Congenital Flail Tricuspid Valve Leaflet, A Challenging Surgical Decision. Case Report, and Literature Review
Article Information
Ali A. Alakhfash1, 2*, Khaled Alhawri2, 3, Abdullah Alqwaiee4, Abdulrahman Almesned4, Bana Nasser5, Marwan Alhawbani5, Mohammed Hasab Elnabi6
1Consultant Pediatric Cardiologist, Department of Pediatric Cardiology, Prince Sultan Cardiac Center-Qassim, Qassim Health Cluster, MOH, Saudi Arabia
2Ph. D. researcher, Cardiovascular Sciences, La Sapienza University, Rome, Italy
3Consultant Pediatric Cardiac Surgeon, Department of Pediatric Cardiology, Prince Sultan Cardiac Center-Qassim, Qassim Health Cluster, MOH, Saudi Arabia
4Consultant Pediatric Cardiologist, Department of Pediatric Cardiology, Prince Sultan Cardiac Center-Qassim, Qassim Health Cluster, MOH, Saudi Arabia
5Consultant Pediatric Cardiac Intensivist, Prince Sultan Cardiac Center-Qassim, Qassim Health Cluster, MOH, Saudi Arabia
6Pediatric Cardiac Surgery, Prince Sultan Cardiac Center-Qassim, Qassim Health Cluster, MOH, Saudi Arabia
*Corresponding Author: Ali A. Alakhfash, Consultant Pediatric Cardiologist, Department of Pediatric Cardiology, Prince Sultan Cardiac Center-Qassim, Qassim Health Cluster, MOH, Saudi Arabia
Received: 03 September 2021; Accepted: 14 September 2021; Published: 01 October 2021
Citation:
Ali A. Alakhfash, Khaled Alhawri, Abdullah Alqwaiee, Abdulrahman Almesned, Bana Nasser, Marwan Alhawbani, Mohammed Hasab Elnabi. Congenital Flail Tricuspid Valve Leaflet, A Challenging Surgical Decision. Case Report, and Literature Review. Journal of Pediatrics, Perinatology and Child Health 5 (2021): 211-220.
Share at FacebookAbstract
Background: Congenital severe tricuspid valve (TV) insufficiency secondary to ruptured papillary muscle or ruptured chordae tendineae is very uncommon. Ruptured chordae and/or papillary muscle will lead to flail leaflet and tricuspid insufficiency, the severity of which depends upon the severity of the affected leaflet(s). The neonatal presentation might simulate that of severe Ebstein anomaly of the TV, with severe cyanosis due to reduced pulmonary blood flow. Compared to Ebstein anomaly, there is no apical displacement of the tricuspid valve leaflets.
Case presentation: We are presenting a neonate with refractory neonatal cyanosis immediately after birth due to congenital flail TV leaflets. A successful surgical repair of the TV improved the TV function with excellent immediate, short and midterm outcomes.
Conclusions: TV repair is the best surgical option for TV dysplasia with ruptured chordae and flail leaflets, with good immediate as well as short and mid-term outcomes.
Keywords
Flail Tricuspid Valve Leaflets, Tricuspid Dysplasia, Functional Pulmonary Atresia, Neonatal Tricuspid Valve Repair
Flail Tricuspid Valve Leaflets articles; Tricuspid Dysplasia articles; Functional Pulmonary Atresia articles; Neonatal Tricuspid Valve Repair articles
Article Details
1. Introduction
In utero papillary muscle rupture secondary to myocardial ischemia or infarction may result in flail tricuspid valve (TV) leaflets and significant valvular insufficiency. This regurge leads to reduced pulmonary blood flow (PBF) due to stealing of the blood from the right ventricle (RV) to the right atrium (RA) instead of being ejected through the right ventricular outflow tract (RVOT) to the lungs [1]. These patients might present with cyanosis immediately after birth. Routine management with medications that lower the pulmonary vascular resistance and to keep the ductus arteriosus patent might be sufficient until the pulmonary vascular resistance (PVR) drops and the forward PBF improves [2].
Sometimes, the valvular insufficiency is severe enough to steal the blood from the RV to the RA with a resultant decrease in the forward PBF. In such cases, keeping the ductus arteriosus patent, by prostaglandin infusion, to provide a source of PBF is mandatory [3]. Surgical repair of the TV might be essential to reduce the tricuspid regurge (TR) and improve the forward BPF [4, 5]. We are presenting a neonate who presents with cyanosis immediately after birth. There was no patent ductus arteriosus (PDA) that did not open up in response to prostaglandin (PG) infusion. After 2 weeks of conservative management, he underwent a successful surgical TV repair.
2. Case Presentation
We are reporting a full-term male newborn, product of vaginal delivery to a Gravida 4 Para 3 mother with uneventful antenatal history and good APGAR score. His birth weight is 3.9 Kg. Immediately after birth, he was observed to have cyanosis (SPO2 down to 65%) that didn't improve with oxygen administration. There was a significant heart murmur with unremarkable other systemic examinations. The patient was intubated & ventilated. Echocardiography was performed and he was started on prostaglandin infusion at a rate of 0.05 mic/kg/min. In the neonatal intensive care unit (NICU), saturation didn’t improve, and was started on inhaled nitric oxide (iNO). The patient was unstable and continued to have desaturation down to 55%. The ductus arteriosus (DA) didn't open up after 12 hr, despite being on PG infusion 0.05 to 0.1 mcg/kg/min. His saturation remained around 70% in the right upper limb and lower limbs. On the 2nd day of life, he had bad desaturation down to 50%, so he was shifted urgently to the cardiac catheterization laboratory for possible probing of the DA and PDA stenting.
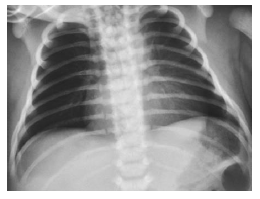
Initial Chest x-ray showed oligaemic lung fields with an almost normal cardiac silhouette (Figure 1).
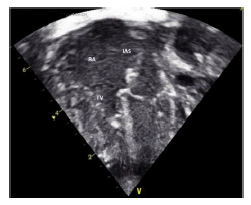
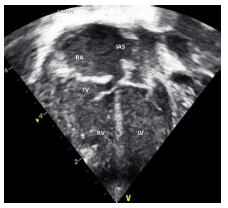
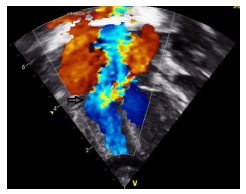
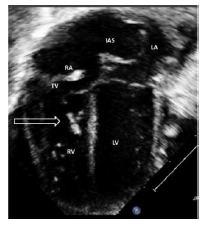
2.1 Echocardiography (Figures 2 A to D)
Figures 2 A to D showed a flail TV anterior leaflet which is shooting beyond the coaptation point. The TV leaflets fail to coapt with severe eccentric tricuspid valve regurgitation (TR), directed toward the interatrial septum (IAS). The TV hinge points are at the normal position, with no evidence of displacement of the TV leaflets. There is a patent foramen ovale (PFO) versus small atrial septal defect (ASD) secondum type with deviation of the IAS to the left side, with moderate right to left atrial shunt. The RA is significantly dilated with mild RV dilatation. The RV systolic function is moderately decreased with evidence of a decrease in the magnitude of the forward PBF (by color and pulsed wave Doppler at the RVOT and the pulmonary arteries (PAs)). There is a decrease in the density of the RVOT / pulmonary valve (PV) color Doppler as well as the PV velocity and time velocity integral by pulsed Doppler spectral display (PW). The TR peak gradient is 30 mmHg. The pulmonary valve annulus is 8 mm with normal main and branch PAs. There is trivial pulmonary insufficiency. The left ventricle (LV) is normal with normal LV systolic function. Left-sided aortic arch with no aortic coarctation. There was no PDA. In addition to the above findings, there were echogenic foci in the RV free wall and interventricular septum (IVS), giving a hint of suspected in-utero ischemia, and possible rupture of the chordae tendineae supporting the TV leaflets.
The conclusion is that the patient is having a dysplastic TV, with a picture of functional pulmonary atresia and a flail anterior leaflet of the TV. No PDA despite giving the patient a high-dose PG infusion. After 24 hr., we realized that he needs an additional source of PBF, so we decided to proceed with PDA stenting (hoping that will be able to probe a functionally closed ductus).
2.1.1 In the cardiac cath: Through the femoral artery using a 4 Fr sheath, an aortogram was performed at the PDA site. The ductus was closed with no flow of blood from the aorta to the PAs. We tried to probe the PDA using coronary wires (regular and Shinobi wires), but the ductus was completely closed and not probe patent. The patient was transferred back to the NICU. We discussed the case thoroughly and agreed to keep the patient in the NICU and start medications used in pulmonary hypertension (Sildenafil, bosentan, and iNO). He received also inotropic support (milrinone and epinephrine infusion), hoping that the PVR will drop and the saturation will improve.
In the NICU he had episodes of hypotension for which he received volume boluses as well as calcium, in addition to epinephrine and milrinone IV infusions. The blood gas was acceptable with frequent episodes of mild metabolic acidosis corrected by sodium bicarbonate and volume boluses. Serum lactate was always around 2. He stayed in the NICU for 2 weeks during which he did not improve. His saturation was low and inotropes could not be weaned. A multi-disciplinary discussion was held and the family counseled and agreed to go for neonatal TV repair. The options discussed include 1) Tricuspid valve repair, with the repair of the ruptured chordae and annuloplasty. 2) Blalock-Taussig (BT) shunt alone without touching the TV. or 3) Conversion to single ventricle with fenestrated patch closure of the TV and BT shunt if the saturation and cardiac output didn’t improve after TV repair.
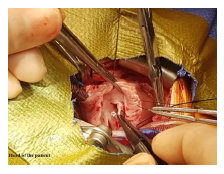
2.2 Surgical findings (Figure 3) and surgical repair technique
The procedure was done under total cardiopulmonary bypass (CPB) using aorto-bicaval cannulation, hypoth-ermia, aortic root cross-clamp, and antegrade cardioplegia. The TV was approached through right atriotomy. The TV leaflets were structurally normal with no displacement of the leaflets' annular attachment. There is evidence of infarction of the head of the anterior papillary muscle with rupture/detachment of the chorda tendineae of the anterior leaflet of the TV leading to flail anterior leaflet. The RV wall surrounding the papillary muscle appeared normal. After visual evaluation and testing of the TV, an artificial chordae was created using CV-6 ePTFE suture (Gore-Tex W. L. Gore and Associates). This constructed chordae was fixed to the anterior papillary muscle and the free edge of the anterior TV leaflet using a 6/0 monofilament polypropylene suture. Although the TV annulus was not dilated, limited posterior annuloplasty using pledgeted 5/0 polypropylene suture was done. The TV was tested to ensure enough inflow using a Hegar dilator size 9 which passed easily through the TV opening. The PFO was left open. The patient was weaned smoothly from CPB with immediate improvement in the saturation that reached 90%. Post-operative epicardial echocardiography showed mild TV regurge with laminar flow across the TV and no evidence of TV stenosis. The aortic cross-clamp and CPB times were 61 and 88 minutes respectively.
2.3 Postoperative ICU course
In the ICU, he maintained his cardiac output and saturation with a normal oxygenation index. He did not require nitric oxide or any other medications to lower the PVR. The only inotropic used was milrinone, which was stopped 24 hours after surgery. We managed to extubate him on the 3rd postoperative day. After 24 hours of extubation, he developed recurrent lung collapse, in both the right and left lungs causing desaturation that necessitates reintubation. A Chest CT scan was done and revealed no specific cause for the recurrent lung collapse This was managed conservatively with chest physiotherapy, mucus dissolving agents, and artificial ventilation, and with time, the lung collapse improved.
The patient's condition improves and was extubated on the 10th post-operative day and discharged home after 2 weeks in stable condition. Follow-up in the clinic at one month, three months, and 6 months after surgery revealed an asymptomatic infant with oxygen saturation of 92%. Follow-up echocardiography revealed a well-functioning TV with good flow across the valve with a mean gradient of 2 mmHg. There is mild TV regurge with a PG of 20 mmHg. The forward PBF is normal, with good RV and LV systolic function.
3. Discussion
Papillary muscle rupture secondary to myocardial ischemia or infarction may result from antepartum or peripartum asphyxia, premature ductal closure, congeni-tal heart disease (CHD), rhesus isoimmunization, congenital endocarditis, thromboembolism, maternal connective tissue disorder, viral infections, traumatic rupture during the birth process, or other more obscure causes [6, 7]. Patients with isolated tricuspid or mitral insufficiency due to ruptured papillary muscles or chordae tendineae constitute in some respects a very favorable group. Such patients generally have well-developed right and left ventricular cavities, no RVOT obstruction, and competent pulmonary and aortic valves without left-sided obstructive lesions [8]. If recognized before the occurrence of irreversible end-organ damage, and with proper surgical repair, these children have an excellent chance for a good outcome [5]. If in utero papillary muscle rupture is identified, the fetus should be carefully monitored until considered viable, and delivered at the first sign of fetal distress. The newborn should be similarly observed, and surgical intervention should be recommended before hemodynamic insta-bility supervenes [6]. The cause of papillary rupture is unclear in our case, however, one might speculate on transient ischemia during the pregnancy. There are echo bright segments of the RV free wall and septal surface with flail anterior TV leaflet immediately after birth, which suggests in utero insult rather than an acute perinatal insult [7] (Figure 2). TV dysplasia is a rare congenital heart problem, with Ebstein's anomaly being the most common TV malformation. Isolated true TV dysplasia is estimated at 1 % of CHD [9]. The anomaly might have a mild degree of TV dysplasia with no clinical consequences, to a severe degree like patients with Ebstein anomaly. It might present with low cardiac output symptoms and signs in which the neonate will not be able to maintain the cardiac output and saturation. The TV regurgitation will steal blood from going forward across the pulmonary valve to the lungs for oxygenation. Because of the usual association with a PFO, there will be a right to left shunt, which maintains the cardiac output at the expense of desaturation.
Some patients with TV dysplasia will be diagnosed with functional pulmonary atresia. These patients will improve with conservative management including vent-ilation and transient administration of PG infusion to keep the DA patent with or without iNO administration until the PVR drops and the forward PBF improves. Some of these patients might need augmentation of the PBF by either PDA stenting or BT shunt. For our pati-ent: he did not improve with conservative management and ventilation. He received PG infusion, iNO, Sildenafil as well as Bosentan. Despite all of these mea-sures, saturation did not improve. The mechanism of TV regurge in this patient was due to a flail anterior TV leaflet, in which there was a significant prolapse and overshooting of the leaflet beyond the coaptation points resulting in severe eccentric TR [10]. What was strange in this patient is the resistance to high doses of PG infusion and even the difficulty of probing the DA. The resistance of DA to PG could be hypothesized by the constriction of the DA and absence of left to right shunt immediately after birth, which limits the ability of the ductus to respond to PG therapy [11]. In patients with tricuspid dysplasia and TR, the forward PBF will decreese. This can be judged from the amount of blood crossing the RVOT and pulmonary valve using color Doppler as well as by calculation of the Qp: Qs by echocardiography utilizing the continuity equation. This should be combined with the clinical condition and the other echocardiographic findings. The density of the pu-lsed Doppler tracing as well as the time velocity integral (TVi) across the RVOT and the pulmonary valve was less than that across the LVOT and aortic valve [12].
For our patient, there was a clear increase in the color Doppler density and the density of PW Doppler and TVi after surgery. Because of similarities between TV dysplasia associated with functional pulmonary atresia and isolated tricuspid dysplasia giving a picture similar to that of functional pulmonary atresia, it might be reasonable to try conservative management including measures to reduce the PVR and to keep the DA patent. If there is no response and there are clear signs of flail TV leaflets, cardiac surgery should be performed [10]. Some might require extracorporeal membrane oxygen-ation (ECMO) as a bridge to surgical intervention [13]. The ideal surgery will be TV repair with the creation of artificial chordae to support the flail leaflets with some annuloplasty and partial closure of the foramen ovale [14]. PDA stenting or BT shunts are other options if TV repair cannot be done (unfit for CPB, or inexperienced surgeon). PDA stent or BT shunt shouldn't be performed in a patient with pulmonary insufficiency to avoid the creation of a loop shunt and stealing of blood from the systemic circulation which might lead to low cardiac output and multi-organ failure with renal and gastroint-estinal hypoperfusion. The reported short and midterm results of TV repair for a ruptured chordae are good [5]. We reviewed the literature for cases diagnosed with TV dysplasia in the neonatal period and found 15 publica-tions, all of which are either case reports or case series. This is represented in Table 1. It is clear from the review that patients with a proven diagnosis of flail TV leaflet will not improve except after repair of the TV. In addition to the regular scheme of management (ventila-tion, PG infusion, and iNO), some of them required ECMO support as a bridge to the surgical intervention (Table 1).
|
No |
Author |
Journal |
Publication date |
Cases description |
Intervention |
ECMO |
|
1 |
Cardiol Young |
2004 Aug |
A neonate with cyanosis & TVR due to ruptured PM |
Repair of the tricuspid valve [15] |
||
|
2 |
Ann Thorac Surg |
2007 Feb |
Two neonates with cyanosis & TVR due to ruptured PM |
Repair of the tricuspid valve [16] |
Yes |
|
|
3 |
Pediatr Cardiol |
2008 Mar |
A 3-month-old infant with a flail tricuspid valve, & auto-immune-mediated heart block with endocardial fibroelastosis diagnosed after birth |
TV repair at 3 months of age [17] |
Yes |
|
|
4 |
Eur J Pediatr |
2010 Feb |
neonate with cyanosis & TVR due to ruptured PM |
Repair of the tricuspid valve [7] |
||
|
5 |
J Saudi Heart Assoc |
2011 Jan |
A neonate with cyanosis & TVR due to ruptured PM |
Supportive conserva-tive management [18] |
||
|
6 |
Korean J Thorac Cardio-vasc Surg |
2014 Aug |
A neonate with cyanosis & TVR due to ruptured PM |
Repair of the tricuspid valve [19] |
Yes |
|
|
8 |
Echocardiography |
2014 May |
A neonate with cyanosis & TVR due to ruptured PM |
Supportive conservative management [20] |
||
|
9 |
Korean Circ J |
2015 Jul |
A neonate with cyanosis & TVR due to ruptured PM |
Repair of the tricuspid valve [4] |
Yes |
|
|
10 |
Tarca AJ, et al |
Cardiol Young |
2017 Sep |
A neonate with cyanosis & TVR due to ruptured PM associated with SLE |
Repair of the tricuspid valve [21] |
|
|
11 |
J Med Ultrason (2001) |
2018 Apr |
A neonate with cyanosis & TVR due to ruptured PM |
Repair of the tricuspid valve [22] |
||
|
12 |
J Thorac Dis |
2018 Mar |
A neonate with cyanosis & TVR due to ruptured PM |
Repair of the tricuspid valve [5] |
||
|
14 |
World J Pediatr Congenit Heart Surg |
2020 Sep |
Five neonates with cyanosis & TVR due to ruptured PM |
Repair of the tricuspid valve [23] |
Table 1: Published case reports and case series of neonates with Tricuspid valve dysplasia and how they were managed.
4. Conclusion
Tricuspid dysplasia with ruptured chordae and flail leaflets might lead to significant cyanosis. Some patients might respond to conservative treatment, giving chance for the PVR to drop and the forward PBF to improve. If no improvement, TV repair is the best surgical option with good immediate as well as the short and mid-term outcome.
Declaration
Ethics approval and consent to participate
Approval from PSCC- Qassim IRB was achieved for the production and publication of this manuscript. Written consent was obtained from the father for the intervention and he was informed that anonymous data will be used for research purposes and publication.
Consent for publication
Written parental consent was taken for anonymous use of patient's data for publication.
Availability of data and material
Echocardiography, Cardiac catheterization, and surgical images are available.
Competing interests
All authors have completed the ICMJE uniform disclosure form. The authors have no conflicts of interest to declare.
Funding
No financial support was received for the production of this manuscript.
References
- Sana M, Edinen A, Siddiqui WJ. Tricuspid Regurgitation. StatPearls [Internet] Treasure Isl StatPearls Publ (2021).
- Altun G, Babaoglu K, Binnetoglu K, et al. Functional pulmonary atresia in newborn with normal intracardiac anatomy: Successful treatment with inhaled nitric oxide and pulmonary vasodilators. Ann Pediatr Cardiol 6 (2013): 83.
- Akkinapally S, Hundalani SG, Kulkarni M, et al. Prostaglandin E1 for maintaining ductal patency in neonates with ductal-dependent cardiac lesions. Cochrane Database Syst Rev (2018).
- Yoon JK, Kim HR, Kwon HW, et al. Ruptured Tricuspid Valve Papillary Muscle in a Neonate with Intractable Persistent Fetal Circulation. Korean Circ J 45 (2015): 340.
- Pan J-Y, Lin C-C, Chang J-P. Successful repair of neonatal tricuspid regurgitation due to chordae rupture. J Thorac Dis 10 (2018): E186-E188.
- Anagnostopoulos P V, Alphonso N, Nölke L, et al. Neonatal Mitral and Tricuspid Valve Repair for In Utero Papillary Muscle Rupture. Ann Thorac Surg 83 (2007): 1458-1462.
- Riede FT, Dähnert I, Razek V, et al. Rupture of the papillary muscle of the tricuspid valve — echocardiographic diagnosis of a rare anomaly leading to critical tricuspid valve regurgitation in the newborn. Eur J Pediatr 169 (2010): 165-166.
- Qureshi MY, Sommer RJ, Cabalka AK. Tricuspid Valve Imaging and Intervention in Pediatric and Adult Patients With Congenital Heart Disease. JACC Cardiovasc Imaging 12 (2019): 637-651.
- Vettukattil JJ, Black D. Tricuspid Valve Dysplasia: From Foetus to Adult. In: Alessandro Giamberti MC, ed. The Tricuspid Valve in Congenital Heart Disease. Milano: Springer Milan (2014): 13-23.
- Loftus PD, Arrington CB, Kaza AK. Neonatal Flail Tricuspid Valve: Diagnosis and Management. Ann Thorac Surg 98 (2014): 1098-1101.
- Clyman RI, Mauray F, Roman C, et al. Factors determining the loss of ductus arteriosus responsiveness to prostaglandin E. Circulation 68 (1983): 433-436.
- Cardoso AA, García-Fernández MA, Moreno M. Calculation of cardiac output using Doppler echocardiography. Rev Port Cardiol 8 (1989): 183-188.
- Arrington CB, Kouretas PC, Mart CR. Extracorporeal membrane oxygenation as a bridge to surgical treatment of flail tricuspid valve in a neonate. Cardiol Young 15 (2005): 660.
- Boon R, Hazekamp M, Hoohenkerk G, et al. Artificial chordae for pediatric mitral and tricuspid valve repair?. Eur J Cardio-Thoracic Surg 32 (2007): 143-148.
- Lim KA, Huh J, Jun T-G. Successful repair of critical tricuspid regurgitation secondary to ruptured papillary muscle in a newborn. Cardiol Young 14 (2004): 450-452.
- Sachdeva R, Fiser RT, Morrow WR, et al. Ruptured Tricuspid Valve Papillary Muscle: A Treatable Cause of Neonatal Cyanosis. Ann Thorac Surg 83 (2007).
- Fleming GA, Scholl FG, Kavanaugh-McHugh A, et al. A Case of an Infant with Flail Tricuspid Valve Due to Spontaneous Papillary Muscle Rupture: Was Neonatal Lupus the Culprit?. Pediatr Cardiol 29 (2008): 442-445.
- Abd El Rahman MY, Al Qurashi MM, Al Khalifeh FA. Unusual cause of neonatal cyanosis. J Saudi Hear Assoc 23 (2011): 45-47.
- Min J, Kim ER, Yang CK, et al. Successful repair of critical tricuspid regurgitation secondary to a ruptured papillary muscle in a neonate. Korean J Thorac Cardiovasc Surg 47 (2014).
- Yemul MA. A flail anterior tricuspid leaflet secondary to anteroposterior papillary muscle rupture in a neonate. Echocardiography 31 (2014).
- Tarca AJ, Eckersley L, Kothari D. Flail anterior tricuspid valve leaflet in a neonate: Association with maternal antiphospholipid syndrome. Cardiol Young 27 (2017).
- Inatomi A, Sasahara J, Ishii K, et al. Prenatal diagnosis of premature constriction of the ductus arteriosus with tricuspid papillary muscle rupture: a case report. J Med Ultrason 45 (2018): 337-340.
- Roy Chowdhuri K, Dutta N, Raja N, et al. Mid-Term Follow-Up of Neonatal Neochordal Reconstruction of Tricuspid Valve for Perinatal Chordal Rupture Causing Severe Tricuspid Valve Regurgitation. World J Pediatr Congenit Hear Surg 11 (2020): 587-594.