |
2236 |
300 |
|
POP4 |
45.2449 |
-112.95 |
2108 |
25-50 |
|
POP5 |
45.7829 |
-112.51 |
2352 |
300 |
|
POP6 |
45.0351 |
-113.56 |
2171 |
30-60 |
|
POP7 |
45.0983 |
-113.97 |
1587 |
30-50 |
|
POP8 |
45.6523 |
-113.65 |
1953 |
3000 |
2.3 GBS library preparation
DNA was quantified via dsDNA fluorometry using Qubit (Thermo Fisher Scientific™, Waltham, MA, USA) and homogenized at 100 ng of DNA per 15 ul. Libraries preparation for genotype-by-sequencing (GBS) follow [26]. Specifically, to digest the DNA the PstI enzyme (New England Biolabs) was used and unique barcodes were ligated to individual samples before pooling all the samples for PCR. Size selection was carried out in a Blue Pippin (LABGENE Scientific SA) for a range of 200-500. The resulting DNA concentration was determined and fragment size distribution was confirmed using a Bioanalyzer and the Agilent High Sensitivity DNA kit (Agilent Technologies, Inc.). Samples were then sent to Novogene Corporation Inc. for paired-end 150 bp Illumina HiSeq 2500 sequencing.
2.4 Read mapping and variant calling
Quality control of the sequencing data was performed with FASTQC [56]. The sequence length was 150 bp and the quality score decreased with sequence length. With FASQC, we also detected the presence of duplicated sequences and adapters. This information was used to inform trimming in the following steps. Samples were demultiplexed and assembled using IPYRAD v0.9.20 (Eaton 2014; Eaton & Overcast 2020). Default parameters were followed with some exceptions: the maximum number of reads per sample was set to 100,000, samples were trimmed to 50 bp, loci were present in at least 50% of the samples, and the minimum sample per locus was set to the number of individuals per population.
2.5 Population Genetics Analysis
The SNP matrix obtained from IPYRAD assembly was used to estimate basic genetic statistics and population structure. The R package “hierfstat” (Goudet 2005) was used to obtain the observed heterozygosity (HO), gene diversity (HS), number of alleles (A), and inbreeding coefficients (FIS) within each population, and to calculate genetic differentiation (FST) between populations [57]. The program Arlequin [58] was used to calculate the number of migrants per generation between populations, which was utilized as a measure of gene flow [59].
Population structure was estimated using ancestry coefficients, a neighbor-joining (NJ) tree, a principal components analysis (PCA), and a discriminant analysis of principal components (DAPC). The goal of using several population structure analyses was to validate the results. Ancestry coefficients were estimated in the R package “LEA” (Frichot & François 2015) which implement the sparse nonnegative matrix factorization algorithms (sNMF) developed by (Frichot et al. 2014). This method is similar to the one implemented in the programs STRUCTURE (Pritchard et al. 2000) and ADMIXTURE (Alexander & Lange 2011), in that they all estimate the ancestry proportion of each individual, but sNMF is more computationally efficient than likelihood algorithms without compromising the accuracy of the estimate (Frichot et al. 2014). We evaluated K 1 to 12 with 10 repetitions per K and used the default for the other parameters. A NJ tree was obtained using the R package “ape” (Paradis & Schliep 2019) which employs the method laid out by Saitou and Nei (1987) to estimate the tree. The PCA were computed using the R packages “adegenet” (Jombart 2008) and then the individual assignment result from the PCA was used to color the tips of the NJ tree. Finally, the DAPC (Jombart et al. 2010) was run in R and the package “adegenet” (Jombart 2008), and the function “find.clusters” was used. This function identifies genetic clusters, without prior information about groups, by maximizing the variation between clusters (K-means method).
To determine if the genetic structure of the populations followed an isolation-by-distance pattern, a multiple matrix regression (MMR) analysis was run [60]. First, the geodesic distance between populations was computed using the package “geosphere” and the function “distGeo” [61]. Then, we followed Wang [60] and computed an MMR with the between population FST as dependent matrix and the geographic distance as the independent matrix. Further, the analysis of molecular variance (AMOVA) was used to comprehend the partitioning of genetic variation within and among-populations utilizing R package “ade4” at 1000 permutations.
2.6 Species Distribution Modeling
We used maximum entropy machine learning to estimate the bioclimatic niche of P. lemhiensis across its known range during the last glacial maximum (25,000-15,000 yr BP), mid-Holocene (6,000 year BP), present (1980-2010), and future (2070-2099 for a “business-as-usual” carbon emission scenario [RCP 8.5]). We followed methods outlined by Philips et al. (2006) and Elith et al. (2011) to fit models using Maxent (version 3.4.3) software. Presence data (431 geographic locations) for P. lemhiensis were obtained from the Montana and Idaho Natural Heritage Programs. Presence data (discrete spatial locations) with acreage amounts of less than 4 hectares were classified as having high spatial accuracy (n=380 geographic locations) and were used in the subsequent modeling. We established an approximation of known range by establishing a 160 km (100 mile) buffer around the convex hull of the species presence data.
Explanatory variables in the model included four biogeophysical and twenty bioclimatic predictor variables (Table S2). Bioclimatic variables were derived [62] for 1980-2010 using PRISM normals [63]. NASA NEX future projections 30-year normals data were used for 2010-2039, 2040-2069, and 2071-2099 8.5 carbon emissions scenario [64]. Historical bioclimatic projections for the Mid Holocene and Last Glacial Maximum were obtained from the WorldClim [61]. Biogeophysical variables, elevation, aspect, slope and northness [39], were derived from digital elevation models (NED).
An initial training model with the high spatial accuracy observations (n = 380 geographic locations) and all explanatory variables was modeled for the present time period. A subsequent final variable reduced model was derived for the historic, present, and future time periods using the explanatory variables having a high contribution percentage (≥1) in the initial model. We used model output to create binary suitable habitat classifications by assigning the default logistic format output (probability of presence; Elith et. al. 2011) a threshold value of ≥ 0.26 that resulted in a fixed 10% omission rate [65].
2.7 Stakeholder Analysis
This study is intended to promote the conservation efforts of P. lemhiensis. However, conservation management, broadly speaking, is complex when multiple stakeholder groups with potentially mis-aligned incentives are required to collaborate [66]. This complexity increases in the face of insufficient scientific data. Diverse stakeholders are unlikely to collaborate in the absence of compelling data, and poorly understood or undervalued population dynamics can facilitate potential conservation policy pitfalls due to misconceptions or misinterpretations between stakeholders and sectors.
Given this, an understanding of the genetic structure and distribution of P. lemhiensis populations is not sufficient in providing appropriate conservation recommendations. It is also necessary to have a firm understanding of the intricate political and economic relationships as they would affect the conservation of P. lemhiensis [67]. Further, with consideration towards any policy decision, it is critical to understand the central role of stakeholders and their influence on organizational actions [68]. This is particularly necessary for decisions as they pertain to conservation actions [69,70,71]. To address this, a stakeholder analysis was conducted to identify and characterize stakeholders and develop a deeper insight into the positions and interests of groups relevant to the conservation of P. lemhiensis.
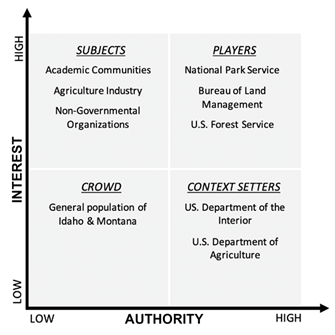
Stakeholders were identified across sectors and characterized based on their respective interest and the power they hold in influencing policy direction as it pertains to conservation management of the species [72,73,74]. This was accomplished through extensive background research and the review of relevant literature, both primary and secondary sources, to understand and assess the influence and interests of organizations. Stakeholders were sorted into four categories – players, subjects, crowd, or context setters – relative to their respective intentions and authority [75]. This identifies the players, organizations or individuals whose interest and power should be taken into consideration when addressing key decisions. Data extrapolated from both GIS and the USGS Protected-Areas of the US (PAD-US) database served to elaborate on direct land ownership and management responsibility. Information generated from this analysis will also be beneficial in perceiving current or future opportunities and threats to conservation management. Coupled with the data from SDM, stakeholder information can aid in strategic conservation planning with consideration for future climatic shifts [76].
3. Results
Estimated population size across the eight populations utilized in this study ranged from 20 to 3000 individuals with 20–31 specimens collected per site (Table 1). The final dataset contained 93 individuals (Table S1), with nine individuals per population for POP1-POP7 and thirty-three for POP8. The SNP matrix consisted of 795 SNPs, 93 individuals and eight populations with a 0.59% missing data, and a total coverage of 848 reads per sample.
3.1 Basic Genetic Statistics and Population Structure
Estimates of population-genetic diversity are summarized in Table 2. The observed heterozygosity (HO) ranged from 0.09 to 0.13. Similarly, the gene diversity estimates were consistent with HO such that populations with high values of gene diversity also had high HO. On the other hand, the number of alleles per population was associated with the number of individuals sampled per population (Table 2). Finally, inbreeding coefficients were low and sometimes negative which is indicative of outbreeding.
Table 2: Penstemon lemhiensis population diversity metrics
|
Population |
N |
A |
HO |
HS |
FIS |
FIS 99% CI |
|
POP1 |
9 |
1036 |
0.095 |
0.098 |
0.013 |
[-0.02, 0.07] |
|
POP2 |
8 |
1071 |
0.113 |
0.112 |
0.001 |
[-0.07, 0.04] |
|
POP3 |
9 |
1076 |
0.111 |
0.11 |
-0.021 |
[-0.06, 0.03] |
|
POP4 |
9 |
1116 |
0.116 |
0.115 |
-0.005 |
[-0.05, 0.04] |
|
POP5 |
9 |
1051 |
0.112 |
0.106 |
-0.039 |
[-0.1, -0.01] |
|
POP6 |
8 |
1014 |
0.093 |
0.091 |
-0.019 |
[-0.07, 0.03] |
|
POP7 |
8 |
1134 |
0.134 |
0.129 |
-0.034 |
[-0.08, -0.02] |
|
POP8 |
33 |
1314 |
0.114 |
0.118 |
0.032 |
[0.01, 0.05] |
Population genetics statistics are as follows: Number of Individuals (N), Inbreeding Coefficient (FIS), Observed Heterozygosity (HO), Gene Diversity (HS), Number of Alleles (A).
The population genetic differentiation followed an isolation-by-distance pattern (Table 3, Figure 1). The results of the MMR analysis indicated that geographic distance explained 47% of the variation in the genetic differentiation (R2 = 0.475, p-value = 0.0001). In particular, the largest FST values were found between POP1 and POP6, which are significantly geographically distant populations as well as physically separated by the Beaverhead Mountain Range. The smallest FST value was found between the two most geographically close populations, POP2 and POP4, approximately six miles apart. Also, the number of migrants per generation (Nm) was consistent with the pattern seen with FST values such that the highest values of FST corresponded to the lowest Nm values. Nevertheless, all populations had Nm values greater than one, which indicates gene flow enough to homogenize the populations [77]. AMOVA analysis further corroborates detected genetic differentiation; only 15.8% of the total genetic diversity was attributable to diversity among populations, whereas 83.9% of total genetic diversity was attributed to variation among individuals while variation between individuals within populations only amounted to 0.24% of total diversity.
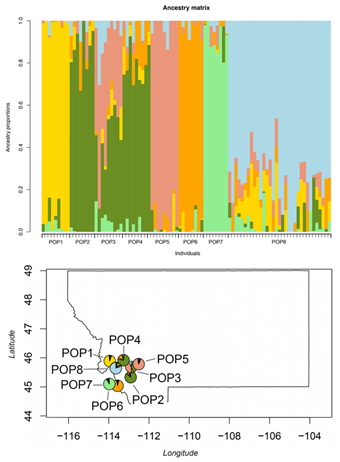
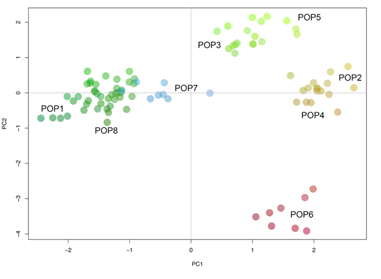
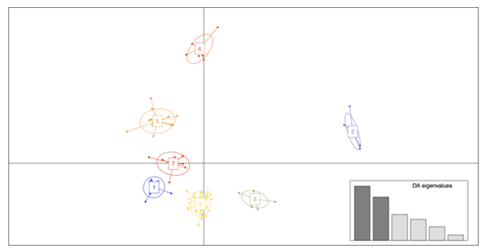
The results from the different population structure analyses used here were consistent with each other. First, the DAPC found seven clusters in the data (Figure S1); each one representing individual populations, except for cluster five which included two populations, POP2 and POP4. Further, POP3 and POP5 are close together but they form independent genetic clusters. Given the geographic proximity to one another, clustering of these populations is not unexpected. Concurrently with the DAPC, the NJ tree (Figure S2) shows that all the individuals in POP2 and POP4 formed one group whereas POP3 and POP5 were genetically similar, but similar to the DAPC, formed unique branches in the tree, unlike the relationship seen between POP2 and POP4 [78]. Individuals from POP8 were genetically more similar to each other than any other population. That was also evident in the ancestry analysis (Figure 2) and the PCA (Figure 3).
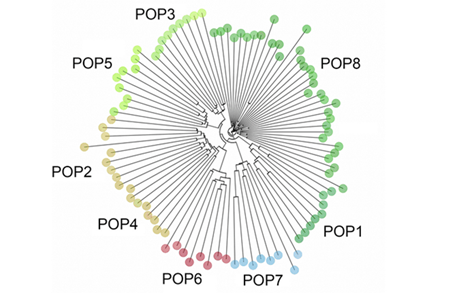
Through the ancestry analysis, we were able to identify six ancestral subpopulations (Figure 2). Overall, while some populations clustered together, we found a heavy degree of admixture across all individuals, despite differences in topography (Figure 2). The results of the ancestry analysis coincided with both the NJ tree and the DAPC; geographically close populations clustered together while geographically distant populations formed distinct groups. Notably, no major distinctions were detected between the three subpopulations of POP8.
Principal component analysis (PCA) is a valuable statistical tool for analyzing high-dimensional data and has been of particular use in understanding population structure and ancestral variation [79]. The results of the PCA further complement the results seen in the ancestry analysis, NJ tree, and DAPC. Clustering of populations was largely a factor of geographic distance. As found in previous analyses, POP2 and POP4 clustered together, as well as POP3 and POP5. Unexpectedly, POP1, POP7, and POP8 clustered together in the PCA, despite significant geographic distance and the presence of physical barriers between populations.
Table 3: Genetic Differentiation and Number of Migrants per Generation
|
POP1 |
POP2 |
POP3 |
POP4 |
POP5 |
POP6 |
POP7 |
POP8 |
|
|
POP1 |
- |
0.218 |
0.207 |
0.21 |
0.226 |
0.28 |
0.212 |
0.123 |
|
POP2 |
1.73 |
- |
0.101 |
0.025 |
0.152 |
0.194 |
0.188 |
0.131 |
|
POP3 |
1.892 |
4.524 |
- |
0.095 |
0.111 |
0.239 |
0.171 |
0.107 |
|
POP4 |
1.862 |
18.88 |
4.76 |
- |
0.138 |
0.171 |
0.179 |
0.124 |
|
POP5 |
1.707 |
2.82 |
4.018 |
3.158 |
- |
0.254 |
0.222 |
0.132 |
|
POP6 |
1.266 |
2.014 |
1.573 |
2.413 |
1.464 |
- |
0.249 |
0.183 |
|
POP7 |
1.852 |
2.158 |
2.546 |
2.292 |
1.83 |
1.486 |
- |
0.156 |
|
POP8 |
3.506 |
3.277 |
4.114 |
3.495 |
3.267 |
2.208 |
2.846 |
- |
Lower diagonal represents the number of migrants per generation and the upper diagonal represents FST values.
Figure 2: (TOP) Ancestry analysis of P. lemhiensis, K = 6. Populations labeled accordingly while each individual is represented by vertical bars. (BOTTOM) Map of the sampled population with pie charts representing population genetic structure based on the ancestry analysis of P. lemhiensis for the "best" value of K = 6. Admixture is present across all populations, but with higher degrees of genetic subdivision evident in geographically distant and isolated populations.
3.2 Species Distribution Modeling
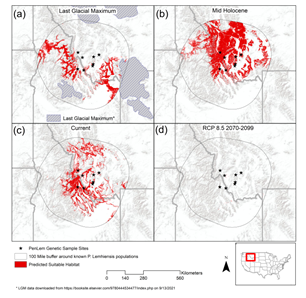
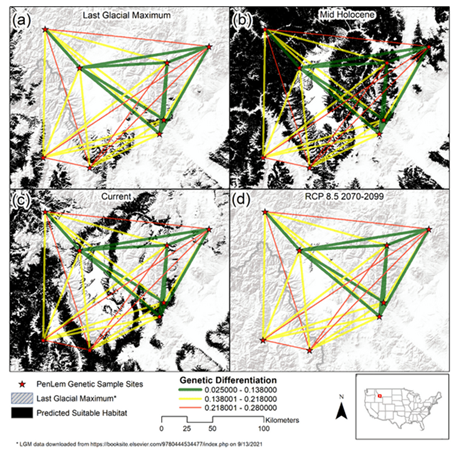
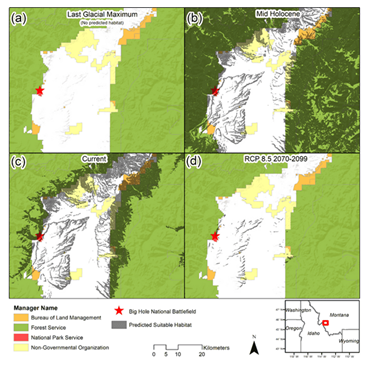
Predictive performance of the model as indicated by the area under the curve of the receiver operating characteristic (AUC) had a mean value of 0.937 across the replicate runs, with a standard deviation of 0.020. Variable contributions in the final model are described in Table S2. Precipitation seasonality (Bio 15), temperature seasonality (Bio 4), slope, and precipitation of warmest quarter (Bio 18) represented the variables with model contributions >10%, and where aspect contributed 9.9%. The results of our SDM (Figure 4) suggest significant range expansion during the mid-Holocene. This historic range expansion is particularly evident north of modern populations, paired with the apparent retreat of modern southern suitable habitats. Results of our SDM also highlight the severe reduction in suitable habitat under a business-as-usual-carbon emissions scenario. POP 8, situated within Big Hole National Battlefield, was the only sampled population anticipated to persist under future climatic conditions.
Figure 4: Species distribution models showcasing range estimations for P. lemhiensis based on climatic suitability across four periods: (a) the Last Glacial Maximum (0.020 Ma); (b) mid Holocene (0.0117 – 0 Ma); (c) current range; (d) future projects (2070-2099) based on representative concentration pathways (RCP) of 8.5. Range estimation is seen in orange.
3.3 Stakeholder Analysis
Based on legislative history, budgetary prioritizations, and general interest, major stakeholders were identified and categorized (Figure 5) as established by [75]. Players in this analysis were defined as individuals or organizations that hold both significant interest and power in shaping the events of a situation. With consideration to this issue, the Bureau of Land Management (BLM), the National Park Service (NPS), and the US Forest Service (USFS) were considered major players. Based on evaluation of genetic data, in conjunction with SDM data (Figure 4), the determination of the Upper Big Hole Valley (POP8) as critical for future species persistence elevated the potential role of NPS as a very significant player. Subjects in this analysis were considered to be individuals or organizations that hold high interest in conservation initiatives, but unlike players, have little to no authority in the situation (Figure 5). In this case, non-governmental organizations (NGOs), the academic community, and private industry represented a few of the most significant subjects in the context of P. lemhiensis conservation. Crowds were designated as individuals or organizations that hold little to no authority and interest in the proceedings of policy issues. Within the framework of P. lemhiensis conservation, this was considered to be the human population settled across both Idaho and Montana. Finally, context setters were defined as organizations or individuals that are within the scope of power to significantly affect conservation management for P. lemhiensis but to have little interest in the process. The prerogative of such organizations to impact action is derived from monetary allocation. In this context, the US Department of the Interior and the US Department of Agriculture set agency priorities based on annual budgetary allocation and specified project appropriations, and were considered context setters (Figure 5).
4. Discussion
Appropriate conservation management of P. lemhiensis, holistically, has presented a challenge for land managers, especially considering the unequal management practices already applied. Big Hole National Battlefield (POP8), which supports the largest known population of P. lemhiensis has applied monitoring procedures since 2009 [80, 39]. Further, the area has undergone controlled burns and spotted knapweed invasion removal. To date, this population is the only area known to have recently applied an active management strategy. Outside of NPS, the species has been monitored by USFS, BLM, the Montana Natural Heritage Program, and the Idaho Native Plant Society [81, 82, 83]. Monitoring has elevated P. lemhiensis as a species of conservation concern (SCC) within USFS, and a Type 3 species within BLM, indicating that the species is moderately endangered. Irrespective of classifications, no direct action or mitigation strategy has been applied by BLM or USFS. Further, these evaluations are less formally structured and haphazardly timed, when compared to those conducted by NPS. The extent to which actions undertaken by NPS have worked to enhance the genetic integrity and diversity of the population within the Battleground is unknown. A firm understanding of how preceding management strategies has impacted populations, negatively or positively, can guide future management in establishing priorities for population conservation and act as a foundation for conservation plan formulation.
4.1 Population genetic diversity and structure
Genetic differentiation (FST) is an estimate that provides insight into the dynamics of population connectivity. Traditionally, FST values >0.15 are considered to represent significant genetic differentiation [84] but more contemporary research suggests that FST values ≥0.20 indicate insufficient gene flow between populations such that harmful effects of inbreeding could manifest within small populations [85]. The largest degree of differentiation, valuing at FST = 0.280, elaborates on the relationship between POP1 and POP6. Given the extent of geographic separation, nearly 62 miles in lateral distance, the approximate 776 meters difference in elevation, and the Beaverhead Mountain Range between both populations, this resulting degree of differentiation is expected. Correlation between genetic differentiation and geographic distance is a well observed pattern [86, 87]. A Nm value of 1.266 between POP1 and POP6, however, indicates some degree of gene flow. This phenomenon was detected in equally distant populations; POP5 and POP6 (FST = 0.254) and POP6 and POP7 (FST = 0.249), as well as the relationship seen between POP1 and every other population (Table 3). While FST values were broadly consistent with the number of migrants per generation, such that populations with the largest degree of differentiation had a smaller number of migrants per generation, and vice versa, it is notable that all values of Nm remain above 1. Based on [77] and ensuing analyses, one migrant individual per local population per generation is considered sufficient to maintain gene flow and impede differentiation [78]. The extent to which some populations are isolated from one another despite the detected gene flow is likely an indicator of the presence of a larger ancestral population in recent geological time. This population is likely undistinguishable in our current analyses due to the recency of divergence of the section in which this species resides, and the genus more broadly [88,89,33,90]. This conclusion is compounded by the remarkably low inbreeding coefficient values seen across all eight populations. Five out of eight populations were found to have negative inbreeding coefficient values, indicating a departure from Hardy-Weinberg equilibrium and an excess of heterozygosity within populations [91]. This, together with the lack of a significant difference between HO and HS values, is suggestive of outbreeding and gene flow amongst populations. Fitness traits were not measured in this study and neither inbreeding nor outbreeding depression could be assessed. In comparison to FIS values observed in other vulnerable species of Penstemon (Table 4), the values detected in this study do not raise immediate concerns that can be applied to conservation management practices. However, given the aforementioned degree of differentiation between populations, the existence of a large, ancestral population is a plausible explanation for the high amount of connectivity detected. While the effects of habitat fragmentation, both negative and positive, are well documented [92,93], the strength of the response detected is dependent on several factors, one of which is time since fragmentation. This is particularly true for measures of FIS, where effects become more pronounced and identifiable as more generations elapse [94].
While we were unable to detect ancestral connectivity across all eight populations, the results of the PCA show similarity across ancestral composition between POP1, POP7, and POP8, despite geographic isolation and physical distance between populations. This connectivity is likely the result of population shifts in response to climatic changes across the landscape, a phenomenon that is common for plant species following post glaciation [95]. Although massive ice sheets such as the Cordilleran existed significantly farther North than extant populations of P. lemhiensis, areas of Western Montana were covered by mountain glaciers throughout the late Pleistocene [96,97]. Further, the areas where both POP1 and POP7 are currently found was once within the Missoula flood extent and within bounds of the Late Wisconsin ice sheet [98]. Thus, the possibility of post-glacial expansion into Northern territory is likely an explanation for ancestral connectivity detected by the PCA. The expansion of suitable habitat detected in the mid-Holocene stands to confirm the likelihood of ancestral connectivity (Figure 4). Following the Last Glacial Maximum retreat, southern populations would expand north into suitable, open habitats [99], as is often seen in Penstemon, a genus notorious for expansion into niches opened by glacial retreat [33, 90]. Suitable habitat estimates through the Last Glacial Maximum (Figure 4) showcase the likelihood of southern populations, which would migrant north with a shifting climate.
Table 4: Inbreeding Coefficients for Penstemon Taxa
|
Taxa |
Estimate Population Size |
FIS |
|
P. scariosus var. albifluvis |
12,215 |
0.23 |
|
P. scariosus var. cyanomontanus |
N/A |
0.2 |
|
P. scariosus var. garrettii |
N/A |
0.29 |
|
P. scariosus var. scariosus |
N/A |
0.37 |
|
P. debilis |
4,000 |
0.23 |
|
P. albomarginatus |
100,000-200,000 |
0.16 |
|
P. caryi |
3,375 |
0.1 |
4.2 Stakeholder Analysis
In addition to an understanding of the genetic integrity of populations, this study aims to evaluate the stakeholders relevant to the conservation of P. lemhiensis in order to better understand the influence of different individuals and organizations in directing appropriate conservation policy in the present and future [72]. The identified major stakeholders, as outlined in Figure 4, have been categorized based on their respective interest and authority in conservation action. Authority is defined, in part according to [100] as the “aggregate of political resources available to an actor”, as well as according to Ackerman & Eden (2011) as having the “capability to influence the delivery” of policy decisions. Interest is defined as having a politically, socially, morally, or economically motivated stake in the outcome of a policy decision [76]. Stakeholders with the most authority and influence can be considered key stakeholders [101] and should be both considered and leveraged in any policy decision regarding P. lemhiensis. We recognize that the stakeholders identified in this paper are by no means an exhaustive list and that other groups or organizations close to the issue, particularly those within marginalized communities, may have been excluded [102]. It is therefore our recommendation that moving forward, conservation efforts are cognizant of this and work to bridge any perceived information gaps.
4.3 Players
Any conservation intervention initiatives for P. lemhiensis must thoroughly consider standing land management policies as well as the organizations with the legal discretion to mandate those policies [103]. The eight populations of P. lemhiensis fall collectively across the jurisdiction of three major land management agencies - the National Park Service, the US Forest Service, and the Bureau of Land Management (Figure 5). Each agency draws its discretion from separate mandates that, in turn, subsequently affects the prioritization of conservation initiatives.
The Bureau of Land Management (BLM) is nested within the Department of the Interior and as mandated by the Federal Land Policy and Management Act (FLPMA) of 1976 (43 U.S.C. §§1701 et seq.) [104] and the National Environmental Policy Act (NEPA) of 1969 (42 U.S.C. § 4321 et seq), balances both multiple use and sustained yield in its’ land management approach. Multiple uses of BLM-managed land include recreation, grazing, timber harvesting, and watershed, wildlife, and fish habitat management, and other conservation practices. Additionally, BLM facilitates federal mineral resource management and energy resource leasing and management. The US Forest Service, under the Department of Agriculture, is similar to BLM in its multiple use approach to land management. Following the discretion of FLPMA, NEPA, and the National Forest Management Act (P.L. 94-588; 16 U.S.C. §§ 1600-1614), the Multiple Use Sustained Yield Act of 1960 (16 U.S.C. §§528-531), and adjacent legislation, follows a land management style analogous to BLM. The Forest Service similarly facilitates a mission in managing land under principles of sustained yield and multiple use. Mandated to preserve the delegated land, water, and wildlife, the Forest Service also administers recreational activities, livestock grazing, timber extraction, and the management of renewable resources. Under the guise of multiple-use land management priorities, conservation priorities are difficult to equitably coincide with factors such as commodity production [105].
The National Park Service (NPS), while also maintaining a dual-purpose mission, focuses on preservation and recreation, as mandated by the National Park Service Organic Act of 1916 (16 U.S.C. §1) [106] , the Wilderness Act of 1964 (16 U.S.C. ch. 23 § 1131 et seq), and the Historic Preservation Act (54 U.S.C. 300101 et seq.). Land and resources managed by the NPS are offered a greater degree of protection than those by the Forest Service or BLM due to differences in management priorities (Congressional Research Service 2019).
While the aforementioned legislations guide land management planning and prioritization, agency authority affords a wide degree of discretion towards actual implementation [106]. Both BLM and the USFS are often faced with competing interests in consideration of a singular land management approach. NPS faces this issue as well in balancing between recreation and conservation, albeit to a lesser degree. Statutes authorizing agency action will influence the priority placed on P. lemhiensis conservation efforts by each respective agency.
Consolidated conservation efforts across different federal and state agencies will be reliant upon cooperation across these agencies. The Council on Environmental Quality, for instance, mandates such cooperation under NEPA in instances of shared projects or interests. However, agencies have reported challenges in executing interagency projects; notably issues regarding timely communication, variation across agency standards, and a lack of memoranda of understanding defining agency roles [107]. Concentrated efforts across the BLM, USFS, and NPS to collectively manage and conserve populations of P. lemhiensis must first acknowledge historic roadblocks to cooperation and actively seek to mitigate them.
4.4 Subjects
Subjects in this analysis are classified as individuals or organizations that hold high interest in conservation initiatives but have little to no authority in the matter (Figure 5). In this case, non-governmental organizations (NGOs), the academic community, and private industry represent a few of the most significant subjects in the context of P. lemhiensis conservation.
The academic community plays a critical role in contributing to the policy decisions of state and federal agencies actively approaching various issues [108], especially issues directly relevant to species conservation [109,110]. This can be accomplished through academic-led research focusing on the genetic diversity of species in question, peer-reviewed publications, and public dissemination of objective information as it impacts species conservation [10, 111,112]. Academics also have a high interest, for a variety of reasons, in the formulation of conservation protocols [113]. Assuring that scientific research is interpreted appropriately and offering feedback on written policy in the form of public comments throughout the Administrative Procedure Act rulemaking process. The academic community does not, however, maintain the legal authority or power to influence decision-making directly, unless through litigation.
Non-governmental organizations (NGOs), specifically those focused on species conservation and environmental integrity, hold a high degree of interest in conservation initiatives broadly [114], but also more specifically for P. lemhiensis. NGOs, such as the Montana Native Plant Society and the Idaho Native Plant Society, work to educate the public on environmental and conservation issues, as well ecological values, and provide the resources to tackle issues firsthand. Similar to the academic community, NGOs lack the legal authority to mandate state or federal conservation management of P. lemhiensis. NGOs, as well as the academic community, hold the ability to litigate on behalf of conservation issues or in response to the absence of agency action. Norton v. Southern Utah Wilderness Alliance (542 U.S. 55, 2004) [115], as well as the preceding lower court cases stand as notable examples of the litigative capacity of NGOs in affecting agency actions.
In addition to organizations with a commitment to conservation initiatives, others carry contradictory interest as it can interfere with their own organizational goals. Most prominently, the agriculture industry in both Idaho and Montana, among other private industries, is highly interested in any potential conservation measures applied towards P. lemhiensis populations. Private organizations retain significant political influence and are themselves subject to governmental regulation [116]. Conservation mandates enacted by either BLM, USFS, or NPS can directly affect the interests of private industries in the area, which in this case, is predominantly private landowners and the agricultural industry within these two states. Both Idaho P. lemhiensis populations occur on land allotted by BLM for grazing - POP7 occurs on the Perreau Creek allotment while POP6 occurs on the Muleshoe allotment [117]. While the Montana populations do not occur on land allotted for grazing directly, POP3 occurs within approximately 130 meters of the Vipond-Glendale grazing allotment and within a reasonable distance such conservation management of the population could impact the neighboring allotment. Grazing allotment places individuals at risk of herbivory and trampling, as well as habitat alteration more broadly [41].
4.5 Crowd
In this analysis, the crowd is designated as individuals or organizations that hold little authority and interest in the proceedings of conservation initiatives for P. lemhiensis (Figure 5). The general civilian population of both Idaho and Montana have little power over the actions of BLM, USFS, and NPS and broadly low interest in their day-to-day priorities. This standing is conditional, and thus is subject to change across individuals based on their unique interests [114]. The citizenry has the capability of reaching out to representatives and public organizations when they feel the agency is not doing their due diligence regarding land management practices and obligations. This is most evident through citizen engagement during the public comment period of the Administrative Procedure Act.
4.6 Context Setters
Context setters are individuals or organizations that are within the scope of power to significantly affect conservation management for P. lemhiensis but have little interest in the process (Figure 5). The prerogative of organizations to impact action is often derived from monetary allocation. In this case, the US Department of the Interior and US Department of Agriculture set agency priorities based on the annual budget and specified project appropriations. For example, the US Department of the Interior’s 2021 budget authorizes $244.1 million for the purpose of conserving, protecting, and enhancing both listed and at-risk fish, wildlife, plants, and their habitats, a 1.71% increase compared to their Fiscal Year 2020 budget [118]. Further, the US Department of the Interior increased their Fiscal Year 2021 budget for listed species recovery by 13.5% when compared to Fiscal Year 2020. The US Department of Agriculture, which allocates annual funding to the US Forest Service, increased their budget for the National Forest System by 2.4% from Fiscal Year 2020 to Fiscal Year 2021 [119]. This prioritizes the implementation of programs that work to increase the health and resilience of Forest Service land while simultaneously meeting multiple-use land mandates. While both the Department of Interior and Department of Agriculture have generally increased their annual budgets for each agency, is it notable that only the Department of Interior specifically addressed the conservation and protection of at-risk or listed plant species within its budget summary. This detail, while incidental, denotes the priority placed by each department unto conservation initiatives. Organizations within the ‘Crowd’ designation are not considered key stakeholders but should be monitored regardless because of the impact they may have on policy decisions [102].
4.7 Conservation Management
The long-term persistence of a species is dependent upon its genetic diversity, a factor responsible for species resilience to deterministic and stochastic events as well as overall fitness [120,121]. Based on the genetic statistics and population structure, it is not immediately evident that P. lemhiensis is threatened or lacking in genetic diversity (Table 2). However, the high degree of physical separation, and the relatively small-estimated population sizes, despite detection of gene flow, is indicative of ancestral connectivity via a larger population. As a case in point, POP1 and POP4, two of the smallest estimated populations and physically distant, are still observed to maintain gene flow between each other. The relative recency of fragmentation makes aspects such as differentiation, inbreeding, and restricted gene flow more difficult to observe in our analyses. The small population sizes seen in a majority of the populations included in this study can contribute to the aforementioned effects and, eventually, to extinction [122]. It is therefore recommended that, in the future, particular populations be monitored for ecological and demographic threats, along with measurements of fitness assessed within each population such that deleterious effects of inbreeding may be better detected [123].
The high degree of differentiation observed between certain populations supports the conservation of as many populations as possible [124]. Priority should be placed upon populations with the most diversity; POP7 and POP8, in this case, albeit small in comparison. Future suitable habitat range, estimated based on climate change projections under a high-carbon emission scenario, severely reduce suitable habitat for P. lemhiensis (Figure 4) such that only POP8 will likely persist (Figure 4). While we advocate for the broadscale preservation of all P. lemhiensis populations, priority could be placed on populations with a high likelihood of survival under a rapidly changing planet.
Following the framework established by Ottewell et al. (2015), populations that are less differentiated from one another and show a high degree of gene flow, that is corroborated by close geographic proximity, should be monitored and managed to reduce threats to the populations – POP2, POP3, and POP4 fall into this category. Given that inbreeding within populations may be more serious than currently detectable, seed migration between close populations may also be more feasible. Seed germination practices should be cognizant of cold treatment and growth hormone requirements [49, 47].
Populations that are highly differentiated from one another should be approached more cautiously. Analogously, these populations should be monitored, and ecological threats reduced. However, translocation of individuals, seeds, or pollen should be avoided due to risks associated with outbreeding depression [125] (Ottewell et al. 2015). Rather, focus should be placed on germplasm conservation. This includes methodology such as in situ and ex situ seed and pollen conservation, in vitro cultures, and cryptopreservation [126,127].
As indicated by the stakeholder analysis, the organizations with the most authority and interest in conservation management of P. lemhiensis are BLM, NPS, and USFS. Shifts in climate and subsequent suitable habitat for P. lemhiensis will likely result in only NPS-managed populations, but nevertheless, inter-agency relationships should be developed and fostered. Given the benefits of inter-agency collaboration in conservation, it is thus paramount that all three agencies work collectively to conserve the species uniformly [128]. A cooperation tactic that may be utilized across agencies is the Landscape Conservation Cooperatives (LCC). Established in 2009, LCCs are intended to streamline interagency cooperation through a formal collaborative framework [129]. Conservation management strategies founded on shared priorities across formal jurisdictions would, theoretically, lead to more effective and successful management. However, despite well intentions, it has become clear that conservation management is still approached independently by agencies. The complex web of federal, state, and local statutes and jurisdiction curtails cooperation and presents institutional challenges, even through programs such as LCCs [129]. It is our recommendation that LCCs not be abandoned but utilized and built upon to ensure interagency cooperation in developing management strategies for P. lemhiensis conservation. Notably, collaborative organizational capacity must be developed in order to succeed [130]. Through the use of gap analysis, disparity in infrastructure and individual agency needs can be identified and remedied through various policy tools. Further, a formal participatory process should be developed and fostered. Expanding beyond the eight populations included within this study, all known, historic, observations of P. lemhiensis broaden the scope of agency involvement from three agencies to twelve, as well as privately managed land. This, together with the stakeholders identified in this study, constructs a need for a strong participatory process. Collaboration between public and private entities is a powerful tool [131] that should be explored.
An interagency communication strategy that allows for engagement of federal, state, tribal, private, and non-governmental stakeholders can be beneficial [132-143] and ensure the longevity of conservation management initiatives. While isolated to the issue of P. lemhiensis conservation, successful cooperative conservation strategies hold the potential to advance the dynamics of how issues of conservation are approached and valued in this country.
Acknowledgments:
Funding for this study was provided by the National Park Service Upper Columbia Basin Network and provided to Ohio State University through a Great Lakes-Northern Forest Cooperative Ecosystem Studies Unit cooperative agreement (P17AC01724). G. Dicus served as the agreement technical representative and supervised NPS authors. J. Salix, Beaverhead-Deer Lodge Forest US Forest Service, and staff from Salmon District Bureau of Land Management and from Oregon State University-Cascades Human & Ecosystem Resilience and Sustainability Lab assisted with P. lemhiensis leaf tissue collection. C. Ott-Hopkins accessioned P. lemhiensis herbarium specimen number 5063 into the Herbarium of Central Oregon Community College, Bend, Oregon
References
- Wake DB, Vredenburg VT. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci USA 105 (2008): 11466–11473.
- Díaz S, Settele J, Brondízio E, et al. Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (2020).
- Newbold T, Hudson LN, Arnell AP, et al. Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Sci 353 (2016): 288-291.
- Bradley BA. Assessing ecosystem threats from global and regional change: hierarchical modeling of risk to sagebrush ecosystems from climate change, land use and invasive species in Nevada, USA. Eco 33 (2010): 198-208.
- Vilà M, Espinar JL, Hejda M, et al. Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol lett 14 (2011): 702-708.
- Wiens J J. Climate-related local extinctions are already widespread among plant and animal species. PLoS biol 14 (2016): 2001104.
- Aguilar R, Ashworth L, Galetto L, et al. Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecology letters 9 (2016): 968-980.
- Kramer A T, & Havens K. Plant conservation genetics in a changing world. Trends in plant science 14 (2009): 599-607.
- Humphreys A M, Govaerts Ficinski R SZ, Lughadha EN, et al. Global dataset shows geography and life form predict modern plant extinction and rediscovery. Nat Ecol Evol 3 (2019): 1043-1047.
- Fisher JR, Wood SA, Bradford M A, et al. Improving scientific impact: How to practice science that influences environmental policy and management. Conserv Sci Prac 2(2020): 210.
- Corlett R T. Plant diversity in a changing world: Status, trends, and conservation needs. Plant diversity 38 (2016): 10–16.
- Cardinale BJ, Matulich K, Hooper D U, et al. The functional role of producer diversity in ecosystems. Am J Botan 98 (2011): 572.
- Hooper DU, Chapin FS, Ewel JJ, et al. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr 75 (2005): 3-35.
- Espeland E K, & Kettenring K M. Strategic plant choices can alleviate climate change impacts: A review. J Environ Management 222 (2018): 316-324.
- Negrón-Ortiz V. Pattern of expenditures for plant conservation under the Endangered Species Act. Biol Conserv 171 (2014): 36-43.
- Male TD, & Bean M J. Measuring progress in us endangered species conservation. Ecol Lett 8 (2005): 986–992.
- Stein BA and Gravuer K. Hidden in Plain Sight: The Role of Plants in State Wildlife Action Plans. Arlington, Virginia: NatureServe (2008).
- Clark JA, Hoekstra JM, Boersma PD, et al. Improving US Endangered Species Act recovery plans: key findings and recommendations of the SCB recovery plan project. Conserv biol 16 (2002): 1510-1519.
- Harvey E, Hoekstra JM, O'Connor RJ, et al. Recovery plan revisions: progress or due process? Ecol Applications 12 (2002): 682–689.
- Hoekstra JM, Fagan WF, and Bradley JE. A critical role for critical habitat in the recovery planning process? Not yet. Ecol Applications 12 (2002): 701–707.
- Lundquist C J J, Diehl M, Harvey E, et al. Factors affecting implementation of recovery plans. Ecol Applications 12 (2002): 713–718.
- Pierson JC, Coates DJ, Oostermeijer JGB, et al. Genetic factors in threatened species recovery plans on three continents. Front Ecol Environ 14 (2016): 433-440.
- Ralls K, Ballou JD, Dudash MR, et al. Call for a paradigm shift in the genetic management of fragmented populations. Conserv Lett 11 (2018): 12412.
- Turchetto C, Segatto A L A, Mäder G, et al. High levels of genetic diversity and population structure in an endemic and rare species: implications for conservation. AoB Plants 8 (2016).
- Ahrens C W, & James E A. Range-wide genetic analysis reveals limited structure and suggests asexual patterns in the rare forb Senecio macrocarpus. Biol J Linne Soci 115 (2015): 256-269.
- Elshire R J, Glaubitz J C, Sun Q, et al. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PloS one 6 (2011): 19379.
- Ahrens C W, Supple M A, Aitken N C, et al. Genomic diversity guides conservation strategies among rare terrestrial orchid species when taxonomy remains uncertain. Ann bot 119 (2017): 1267-1277.
- Beissinger T M, Hirsch CN, Sekhon RS, et al. Marker density and read depth for genotyping populations using genotyping-by-sequencing. Gen 193 (2013): 1073-1081.
- He J, Zhao X, Laroche A, et al. Genotyping-by-sequencing (GBS), an ultimate marker-assisted selection (MAS) tool to accelerate plant breeding. Front plant sci 5 (2014): 484.
- Narum S R, Buerkle CA, Davey J W, et al. Genotyping-by-sequencing in ecological and conservation genomics. Mol ecol 22 (2013): 2841.
- Fu Y B, Cheng B, & Peterson G W. Genetic diversity analysis of yellow mustard (Sinapis alba L.) germplasm based on genotyping by sequencing. Genet Res Crop Evol 61 (2017): 579-594.
- Poland JA, & Rife T W. Genotyping-by-sequencing for plant breeding and genetics. The Plant Genome 5 (2012).
- Wolfe AD, Randle CP, Datwyler SL, et al. Phylogeny, taxonomic affinities, and biogeography of Penstemon (Plantaginaceae) based on ITS and cpDNA sequence data. Am J Bot 93 (2006): 1699–1713.
- S. Fish and Wildlife Service (USFWS). Endangered and threatened wildlife and plants; review of plant taxa for listing as endangered or threatened species. Fed Reg 58 (1993): 51144–51180.
- Fish and Wildlife Service (USFWS). Final rule to determine Penstemon haydenii (blowout penstemon) to be an endangered species. Fed Reg 54 (1987).
- S. Fish and Wildlife Service (USFWS). Endangered and Threatened Wildlife and Plants; Final Rule to Determine Astragalus osterhouti and Penstemon penlandi to be Endangered Species. Fed Reg 54 (1989).
- S. Fish and Wildlife Service (USFWS). Endangered and Threatened Wildlife and Plants; Determination of Endangered Status for Ipomopsis polyantha (Pagosa Skyrocket) and Threatened Status for Penstemon debilis (Parachute Beardtongue) and Phacelia submutica (DeBeque Phacelia). Fed Reg 76 (2011).
- Wolfe AD, Necamp T, Fassnacht S, et al. Population genetics of Penstemon albomarginatus (Plantaginaceae), a rare Mojave Desert species of conservation concern. Conserv Genet 17 (2016): 1245-1255.
- Stucki DS, Rodhouse T J, Lyon J W, et al. Natural Resource Conservation in a Cultural Park: Evaluating the Importance of Big Hole National Battlefield to the Endemic Lemhi Penstemon (Penstemon lemhiensis). Nat are J 33 (2013): 50-58.
- Rodhouse TJ. Monitoring Lemhi penstemon (Penstemon lemhiensis) and spotted knapweed (Centaurea stoebe) in Big Hole National Battlefield: 2009-2014. Natural Resource Report (2016).
- Elzinga C. Habitat conservation assessment and conservation strategy for Penstemon lemhiensis (Lemhi Penstemon). Missoula, Montana (1997).
- Heidel B, & Shelly JS. The Effects of Fire on Lemhi Penstemon (Penstemon Lemhiensis) Final Monitoring Report. Report to the Beaverhead-Deerlodge National Forest and the Dillon Field Office - Bureau of Land Management. Mont Nat Heritage Pro (2001).
- Moseley RK, Mancuso M, & Hilty J. Field investigation and status survey of Penstemon lemhiensis (Lemhi penstemon) in Idaho. Unpublished report on file at the Idaho Department of Fish and Game, Conservation Data Center 5 (1990).
- Shelly JS and Heidel BL. Demographic monitoring of Penstemon lemhiensis in southwest Montana - final report. Unpublished report to the Beaverhead National Forest and the Bureau of Land Management. Mont Nat Heritage Pro 10 (1995): 1-26.
- Heidel BL and JS Shelly. Demographic monitoring of Penstemon lemhiensis, Dillon Resource Area, Bureau of Land Management: 1992 Progress Report. Unpublished Report to the Bureau of Land Management, Dillon Resource Area. Montana Natural Heritage Program, Helena 4 (1998): 1-18.
- Achuff P L. Demographic monitoring of Penstemon lemhiensis. Unpublished report for the Bureau of Land Management. Montana Natural Heritage Program 5 (1992): 1-11.
- Shelly JS. Status review update and establishment of demographic monitoring studies: Penstemon lemhiensis. Prepared for U.S. Department of Agriculture, Forest Service - Region 1, Beaverhead and Bitterroot National Forests, Montana 10 (1990): 1-61.
- Freeman CC. Penstemon. In: Flora of North America Editorial Committee, eds. 1993+. Flora of North America North of Mexico. 21+ vols. New York and Oxford 17 (2019): 82-255.
- Ramstetter J. "An ecological study of the regional endemic Penstemon lemhiensis (Keck) Keck & Cronq. (Scrophulariaceae)" Graduate Student Theses, Dissertations, & Professional Papers (1983): 7402.
- Strickler D. Northwest Penstemons: 80 species of Penstemon native to the pacific northwest. Columbia Falls, MT: Flower Press (1997).
- Doyle J, Doyle JL. DNA Isolation from small amounts of plant tissue. Phytochem. Bull 57 (1991): 13-15.
- Wolfe A D. Issr techniques for evolutionary biology. Methods in Enzymology 395 (2005): 134–144.
- Nazareno AG, Bemmels J B, Dick CW, et al. Minimum sample sizes for population genomics: an empirical study from an Amazonian plant species. Mol Ecol Res 17 (2017): 1136-1147.
- De Mita S, Thuillet A C, Gay L, et al. Detecting selection along environmental gradients: analysis of eight methods and their effectiveness for outbreeding and selfing populations. Mol ecol 22 (2013): 1383-1399.
- Alex Buerkle C, & Gompert Z. Population genomics based on low coverage sequencing: how low should we go?. Mol ecol 22 (2013): 3028-3035.
- Andrews S. FastQC: a quality control tool for high throughput sequence data. Babraham Bio (2010).
- Weir BS, & Cockerham CC. Estimating F-statistics for the analysis of population structure. Evol (1984): 1358-1370.
- Excoffier L, & Lischer HE. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol ecol res 10 (2010): 564-567.
- Serbezov D, Jorde P E, Bernatchez L, et al. Short-term genetic changes: evaluating effective population size estimates in a comprehensively described brown trout (Salmo trutta) population. Genet 191 (2012): 579-592.
- Wang IJ. Examining the full effects of landscape heterogeneity on spatial genetic variation: a multiple matrix regression approach for quantifying geographic and ecological isolation. Evol 67 (2013): 3402-3411.
- Fick SE and Hijmans RJ. WorldClim 2: new 1km spatial resolution climate surfaces for global land areas. Inter J Climatol 37 (2017): 4302-4315.
- O’Donnell MS, and Ignizio DA. Bioclimatic predictors for supporting ecological applications in the conterminous United States: U.S. Geological Survey Data Series 691 (2012): 10.
- PRISM Climate Group, Oregon State University, 30 Year Normals 1980-2010, 800 meter (2015).
- NASA Earth Exchange Monthly 30 Year Climatologies from 2010-2039 for PPT, TMAX and TMIN, derived from NASA Earth Exchange (NEX) downscaled climatic projections (DCP) data (NEX DCP30) (2013).
- Ortega-Andrade HM, Torres-Priteo DA, Gómez-Lora I, et al. Ecological and Geographical Analysis of the Distribution of the Mountain Tapir (Paprius pinchaque) in Ecuador:Importance of Protected Areas in Future Scenarios of Global Warming, PLOS ONE 23 (2015).
- Charnley S, Fischer A P, & Jones E T. Integrating traditional and local ecological knowledge into forest biodiversity conservation in the Pacific Northwest. Forest ecol manage 246 (2007): 14-28.
- Scott JM, Tear T H, & Mills L S. Socioeconomics and the recovery of endangered species: biological assessment in a political world. Conservation Biology 9 (1995): 214-216.
- Ramirez R. Stakeholder analysis and conflict management. In Cultivating peace: conflict and collaboration in natural resource management. IDRC, Ottawa, ON, CA (1996).
- Mushove P, & Vogel C. Heads or tails? Stakeholder analysis as a tool for conservation area management. Global environmental change 15 (2005): 184-198.
- Welp M, de la Vega-Leinert A, Stoll-Kleemann S, et al. Science-based stakeholder dialogues: theories and tools. Global Environmental Change. Human and Policy Dimensions 16 (2006): 170–181.
- Young JC, Jordan A, Searle KR, et al. Does stakeholder involvement really benefit biodiversity conservation?. Biol Conserv 158 (2013): 359-370.
- Bryson J. Strategic planning for public and nonprofit organizations: A guide to strengthening and sustaining organizational achievement Hoboken, New Jersey: John Wiley & Sons (2018): 544.
- Freeman RE. Strategic Management: a Stakeholder Approach. SSRN Electro J (2001).
- Heidrich O, Harvey J, & Tollin N. Stakeholder analysis for industrial waste management systems. Waste Management 29 (2009): 965-973.
- Ackermann F & Eden C. Strategic management of stakeholders: Theory and practice. Long range planning 44 (2011): 179-196.
- Bryson JM. Strategic planning for public and nonprofit organizations: A guide to strengthening and sustaining organizational achievement. Wiley (2018).
- Wright S. Evolution in Mendelian populations. Genet 16(1931): 197-259.
- Mills LS and Allendorf EW. The one-migrant-per-generation rule in conservation and management. Conserv Biol 6 (1996): 1509-1518.
- Privé F, Luu K, Blum M G, et al. Efficient toolkit implementing best practices for principal component analysis of population genetic data. Bio 36 (2020): 4449-4457.
- Rodhouse TJ, Stucki DS, Lyon J W, et al. Population monitoring protocol for Lemhi penstemon (Penstemon lemhiensis) and spotted knapweed (Centaurea stoebe) in Big Hole National Battlefield: Natural Resource Report (2018).
- Bureau of Land Management (BLM). BLM Idaho Special Status Plant List - FINAL August 12, 2016 (2016).
- Brown B & Proctor J. Lemhi Penstemon. USFS (United States Department of Agriculture, Forest Service) (2018).
- Idaho Native Plant Society (INPS). Idaho Native Plant Society Rare Plant List (2020).
- Frankham R, Briscoe DA, Ballou JD. Introduction to conservation genetics. Cambridge University Press (2002).
- Lowe WH & Allendorf FW. What can genetics tell us about population connectivity?. Mol ecol 19 (2010): 3038-3051.
- Bontrager M & Angert A L. Genetic differentiation is determined by geographic distance in Clarkia pulchella. BioRx (2018).
- Hou Y & Lou A. Population genetic diversity and structure of a naturally isolated plant species, Rhodiola dumulosa (Crassulaceae). PLoS One 6 (2011): 24497.
- Blischak PD, Thompson CE, Waight E M, et al. Inferring Patterns of Hybridization and Polyploidy in the Plant Genus Penstemon (Plantaginaceae). bioRx (2020).
- Wessinger C A, Freeman C C, Mort M E, et al. Multiplexed shotgun genotyping resolves species relationships within the North American genus Penstemon. Am J Bot 103 (2016): 912-922.
- Wolfe A D, Blischak P D & Kubatko L. Phylogenetics of a rapid, continental radiation: diversification, biogeography, and circumscription of the beardtongues (Penstemon; Plantaginaceae). bioRx (2020).
- Johnson MG, & Shaw A J. Genetic diversity, sexual condition, and microhabitat preference determine mating patterns in Sphagnum (Sphagnaceae) peat-mosses. Biol J Linne Soci 115 (2015): 96-113.
- Fahrig L. Effects of habitat fragmentation on biodiversity. Ann rev ecol evol sys 34 (2016): 487-515.
- Fahrig L. Ecological responses to habitat fragmentation per se. Ann Rev Ecol Evol Sys 48 (2017): 1-23.
- Schlaepfer D R, Braschler B, Rusterholz H P, et al. Genetic effects of anthropogenic habitat fragmentation on remnant animal and plant populations: a meta-analysis. Eco 9 (2010): 02488.
- Huntley B & Webb III T. Migration: species' response to climatic variations caused by changes in the earth's orbit. J Bio (1989): 5-19.
- Alden W C. Physiography and glacial geology of western Montana. U.S. Geological Survey, Professional Paper 231 (1953): 200.
- Locke WW. Late Pleistocene glaciers and the climate of western Montana, USA, Arctic and Alpine Research 22 (2007): 1-13.
- Cerling TE, Poreda RJ Rathburn SL. Cosmogenic 3He and 21Ne age of the Big Lost River flood, Snake River Plain, Idaho: Geo 22 (1994): 227-230.
- Hewitt G. The genetic legacy of the quaternary ice ages. Nat 405 (2000): 907–913.
- Cox RW, Jacobson HK.The Anatomy of Influence: Decision Making in International Organization. New Haven, CT; London: Yale University Press (1973).
- Eckhard S & Jankauskas V. The politics of evaluation in international organizations: A comparative study of stakeholder influence potential. Eval 25 (2015): 62-79.
- Reed M S, Graves A, Dandy N, et al. Who's in and why? A typology of stakeholder analysis methods for natural resource management. J Environ Manage 90 (2009): 1933-1949.
- Koontz T M & Bodine J. Implementing ecosystem management in public agencies: lessons from the u.s. bureau of land management and the forest service. Conserv Biol 22 (2008): 60–69.
- National Forest Management Act of 1976. U.S.C 16 (1990).
- Robbins K. The laws of nature: reflections on the evolution of ecosystem management law and policy (First, Ser. Law, legal thought across disciplines). University Of Akron Press (2016).
- Congressional Research Service. The Federal Land Management Agencies 16 (2021).
- Stern MJ & Mortimer MJ. Exploring National Environmental Policy Act processes across federal land management agencies. Gen. Tech. Rep. PNW-GTR-799. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station (2009): 106.
- Clancy CM, Glied SA & Lurie N. From research to health policy impact. Health Serv Res 47 (2012): 337–343.
- Posner S M, McKenzie E & Ricketts T H. Policy impacts of ecosystem services knowledge. Pro Natl Acad Sci U S A 113 (2016): 1760-1765.
- Rose DC, Amano T, González-Varo JP, et al. Calling for a new agenda for conservation science to create evidence-informed policy. Biol Conserv 238 (2019): 108222.
- Taft H R, McCoskey D N, Miller J M, et al. Research–management partnerships: An opportunity to integrate genetics in conservation actions. Conserv Sci Prac 2 (2020): 218.
- Walsh J C, Dicks L V & Sutherland W J. The effect of scientific evidence on conservation practitioners’ management decisions. Conserv Biol 29 (2015): 88-98.
- Evans MC, Cvitanovic C. An introduction to achieving policy impact for early career researchers. Palgrave Commun 4 (2018): 88.
- Boiral O & Heras-Saizarbitoria I. Managing biodiversity through stakeholder involvement: why, who, and for what initiatives?. J Business Ethics 140 (2017): 403-421.
- Norton v. Southern Utah Wilderness Alliance. U. S 55 (2004)
- Rainey H. Understanding and managing public organizations (2nd ed.,The jossey-bass public nonprofit and public management series). Jossey-Bass (1997).
- Bureau of Land Management (BLM). BLM National Grazing Allotment Polygons U.S. Department of the Interior (2020).
- US Department of the Interior. Fiscal Year 2021 - The Interior Budget in Brief (2020).
- US Department of Agriculture. FY2021 Budget Summary (2020).
- Bouzat J L. Conservation genetics of population bottlenecks: the role of chance, selection, and history. Conserv Genet 11(2010): 463-478.
- Frankham R. Genetics and extinction. Biol Conserv 126 (2005): 131-140.
- Markert JA, Champlin D M, Gutjahr-Gobell R, et al. Population genetic diversity and fitness in multiple environments. BMC Evol Biol 10 (2020): 1-13.
- Reed D H & Frankham R. Correlation between fitness and genetic diversity. Conserv Biol 17 (2003): 230-237.
- Gaudeul M, Taberlet P & Till-Bottraud I. Genetic diversity in an endangered alpine plant, Eryngium alpinum L. (Apiaceae), inferred from amplified fragment length polymorphism markers. Mol Ecol 9 (2001): 1625-1637.
- Frankham R, Ballou JD, Eldridge M D, et al. Predicting the probability of outbreeding depression. Conserv Biol 25 (2011): 465-475.
- Oseni O M, Pande V & Nailwal T K. A review on plant tissue culture, a technique for propagation and conservation of endangered plant species. Int J Curr Microbiol App Sci 7 (2018): 3778-3786.
- Paunescu A. Biotechnology for endangered plant conservation: a critical overview. Roman Biotech Lett 14 (2009): 4095-4103.
- Maier C & Wirth K. The world (s) we live in–Inter-agency collaboration in forest management. Forest Policy and Economics 96 (2018): 102-111.
- National Academies of Sciences, Engineering, and Medicine (NASEM). A review of the landscape conservation cooperatives. National Academies Press (2016).
- Foster-Fishman PG, Berkowitz S L, Lounsbury DW, et al. Building collaborative capacity in community coalitions: a review and integrative framework. Am J Commun Psychol 29 (2001): 241–261.
- Ansell C & Gash A. Collaborative governance in theory and practice. J Public Admin Res Theory 18 (2008): 543-571.
- Kark S, Tulloch A, Gordon A, et al. Cross-boundary collaboration: key to the conservation puzzle. Curr Opinion Environ Sustain 12 (2015): 12-24.
- Endangered and Threatened Wildlife and Plants: Review of Plant Taxa for Listing as Endangered or Threatened Species. Fed Reg 58 (1993).
- Franklin J, Davis F W, Ikegami M, et al. Modeling plant species distributions under future climates: how fine scale do climate projections need to be? Global Change biol 19 (2013): 473-483.
- Guillory WX & Brown J L. A new method for integrating ecological niche modeling with phylogenetics to estimate ancestral distributions. System Biol 15 (2021).
- Niu YT, Ye JF, Zhang JL, et al. Long-distance dispersal or postglacial contraction? Insights into disjunction between Himalaya–Hengduan Mountains and Taiwan in a cold-adapted herbaceous genus, Triplostegia. Ecol Evol 8 (2018): 1131-1146.
- Thomas CW. Bureaucratic landscapes: Interagency cooperation and the preservation of biodiversity. Mit Press (2003).
- S. Department of Agriculture. National monitoring trends and burn severity (MTBS) burned area boundaries dataset (2015).
- S. Department of Agriculture. Soil survey geographic database (SSURGO) version 2.2 for select counties in Idaho, Montana, and Orgeon. U S Dept Agriculture Nat Res Conserv Ser (2015).
- S. Department of Agriculture. State Soil Geographic database (STASTO) version 2.2 (STASTO) statewide for Idaho, Montana, and Oregon. U S Dept Agriculture Nat Res Conserv Ser (2015)
- Conservation Service (2015)
- S. Department of Agriculture. Gridded Soil Survey Geographic (gSSURGO) Database for Idaho and Montana. United States Department of Agriculture, Natural Resources Conservation Service (2015).
- Wang IJ. Application of the one-migrant-per-generation rule to conservation and management. Conserv Biol 18 (2004): 332–343.
Supplementary Materials:
Supplementary Table S1: Location information for P. lemhiensis samples utilized for genetic analysis.
|
POPULATION |
LONGITUDE |
LATITUDE |
|
Population 1 - Sula |
-113.953 |
45.8894 |
|
Population 1 - Sula |
-113.953 |
45.88941 |
|
Population 1 - Sula |
-113.953 |
45.88978 |
|
Population 1 - Sula |
-113.953 |
45.88978 |
|
Population 1 - Sula |
-113.953 |
45.88974 |
|
Population 1 - Sula |
-113.953 |
45.88975 |
|
Population 1 - Sula |
-113.953 |
45.88958 |
|
Population 1 - Sula |
-113.953 |
45.8896 |
|
Population 1 - Sula |
-113.953 |
45.8896 |
|
Population 2 – French Creek |
-112.909 |
45.33152 |
|
Population 2 – French Creek |
-112.9 |
45.34014 |
|
Population 2 – French Creek |
-112.903 |
45.33853 |
|
Population 2 – French Creek |
-112.909 |
45.33176 |
|
Population 2 – French Creek |
-112.91 |
45.33171 |
|
Population 2 – French Creek |
-112.904 |
45.33566 |
|
Population 2 – French Creek |
-112.91 |
45.32586 |
|
Population 2 – French Creek |
-112.909 |
45.33039 |
|
Population 3 – Vipond Park |
-112.878 |
45.68473 |
|
Population 3 – Vipond Park |
-112.878 |
45.68476 |
|
Population 3 – Vipond Park |
-112.878 |
45.68471 |
|
Population 3 – Vipond Park |
-112.879 |
45.68396 |
|
Population 3 – Vipond Park |
-112.879 |
45.68393 |
|
Population 3 – Vipond Park |
-112.879 |
45.68393 |
|
Population 3 – Vipond Park |
-112.879 |
45.68393 |
|
Population 3 – Vipond Park |
-112.879 |
45.68394 |
|
Population 3 – Vipond Park |
-112.88 |
45.68397 |
|
Population 4 – Badger Pass |
-113.265 |
45.91721 |
|
Population 4 – Badger Pass |
-113.265 |
45.91721 |
|
Population 4 – Badger Pass |
-112.946 |
45.24489 |
|
Population 4 – Badger Pass |
-112.946 |
45.24499 |
|
Population 4 – Badger Pass |
-112.946 |
45.24503 |
|
Population 4 – Badger Pass |
-112.946 |
45.24462 |
|
Population 4 – Badger Pass |
-112.946 |
45.24515 |
|
Population 4 – Badger Pass |
-112.946 |
45.24504 |
|
Population 4 – Badger Pass |
-112.946 |
45.24497 |
|
Population 5 - Highland |
-112.511 |
45.78288 |
|
Population 5 - Highland |
-112.51 |
45.78333 |
|
Population 5 - Highland |
-112.51 |
45.78335 |
|
Population 5 - Highland |
-112.51 |
45.78339 |
|
Population 5 - Highland |
-112.51 |
45.78343 |
|
Population 5 - Highland |
-112.51 |
45.78343 |
|
Population 5 - Highland |
-112.51 |
45.78344 |
|
Population 5 - Highland |
-112.51 |
45.78349 |
|
Population 5 - Highland |
-112.51 |
45.78345 |
|
Population 6 – Sharkey Hot Springs |
-113.56 |
45.03511 |
|
Population 6 – Sharkey Hot Springs |
-113.56 |
45.03512 |
|
Population 6 – Sharkey Hot Springs |
-113.56 |
45.03512 |
|
Population 6 – Sharkey Hot Springs |
-113.56 |
45.03513 |
|
Population 6 – Sharkey Hot Springs |
-113.56 |
45.03512 |
|
Population 6 – Sharkey Hot Springs |
-113.56 |
45.03517 |
|
Population 6 – Sharkey Hot Springs |
-113.56 |
45.03506 |
|
Population 6 – Sharkey Hot Springs |
-113.56 |
45.03529 |
|
Population 7 – William Creek |
-113.97 |
45.09835 |
|
Population 7 – William Creek |
-113.97 |
45.09834 |
|
Population 7 – William Creek |
-113.974 |
45.09851 |
|
Population 7 – William Creek |
-113.974 |
45.09851 |
|
Population 7 – William Creek |
-113.974 |
45.09852 |
|
Population 7 – William Creek |
-113.974 |
45.09854 |
|
Population 7 – William Creek |
-113.974 |
45.09858 |
|
Population 7 – William Creek |
-113.97 |
45.09833 |
|
Population 8 – Big Hole |
-113.649 |
45.65233 |
|
Population 8 – Big Hole |
-113.649 |
45.65169 |
|
Population 8 – Big Hole |
-113.649 |
45.65248 |
|
Population 8 – Big Hole |
-113.647 |
45.65411 |
|
Population 8 – Big Hole |
-113.647 |
45.65337 |
|
Population 8 – Big Hole |
-113.653 |
45.6484 |
|
Population 8 – Big Hole |
-113.653 |
45.64875 |
|
Population 8 – Big Hole |
-113.649 |
45.65324 |
|
Population 8 – Big Hole |
-113.65 |
45.65063 |
|
Population 8 – Big Hole |
-113.651 |
45.65091 |
|
Population 8 – Big Hole |
-113.655 |
45.64487 |
|
Population 8 – Big Hole |
113.6593 |
45.64367 |
|
Population 8 – Big Hole |
-113.657 |
45.64559 |
|
Population 8 – Big Hole |
-113.657 |
45.64559 |
|
Population 8 – Big Hole |
-113.658 |
45.64324 |
|
Population 8 – Big Hole |
-113.656 |
45.64439 |
|
Population 8 – Big Hole |
-113.657 |
45.64365 |
|
Population 8 – Big Hole |
-113.656 |
45.64506 |
|
Population 8 – Big Hole |
-113.656 |
45.64546 |
|
Population 8 – Big Hole |
-113.658 |
45.64324 |
|
Population 8 – Big Hole |
-113.646 |
45.64186 |
|
Population 8 – Big Hole |
-113.646 |
45.64186 |
|
Population 8 – Big Hole |
-113.646 |
45.64186 |
|
Population 8 – Big Hole |
-113.646 |
45.64186 |
|
Population 8 – Big Hole |
-113.646 |
45.64186 |
|
Population 8 – Big Hole |
-113.646 |
45.64186 |
|
Population 8 – Big Hole |
-113.646 |
45.64186 |
|
Population 8 – Big Hole |
-113.646 |
45.64186 |
|
Population 8 – Big Hole |
-113.646 |
45.64186 |
|
Population 8 – Big Hole |
-113.646 |
45.64186 |
|
Population 8 – Big Hole |
-113.646 |
45.64186 |
|
Population 8 – Big Hole |
-113.646 |
45.64186 |
|
Population 8 – Big Hole |
-113.646 |
45.64186 |
Supplementary Table S2: Biogeophysical, disturbance, bioclimatic predictor variables, descriptions and statistics across the whole study area, and the Lemhi observation sites (n=380). Variables retained in the final model are in bold. ** were removed due to lack of data in historical and or future projections. **^variable replaced *^ variable in the final model due to data availability in WorldClim historic time periods.
Supplementary Table S3: Species distribution model variable contributions across replicate maxent runs.
|
Variable |
Percent contribution |
Permutation importance |
|
Precipitation Seasonality |
19.6 |
11.2 |
|
Temperature Seasonality |
17.3 |
12.2 |
|
Slope |
15.8 |
7.9 |
|
Precipitation of Warmest Quarter |
11.3 |
14.9 |
|
Aspect |
9.9 |
4.3 |
|
Mean Temperature of Wettest Quarter |
7.3 |
8.8 |
|
Min Temperature of Coldest Month |
6.3 |
6.8 |
|
Elevation |
5.1 |
5.5 |
|
Precipitation of Driest Quarter |
2.5 |
10.3 |
|
Isothermality |
2.4 |
1.6 |
|
Precipitation of Wettest Month |
1.5 |
6.3 |
|
Mean Temperature of Coldest Quarter |
0.9 |
10.3 |
Supplementary Figure S3: Species distribution models showcasing range estimations for P. lemhiensis based on climatic suitability across four periods: (a) the Last Glacial Maximum (0.020 Ma); (b) mid Holocene (0.0117 – 0 Ma); (c) current range; (d) future projects (2070-2099) based on representative concentration pathways (RCP) of 8.5. FST (genetic differentiation) estimates between populations overlayed.
Supplementary Figure S4: Change in Penstemon lemhiensis suitable habitat over time from a) last glacial maximum, b) mid-Holocene, c) current, to b) potential future in the Upper Big Hole valley, Montana, US. Key players are shown, with Big Hole National Battlefield (NPS) as the potential central nexus for catalyzing collaborative conservation.