Comprehensive Comparison of Diagnostic Accuracy of Ultrasound and Mammography in Young Women with Radiographically Dense Breasts
Article Information
Jitendra Parmar1*, Sumita Choudhary1, Anagha Zope2, Tapan Patel1, Nishith Chaudhari1, Sandip Shah1, Maulik Vora3, Harsh Vyas1
1Department of Radiology, Apollo Hospitals International Limited, Ahmedabad, India
2Department of Surgical Oncology, Apollo Hospitals International Limited and Apollo Comprehensive Blood & Cancer Center, India
3Department of Radiology, Indira Gandhi Medical College, Shimla, India
*Corresponding author: Jitendra Parmar, Department of Radiology, Apollo Hospitals International Limited, Ahmedabad, India.
Received: 28 February 2022; Accepted: 08 March 2022; Published: 16 March 2022
Citation: Jitendra Parmar, Sumita Choudhary, Anagha Zope, Tapan Patel, Nishith Chaudhari, Sandip Shah, Maulik Vora, Harsh Vyas. Comprehensive Comparison of Diagnostic Accuracy of Ultrasound and Mammography in Young Women with Radiographically Dense Breasts. Archives of Clinical and Biomedical Research 6 (2022): 308-321.
Share at FacebookAbstract
Purpose: To study the comparative accuracy of mammography and ultrasound in young females of 45 years or younger with dense breasts.
Material and Methods: All 134 patients have undergone mammography followed by ultrasound, which was independently analyzed by two different teams of radiologists. The measures of diagnostic accuracy were calculated keeping histopathology as the gold standard. The sensitivity and specificity of the two tests were compared in all subjects using McNemar’s chi-square test.
Results: Out of 134 patients included, 44 patients were positive and 90 patients were negative for breast malignancy. Invasive ductal carcinoma was the most common malignancy (36 patients). The combined assessment (Mammography + Ultrasound) had the highest sensitivity (97.7%) and specificity (95.6%) in the diagnosis of breast malignancy. Ultrasound had higher sensitivity (95.5%) than mammography (79.5%). However, mammography had higher specificity (94.4%) than ultrasound (91.1%). The results between mammography and ultrasound were statistically significant with a p-value of <0.05.
Conclusion: In conclusion, breast ultrasound is more accurate than mammography in young females with radiographically dense breast tissue and may be an appropriate initial imaging test in these women.
Keywords
Breast Malignancy; Dense Breast; Mammography; Ultrasound; Young female
Breast Malignancy articles; Dense Breast articles; Mammography articles; Ultrasound articles; Young female articles
Article Details
1. Introduction
Breast malignancy is the most common malignancy in the world and also the most common type of malignancy among women [1]. The rising malignancy rates will be disproportionately higher in developing countries to increase in incidence and greater mortality in coming years [2-4]. According to Globocon 2018, the number of new cases of breast malignancy among women of all ages in India was found to be 27.7% of all malignancies [5]. These statistics are even worse in Ahmedabad, a metro city of Gujarat state, where it represents an incidence rate of approximately
31.5 % among all malignancy types in women. Data from the National Cancer Registry Programme of 2012 to 2014 shows, there are increasing cases of breast malignancy in young women [6]. Breast malignancy may exhibit more aggressive behavior and poor prognosis in young women [7,8]. Factors connected with worse prognosis are often seen in younger individuals, which includes larger tumor size, higher histological grading, involvement of vessels or lymph nodes, no hormone receptors, tumors with a high S-phase fraction [9-15] and some studies suggest age as an independent prognostic factor of local and distal recurrences [12]. In this group, the delay in the diagnosis of breast malignancy is a common problem by many factors such as lack of awareness about the disease and consequent delay in health care demand, lack of screening programs in this age group, rapid tumor growth and dense breast parenchyma, which can hinder the identification of lesions in clinical examinations [16,17]. Mammography is the gold standard imaging modality for breast malignancy screening worldwide and with advances in treatment, resulted in reduced mortality. However, dense fibro glandular tissue is the most important inherent limitation of mammography, which may lower the sensitivity to as low as 30-48% and can hinder the early diagnosis of breast malignancy, thus often requiring additional modalities [18,19]. Moreover, dense fibro-glandular tissue per se is considered a high risk for developing malignancy [19,20]. A comprehensive review of the literature found very little evidence about the comparative accuracy of mammography and ultrasound in young women of 45 years or younger with dense breasts [21,22]. With an increasing incidence of breast malignancy in young women of 45 years old or young, dense fibro glandular tissue, lack of proper screening modality and well-recognized limitations of mammography, there is a need to formulate a better screening and diagnostic tool for breast malignancy in this age group.
2. Material and Methods
2.1. Aim of the Study
To study the comparison of diagnostic accuracy of mammography and ultrasound in women of 45 years or younger with radiographically dense breasts.
2.2. Study Design
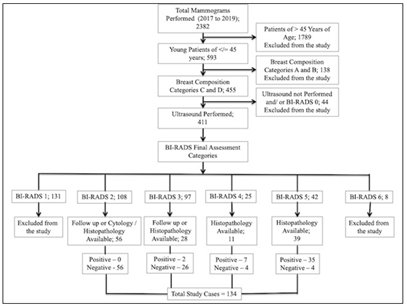
Potential subjects included all female patients of 45 years of age or younger referred for breast imaging to the radiology department from 2017 to 2019. Women referred for mammograms were X-Rayed by the institutional breast cancer clinical guidelines. The patients with radiographically dense breasts (American College of Radiology (ACR) Breast Imaging-Reporting and Data System (BI-RADS) density category of C and D) were consecutively recruited upon obtaining informed written consent to participate in the study [23]. The detailed study flow chart is depicted in (Figure 1). Mammograms were performed with Siemens Mammomat Select. Craniocaudal & mediolateral oblique views were taken in each case. Additional views were performed in case of any pathology, e.g., spot compression view for better visualization of lesions margins and magnification view for microcalcifications. The mammograms were subsequently viewed systematically on a dedicated mammography film viewer box by a Consultant Radiologist. A GE Logiq S7 with XDclear Ultrasound Machine, with a 7.5-12-Megahertz linear probe was used to scan the breasts by another Consultant Radiologist, who was blinded to the mammographic findings. Breast imaging interpretations were performed by two different Consultant Radiologists each for mammography and ultrasound, with BI-RADS atlas available for reference to ensure correct interpretation was made [23-25]. The reports were recorded on the standardized form using the BIRADS categorical scale of 1–5 with an increasing level of suspicion (1 = no significant abnormality, 2 = benign, 3 = indeterminate, 4 = suspicious, and 5 = malignant). The patients with benign and probably benign pathology on imaging and who did not undergo surgery or biopsy were followed up for at least 1 year and a maximum of 2 years to confirm the stability of the disease. Seventeen eligible patients (3 patients of BI-RADS 5 and 14 patients of BI-RADS 4) were excluded because histopathology or follow up was not available. Patients with malignancy included all women in the defined inclusion criteria proven to have a breast malignancy on histopathology. The patients were classified as not having breast malignancy based on the diagnostic assessment, stability on follow-up imaging and/or histopathology. Ethics statement: The institutional ethics committee and scientific research committee approved the study. Informed consent: All the subjects provided informed written consent for the study and publication.
2.3. Statistical Analysis
2.3.1. Type of study – Prospective observational study.
2.3.2. Statistical Methods and Data Analysis
Logistic regression analysis and cross-tabulations were performed using ‘Statistical Package for the Social Sciences’ Version 26. BI-RADS categories 2 and 3 were considered negative for malignancy, and BI-RADS categories 4-5 were positive for malignancy. The overall measures of diagnostic accuracy, including the sensitivity and specificity, Positive and Negative Predictive Value (PPV and NPV), Likelihood Ratio (LR+ and LR-), the area under the Receiver Operating Characteristic (ROC) curve, Youden's index and diagnostic odds ratio were calculated keeping histopathology as the gold standard. The sensitivity and specificity of the two tests were compared in all subjects using McNemar’s chi-square test for paired proportions (accuracy of the two imaging tests in subjects); the 95% Confidence Interval (CI) for the difference between paired proportions was also calculated.
3. Results
A total of 2382 mammograms were performed during 2 years of the period from 2017 to 2019. 1789 patients were older than 45 years of age and excluded from the study. Out of the remaining 593 mammograms of younger patients less than 45 years of age, 138 had Breast Composition Categories of A and B based on ACR BI-RADS® Atlas and were excluded from the study. Mammograms of 411 patients out of the remaining 455 were of Breast Composition Categories of C and D, who underwent ultrasound scan and were assigned BI-RADS final assessment category. BI-RADS category 1 (131 mammograms) and 6 (8 mammograms) were excluded from the study. Among the total 272 mammograms of BI-RADS final assessment category 2, 3, 4 and 5, 62 patients (39 of BI-RADS 5, 11 of BI-RADS 4 and 12 of BI-RADS 3) underwent Tru-cut biopsy for histopathological correlation, and 72 (rest of 16 patients of BI-RADS 3 and 56 patients of BI- RADS 2) were followed subsequently to confirm the stability of the disease. Thus, a total of 134 patients were included in the study who were less than 45 years of age with Breast Composition Categories of C and D, who underwent ultrasound scan and had an outcome either by Tru-cut biopsy or by follow up ultrasound scan for the stability of the disease. 134 patients were enrolled in the study from a total of 2382 mammograms performed during 2 years of the period from 2017 to 2019. Out of 134 cases, 44 cases were positive for breast cancer based on histopathological analysis. The age ranged from 21 to 45 years with a mean age of 38.45 years. The youngest patient diagnosed with malignancy was about 24 years of age. Breast lump with or without discomfort or pain was found to be the most common presentation and was present in 73 patients followed by pain or localized discomfort in 31 patients, localized lumpiness or nodularity in 23 patients, nipple discharge (non-bloody) in 6 patients and nipple discharge (bloody) in 1 patient. Lump most commonly involved upper outer quadrant followed by upper inner; lower outer and lower inner quadrants in that sequence. Based on BI-RADS mammographic descriptors, 40 mammograms were suggestive or suspicious of malignancy. 32 mammograms had identifiable mass, 6 mammograms had an asymmetric density and only microcalcifications were present in 2 mammograms. Based on BIRADS ultrasound descriptors, 50 cases were suggestive or suspicious of malignancy. The combined BI-RADS assessment category (Mammography + Ultrasound) was also assessed and 47 cases were suspected or suggestive of malignancy. The comparative frequency of breast lesions based on each modality is given in (Table 1).
|
Mammography |
Ultrasound |
Combined |
Histopathology |
||
|
Malignant |
40 (29.85%) |
50 (37.31%) |
47 (35.1%) |
44 (32.83%) |
|
|
Non – Malignant |
94 (70.15%) |
84 (62.69%) |
87 (64.9%) |
90 (67.17%) |
|
|
Total |
134 (100%) |
134 (100%) |
134 (100%) |
134 (100%) |
|
|
Note – Percentages indicate the proportion of all cases. |
|||||
Table 1: Breast Lesions on Mammography, Ultrasound and Combined Assessment.
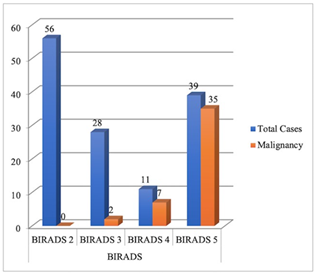
Subsequently, 62 patients (39 of BI-RADS 5, 11 of BI-RADS 4 and 12 of BI-RADS 3) underwent a Tru-cut biopsy for histopathological correlation, out of which 43 lesions turned out to be malignant (35 of BI-RADS 5, 7 of BI-RADS 4 and 1 of BI-RADS 3). The rest of 16 patients of BI-RADS 3 and 56 patients of BI-RADS 2 were followed subsequently to confirm the stability of the disease. Out of which, 1 patient of BI-RADS 3 revealed an increase in the size of the lesion and was subsequently diagnosed with having malignancy. Thus, the final number of cases of malignant pathology was 44, and 90 cases were of non-malignant pathology. The malignancy yield is comparable to the expected rate of malignancy in BI-RADS final assessment categories (Figure 2). Invasive Ductal Carcinoma (IDC) was the most common malignancy in 36 patients followed by Ductal Carcinoma in Situ (DCIS) in 4 patients. Among the non-malignant pathologies, Fibroadenoma (Including Typical, Atypical and Involuted) was the most common pathology in 29 patients followed by Fibrocystic Disease in 28 patients. The mammography and ultrasound features of histopathologically proven malignancy are summarized in (Table 2).
|
Mammography |
N |
Ultrasound |
N |
|
Breast Composition |
Tissue Composition |
||
|
Heterogeneously dense parenchyma |
35 |
Predominantly fibro-glandular tissue |
6 |
|
Extremely dense parenchyma |
9 |
Heterogeneous echotexture |
38 |
|
Mass |
Mass |
||
|
Mass Characteristics |
Mass Characteristics |
||
|
Shape -Round |
2 |
Shape -Round |
3 |
|
-Oval |
10 |
-Oval |
16 |
|
-Irregular |
20 |
-Irregular |
25 |
|
Margin -Circumscribed |
4 |
Margin -Circumscribed |
8 |
|
-Obscured |
4 |
-Microlobulated |
5 |
|
-Microlobulated |
4 |
-Indistinct |
9 |
|
-Indistinct |
4 |
-Spiculated |
22 |
|
-Spiculated |
16 |
||
|
Density -High |
28 |
Echo pattern -Anechoic |
1 |
|
-Equal |
4 |
-Hypoechoic |
28 |
|
-Low |
0 |
-Heterogenous |
15 |
|
Suspicious Calcifications |
15 |
Calcifications |
19 |
|
Asymmetric Density |
6 |
Vascularity |
18 |
|
Architectural Distortion |
12 |
Posterior Acoustic Shadowing |
28 |
|
Axillary Lymphadenopathy |
17 |
Axillary Lymphadenopathy |
25 |
|
Associated Features |
Elasticity -Soft |
1 |
|
|
Skin Thickening |
12 |
-Intermediate |
27 |
|
Nipple Retraction |
3 |
-Hard |
18 |
Table 2: Imaging Features of Malignant Cases.
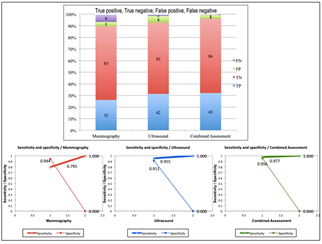
The type and frequency of non-malignant and malignant pathologies are summarized in Table 3. The combined assessment had the highest sensitivity (97.7%) and specificity (95.6%) in the diagnosis of breast malignancy. Ultrasound had higher sensitivity (95.5%) than mammography (79.5%). However, mammography had higher specificity (94.4%) than ultrasound (91.1%). True Positive (TP), True Negative (TN), False Positive (FP), and False Negative (FN) cases and comparative sensitivity and specificity of mammography, ultrasound and combined assessment are given in (Figure 3).
|
Malignant Pathology |
N (%) |
Non-Malignant Pathology |
N (%) |
|
Invasive Ductal Carcinoma |
36 (81.8) |
Fibroadenoma |
29 (32.2) |
|
Ductal Carcinoma In Situ |
4 (9) |
Fibrocystic Disease |
28 (31.1) |
|
Lobular |
1 (2.3) |
Duct Ectasia |
4 (4.4) |
|
Mucinous |
1 (2.3) |
Papilloma |
3 (3.3) |
|
Papillary |
1 (2.3) |
Hamartoma |
3 (3.3) |
|
Malignant Phyllodes |
1 (2.3) |
Others* |
23 (25.6) |
|
Total |
44 |
Total |
90 (100) |
|
Others* include Multiple Large Rod-Like Calcifications, Oil Cysts, Fat necrosis, Bilateral Lymph nodes (3 cases of each pathology); Sebaceous Cyst, Fibrosclerosis, Lipoma, Mastitis (2 cases of each pathology); Galactocele, Fibro-adenomatoid Hyperplasia, Radial Scar (1 case of each pathology). Note - Percentages indicate the proportion of all cases |
|||
Table 3: Malignant and Non-Malignant Pathology.
The difference between the results of combined assessment and ultrasound were not statistically significant with a p-value of >0.05. However, the results between combined assessment and mammography as well as between ultrasound and mammography were statistically significant with a p-value of <0.05. The 2 x 2 contingency table and comparative sensitivity and specificity of ultrasound and mammography are given in Table 4. The measures of diagnostic accuracy of each modality are given in detail in Table 5.
|
Ultrasound |
||||||
|
Positive (%) |
Negative (%) |
Total (%) |
||||
|
Mammography |
Positive (%) |
38 (28.4) |
2 (1.5) |
40 (29.9) |
79.50% |
|
|
Negative (%) |
12 (8.9) |
82 (61.2) |
94 (70.1) |
94.40% |
||
|
Total (%) |
50 (37.3) |
84 (62.7) |
134 (100) |
|||
|
95.5%a |
91.1%b |
|||||
|
Note – Percentages indicate the proportion of all cases. |
||||||
Table 4: 2x2 Contingency Table-Comparative Sensitivity and Specificity of Ultrasound and Mammography.
|
Accuracy Measures |
Combined (95 % CI) |
Ultrasound (95 % CI) |
Mammography (95 % CI) |
|
Accuracy |
0.963 (0.995 - 0.931) |
0.925 (0.970 - 0.881) |
0.890 (0.947 - 0.844) |
|
Misclassification |
0.037 (0.069 - 0.005) |
0.075 (0.119 - 0.030) |
0.104 (0.156 - 0.053) |
|
Sensitivity |
0.977 (1.000 - 0.869) |
0.955 (0.995 - 0.838) |
0.795 (0.890 - 0.652) |
|
Specificity |
0.956 (0.986 - 0.887) |
0.911 (0.956 - 0.831) |
0.944 (0.979 - 0.872) |
|
False positive rate |
0.044 (0.860 - 0.003) |
0.089 (0.146 -0.031) |
0.056 (0.102 - 0.009) |
|
False negative rate |
0.023 (0.065 - 0.000) |
0.045 (0.104 - 0.000) |
0.204 (0.319 - 0.090) |
|
Prevalence |
0.328 (0.408 - 0.249) |
0.328 (0.408 - 0.249) |
0.328 (0.408 - 0.249) |
|
PPV |
0.915 (0.995 - 0.835) |
0.840 (0.942 - 0.738) |
0.875 (0.977 - 0.773) |
|
NPV |
0.989 (1.000 - 0.966) |
0.976 (1.000 - 0.944) |
0.904 (0.845 - 0.964) |
|
LR+ |
21.99 (57.37 - 8.428) |
10.74 (20.87 - 5.525) |
14.32 (34.00 - 6.029) |
|
LR- |
0.024 (0.165 - 0.003) |
0.050 (0.194 - 0.013) |
0.217 (0.389 - 0.121) |
|
Relative risk |
79.60 (389.9 - 16.25) |
35.28 (120.3 - 10.34) |
66.11 (202.8 - 21.56) |
|
Odds ratio |
924.5 (6079.6 - 14.5) |
35.28 (120.3 - 10.34) |
66.11 (202.8 - 21.56) |
|
Youden's index |
0.933 |
0.866 |
0.739 |
|
Note-CI: Confidence Interval; PPV: - Positive Predictive Value; NPV: Negative Predictive Value; LR+ : Positive Likelihood Ratio; LR: Negative Likelihood Ratio |
|||
Table 5: Measures of Diagnostic Accuracy.
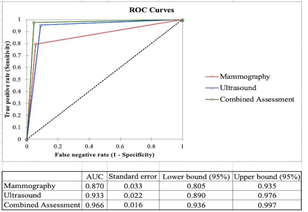
Comparative ROC analysis is given in Figure 4. It demonstrated Area Under Curve (AUC) is more than 0.9 for combined assessment and ultrasound, suggesting excellent tests for diagnosis of breast malignancy as compared to mammography, which has an AUC of 0.87, suggesting a good test.
4. Discussion
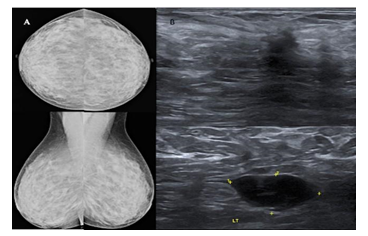
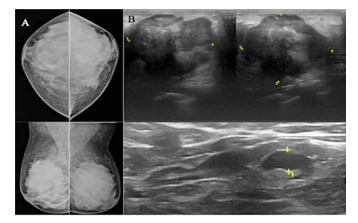
The definition of a “young woman” in the breast oncology field varies, with most literature referring to women under ages 35, 40, or 45 as “young” [3]. The present study used a comparative prospective study design of the two imaging tests in young women (45 years old or younger) with radiographically dense breasts (BI-RADS C & D), which allowed the calculation of the measures of diagnostic accuracy of each test independently of the other. We carried out short-term interval follow up of breast mass lesions categorized as benign and probably benign (BI-RADS 2 and BI- RADS 3 respectively) to ascertain radiological and clinical stability of these mass lesions. Few of them (12 of BI-RADS 3 lesions) underwent biopsy as well; hence sensitivity and specificity of breast ultrasound and mammography could be calculated. The performance of mammography in young women with dense breasts is poor, because of a similar density of tumors and glandular tissue [26-30]. In our study, the total malignancy rate was 32.83%, significantly higher as compared to previous studies by Okello et al. [21] and Paulo et al. [31] who reported prevalence of 14.9% and 4.2%, respectively among symptomatic patients with dense mammograms. The sensitivity of ultrasound is higher than mammography and with nearly similar specificity in young women. Overall, the difference in the sensitivity of the two tests in subjects is statistically significant (p - <0.05). Houssami et al. [22] published a similar kind of comparative study of the two imaging tests in symptomatic young women in 2003, however, the study was retrospective and included lesions in all types of breast density. It concluded ultrasound as a more accurate imaging modality in women 45 years or younger as the sensitivity of ultrasound was 13.2% greater than that of mammography. In this study, the sensitivity of ultrasound is 16% greater than that of mammography, confirming a similar experience. Figure 5 shows a case of missed mass lesion on a mammogram in a 37-year- old patient who presented with a painful lump in the left breast, which was picked up comfortably on ultrasound. Mammography was marginally (3.3%) more specific than ultrasound, however, this difference was not statistically significant (p - >0.05). This fact is consistent with the study published by Houssami et al. [22]. However, some have reported a greater specificity for ultrasound than for mammography [32-34], and others reporting a greater specificity for mammography than for ultrasound [35,36]. Combined assessment (mammography and ultrasound) had a greater sensitivity and specificity (97.7% & 95.6%) than either ultrasound alone (95.5% & 91.1%) or mammography alone (79.5% & 94.4%) in this study. These findings are contradictory to other studies of the accuracy of combined mammography and ultrasound, which suggest higher sensitivity, but lesser specificity when combined [22,37]. The negative likelihood ratio is higher in mammography (0.217) than ultrasound (0.050), which confers that mammography alone, does not help rule out malignancy; hence it missed more cancers, so requires additional imaging (ultrasound or Magnetic Resonance Imaging) to rule out malignancy. The negative likelihood ratio of combined assessment and ultrasound is significantly lower, which means negative ultrasound helps in ruling out malignancy in most cases [38]. (Figure 6) shows a case of poorly appreciated mass lesion in a 25-year-old patient with extremely dense breast presented with a painless lump in the left breast, which was well appreciated on ultrasound.
Figure 5: 37-year-old patient presented with a painful lump in the left breast. Mammogram (a) of both breasts shows heterogeneously dense breast parenchyma without any obvious abnormality. Ultrasound (b) of the left breast and left axillary region shows an irregular shaped hypoechoic taller lesion with spiculated margins and posterior acoustic shadowing in the left breast with a hypoechoic lymph node with heterogeneous and thickened cortex in the axillary region. On histopathology, it turned out to be grade II invasive ductal carcinoma with mucinous differentiation with metastatic axillary lymphadenopathy.
According to the latest BI-RADS atlas, it is mandatory to have a further additional imaging evaluation in a mammographically dense breast to make a conclusive radiological diagnosis, most commonly accomplished by high-frequency breast ultrasound and rarely requires an MRI scan [19,31]. In addition, several adverse factors may limit the optimum use of mammography in breast cancer detection, such as increasing incidence of breast cancer in younger women, who frequently have dense fibro-glandular tissue and are more sensitive to ionizing radiation. In contrast, ultrasound has several inherent advantages, which justifies its use over mammography in young women, including cost-effectiveness, easy availability, no significant limitation by fibro-glandular breast composition, use for image-guided procedures, and easy portability [22, 28-30].
Figure 6: 25-year-old-patient presented with a painless lump in the left breast. Mammogram (a) of both breasts shows extremely dense breast parenchyma. A poorly visualized lesion is seen in the lateral quadrant of the left breast, only visualized on the CC view and not visualized on the MLO view. Ultrasound (b) of the left breast and left axillary region shows a well-defined, irregular shaped, heterogeneous mass lesion with microlobulated margins and posterior acoustic shadowing in the lateral quadrant of the left breast with a solitary lymph node showing focal cortical thickening in the axillary region. On histopathology, it turned out to be triple-negative, grade III invasive ductal carcinoma with undifferentiated components.
5. Conclusion
In conclusion, ultrasound is more accurate and sensitive than mammography with nearly similar specificity in young women (45 years or younger) with dense breast tissue and may be an appropriate initial imaging test in those women.
Declarations
Ethics approval and consent to participate
The institutional ethics committee and scientific research committee approved the study. Written informed consent was taken from all the participants and they were informed of the ethical codes of conduct.
Consent for publication
The written informed consent was taken from all the participants.
Availability of data and materials
All data is available based on a reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
This Research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgement
We are very much thankful to the patients who gave their consent to be a part of the study and appreciate their help and sharing the details at various stages of this research. We wish all of them a long disease-free healthy life.
References
- Kelly KM, Dean J, Comulada WS, et al. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol 20 (2010): 734-742.
- Agarwal G, Pradeep PV, Aggarwal V, et al. Spectrum of breast cancer in Asian women. World J Surg 31 (2007): 1031-1040.
- Villarreal-Garza C, Aguila C, Magallanes-Hoyos MC, et al. Breast cancer in young women in Latin America: an unmet, growing burden. Oncologist 18 (2013): 1298-1306.
- Anderson BO, Cazap E, El Saghir NS, et al. Optimisation of breast cancer management in low-resource and middle-resource countries: executive summary of the Breast Health Global Initiative consensus, 2010. Lancet Oncol 12 (2011): 387-398.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 68 (2018): 394-424.
- National cancer registry programme (2016). Three-year report of population based cancer registries: (2012-2014).
- Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression [published correction appears in J Clin Oncol 26 (2008): 3324-3330.
- Gewefel H, Salhia B. Breast cancer in adolescent and young adult women. Clin Breast cancer 14 (2014): 390-395.
- Gajdos C, Tartter PI, Bleiweiss IJ, et al. Stage 0 to stage III breast cancer in young women. J Am Coll Surg 190 (2000): 523-529.
- Vrieling C, Collette L, Fourquet A, et al. Can patient-, treatment- and pathology-related characteristics explain the high local recurrence rate following breast-conserving therapy in young patients?. Eur J Cancer 39 (2003): 932-944.
- Chung M, Chang HR, Bland KI, et al. Younger women with breast carcinoma have a poorer prognosis than older women. Cancer 77 (1996): 97-103.
- Bertheau P, Steinberg SM, Cowan K, et al. Breast cancer in young women: clinicopathologic correlation. Semin Diagn Pathol 16 (1999): 248-256.
- Sidoni A, Cavaliere A, Bellezza G, et al. Breast cancer in young women: clinicopathological features and biological specificity. Breast 12 (2003): 247-250.
- Sundquist M, Thorstenson S, Brudin L, et al. Incidence and prognosis in early onset breast cancer. Breast 11 (2002): 30-35.
- Dieci MV, Orvieto E, Dominici M, et al. Rare breast cancer subtypes: histological, molecular, and clinical peculiarities. Oncologist 19 (2014): 805-813.
- Samphao S, Wheeler AJ, Rafferty E, et al. Diagnosis of breast cancer in women age 40 and younger: delays in diagnosis result from underuse of genetic testing and breast imaging. Am J Surg 198 (2009): 538-543.
- Lee HB, Han W. Unique features of young age breast cancer and its management. J Breast Cancer 17 (2014): 301-307.
- Kelemen LE, Pankratz VS, Sellers TA, et al. Age-specific trends in mammographic density: the Minnesota Breast Cancer Family Study. Am J Epidemiol 167 (2008): 1027-1036.
- Hersh MR. Imaging the dense breast. Appl Radiol 33 (2004): 22.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med 356 (2007): 227-236.
- Okello J, Kisembo H, Bugeza S, et al. Breast cancer detection using sonography in women with mammographically dense breasts. BMC Med Imaging 14 (2014): 41.
- Houssami N, Irwig L, Simpson JM, et al. Sydney Breast Imaging Accuracy Study: Comparative sensitivity and specificity of mammography and sonography in young women with symptoms. AJR Am J Roentgenol 180 (2003): 935-940.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS® In: ACR BI- RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology (2013).
- Mendelson EB, Böhm-Vélez M, Berg WA, et al. ACR BI-RADS ultrasound. In: D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA, editors. ACR BI-RADS Atlas: Breast Imaging Reporting and Data System. Reston: American College of Radiology (2013).
- D’Orsi CJ, Sickles EA, Mendelson EB, et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology (2013).
- Galukande M, Kiguli-Malwadde E. Rethinking breast cancer screening strategies in resource-limited Settings. Afr Health Sci 10 (2010): 89-92.
- Buist DS, Porter PL, Lehman C, et al. Factors contributing to mammography failure in women aged 40-49 years. J Natl Cancer Inst 96 (2004): 1432-1440.
- Bevers TB, Anderson BO, Bonaccio E, et al. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw 7 (2009): 1060-1096.
- Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 299 (2008): 2151-2163.
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst 92 (2000): 1081-1087.
- Zanello PA, Robim AF, Oliveira TM, et al. Breast ultrasound diagnostic performance and outcomes for mass lesions using Breast Imaging Reporting and Data System category 0 mammogram. Clinics 66 (2011): 443-448.
- Stavros AT, Thickman D, Rapp CL, et al. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology 196 (1995): 123-134.
- Devolli-Disha E, Manxhuka-Kërliu S, Ymeri H, et al. Comparative accuracy of mammography and ultrasound in women with breast symptoms according to age and breast density. Bosn J Basic Med Sci 9 (2009): 131-136.
- Negri S, Bonetti F, Capitanio A, et al. Preoperative diagnostic accuracy of fine-needle aspiration in the management of breast lesions: comparison of specificity and sensitivity with clinical examination, mammography, echography, and thermography in 249 patients. Diagn Cytopathol 11 (1994): 4-8.
- Moss HA, Britton PD, Flower CD, et al. How reliable is modern breast imaging in differentiating benign from malignant breast lesions in the symptomatic population?. Clin Radiol 54 (1999): 676-682.
- Smallwood JA, Guyer P, Dewbury K, et al. The accuracy of ultrasound in the diagnosis of breast disease. Ann R Coll Surg Engl 68 (1986): 19-22.
- Houssami N, Irwig L, Loy C. Accuracy of combined breast imaging in young women. Breast 11 (2002): 36-40.
- Šimundic AM. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC 19 (2009): 203-211.