Comparison of Different Schedules of Rituximab and Chlorambucil in Previously Untreated Chronic Lymphocytic Leukemia: A Retrospective Study of Krohem
Article Information
Sandra Bašic-Kinda1, Ida Hude1, Ena Rankovic1*, Slobodanka Ostojic-Kolonic2, 3, Vlatko Pejša2, 4, Ivo Radman1, Martina Bogeljic-Patekar2, Ozren Jakšic2, 4, Klara Dubravcic1, Ivana Franic-Šimic1, Igor Aurer1, 2
1University Hospital Centre, Zagreb, Croatia
2Medical School, University of Zagreb, Zagreb, Croatia
3University Hospital Merkur, Zagreb, Croatia
4University Hospital Dubrava, Zagreb, Croatia
*Corresponding Author: Ena Rankovic, Division of Hematology, Department of Internal Medicine, University Hospital Centre Zagreb, Kispaticeva 12, HR-10000 Zagreb, Croatia
Received: 18 July 2021; Accepted: 14 September 2021; Published: 01 October 2021
Citation:
Sandra Bašić-Kinda, Ida Hude, Ena Ranković, Slobodanka Ostojić-Kolonić, Vlatko Pejša, Ivo Radman, Martina Bogeljić-Patekar, Ozren Jakšić, Klara Dubravčić, Ivana Franić-Šimić, Igor Aurer. Comparison of Different Schedules of Rituximab and Chlorambucil in Previously Untreated Chronic Lymphocytic Leukemia: A Retrospective Study of Krohem. Journal of Cancer Science and Clinical Therapeutics 5 (2021): 426-433.
Share at FacebookAbstract
Due to age and comorbidities many patients with CLL receive chlorambucil as front-line treatment. Doses and schedules of this drug vary widely but it is not clear whether this affects outcomes. We performed this retrospective analysis to compare the efficacy and toxicity of continuous high-dose chlorambucil (12-20 mg daily until response or toxicity) (cHD-Clb-R) and intermittent high dose chlorambucil (8-10 mg/m2 daily for 7 days q 4 wk) (iClb-R) in combination with rituximab (375 mg/m2/cycle for 8 cycles) in previously untreated CLL patients. Thirtysix patients received cHD-Clb-R and 32 iClb-R. Median age was 66 years (range 41-80); 24 were women and 44 men; 24 had Binet stage A, 27 B and 17 C; 5 had del(17p). Most common severe adverse events were granulocytopenia, occurring in 14; and infections in 7 patients, one of whom died. One patient stopped treatment due to hepatotoxicity. Both schedules resulted in similar toxicity and efficacy (cHD-Clb-R vs. iClb-R overall survival, progression-free survival and survival without next treatment at 30 mo. 70% vs. 83%, 49% vs. 55% and 67% vs. 75% respectively). Combinations of rituximab and chlorambucil are well tolerated and effective treatments for patients ineligible for fludarabine-based regimens. Outcomes seem to be related more to total drug doses than schedules.
B-chronic Lymphocytic Leukemia articles; Chlorambucil articles; Rituximab articles; Lymphoproliferative Disorder articles
B-chronic Lymphocytic Leukemia articles B-chronic Lymphocytic Leukemia Research articles B-chronic Lymphocytic Leukemia review articles B-chronic Lymphocytic Leukemia PubMed articles B-chronic Lymphocytic Leukemia PubMed Central articles B-chronic Lymphocytic Leukemia 2023 articles B-chronic Lymphocytic Leukemia 2024 articles B-chronic Lymphocytic Leukemia Scopus articles B-chronic Lymphocytic Leukemia impact factor journals B-chronic Lymphocytic Leukemia Scopus journals B-chronic Lymphocytic Leukemia PubMed journals B-chronic Lymphocytic Leukemia medical journals B-chronic Lymphocytic Leukemia free journals B-chronic Lymphocytic Leukemia best journals B-chronic Lymphocytic Leukemia top journals B-chronic Lymphocytic Leukemia free medical journals B-chronic Lymphocytic Leukemia famous journals B-chronic Lymphocytic Leukemia Google Scholar indexed journals Chlorambucil articles Chlorambucil Research articles Chlorambucil review articles Chlorambucil PubMed articles Chlorambucil PubMed Central articles Chlorambucil 2023 articles Chlorambucil 2024 articles Chlorambucil Scopus articles Chlorambucil impact factor journals Chlorambucil Scopus journals Chlorambucil PubMed journals Chlorambucil medical journals Chlorambucil free journals Chlorambucil best journals Chlorambucil top journals Chlorambucil free medical journals Chlorambucil famous journals Chlorambucil Google Scholar indexed journals Rituximab articles Rituximab Research articles Rituximab review articles Rituximab PubMed articles Rituximab PubMed Central articles Rituximab 2023 articles Rituximab 2024 articles Rituximab Scopus articles Rituximab impact factor journals Rituximab Scopus journals Rituximab PubMed journals Rituximab medical journals Rituximab free journals Rituximab best journals Rituximab top journals Rituximab free medical journals Rituximab famous journals Rituximab Google Scholar indexed journals Lymphoproliferative Disorder articles Lymphoproliferative Disorder Research articles Lymphoproliferative Disorder review articles Lymphoproliferative Disorder PubMed articles Lymphoproliferative Disorder PubMed Central articles Lymphoproliferative Disorder 2023 articles Lymphoproliferative Disorder 2024 articles Lymphoproliferative Disorder Scopus articles Lymphoproliferative Disorder impact factor journals Lymphoproliferative Disorder Scopus journals Lymphoproliferative Disorder PubMed journals Lymphoproliferative Disorder medical journals Lymphoproliferative Disorder free journals Lymphoproliferative Disorder best journals Lymphoproliferative Disorder top journals Lymphoproliferative Disorder free medical journals Lymphoproliferative Disorder famous journals Lymphoproliferative Disorder Google Scholar indexed journals fludarabine articles fludarabine Research articles fludarabine review articles fludarabine PubMed articles fludarabine PubMed Central articles fludarabine 2023 articles fludarabine 2024 articles fludarabine Scopus articles fludarabine impact factor journals fludarabine Scopus journals fludarabine PubMed journals fludarabine medical journals fludarabine free journals fludarabine best journals fludarabine top journals fludarabine free medical journals fludarabine famous journals fludarabine Google Scholar indexed journals hepatotoxicity articles hepatotoxicity Research articles hepatotoxicity review articles hepatotoxicity PubMed articles hepatotoxicity PubMed Central articles hepatotoxicity 2023 articles hepatotoxicity 2024 articles hepatotoxicity Scopus articles hepatotoxicity impact factor journals hepatotoxicity Scopus journals hepatotoxicity PubMed journals hepatotoxicity medical journals hepatotoxicity free journals hepatotoxicity best journals hepatotoxicity top journals hepatotoxicity free medical journals hepatotoxicity famous journals hepatotoxicity Google Scholar indexed journals chemotherapy articles chemotherapy Research articles chemotherapy review articles chemotherapy PubMed articles chemotherapy PubMed Central articles chemotherapy 2023 articles chemotherapy 2024 articles chemotherapy Scopus articles chemotherapy impact factor journals chemotherapy Scopus journals chemotherapy PubMed journals chemotherapy medical journals chemotherapy free journals chemotherapy best journals chemotherapy top journals chemotherapy free medical journals chemotherapy famous journals chemotherapy Google Scholar indexed journals lymphomas articles lymphomas Research articles lymphomas review articles lymphomas PubMed articles lymphomas PubMed Central articles lymphomas 2023 articles lymphomas 2024 articles lymphomas Scopus articles lymphomas impact factor journals lymphomas Scopus journals lymphomas PubMed journals lymphomas medical journals lymphomas free journals lymphomas best journals lymphomas top journals lymphomas free medical journals lymphomas famous journals lymphomas Google Scholar indexed journals rituximab monotherapy articles rituximab monotherapy Research articles rituximab monotherapy review articles rituximab monotherapy PubMed articles rituximab monotherapy PubMed Central articles rituximab monotherapy 2023 articles rituximab monotherapy 2024 articles rituximab monotherapy Scopus articles rituximab monotherapy impact factor journals rituximab monotherapy Scopus journals rituximab monotherapy PubMed journals rituximab monotherapy medical journals rituximab monotherapy free journals rituximab monotherapy best journals rituximab monotherapy top journals rituximab monotherapy free medical journals rituximab monotherapy famous journals rituximab monotherapy Google Scholar indexed journals articles Research articles review articles PubMed articles PubMed Central articles 2023 articles 2024 articles Scopus articles impact factor journals Scopus journals PubMed journals medical journals free journals best journals top journals free medical journals famous journals Google Scholar indexed journals
Article Details
1. Introduction
Chlorambucil (Clb), an oral alkylating agent, was the mainstay of B-chronic lymphocytic leukemija (CLL) treatments from the fifties. It lost popularity to fludarabine in the nineties, only to resurface as the best chemotherapy option for elderly and unfit, which comprise the majority of CLL patients, after a study showed that patients over 70 had better overall survival (OS) if treated with Clb than fludarabine, despite inferior disease control [1, 2]. Although it has been used for years, there is still no consensus regarding its dose and schedule. Published regimens vary from 0.4 mg/kg q 4 wk (used in the USA) to 2 mg/kg q 2 wk (used in Germany) to 8-10 mg/m2 daily for 7 days q 4 wk (used in Britain) to 8-10 mg/m2 daily until response or toxicity (used in EORTC and IGCI studies) [1, 3]. The latter schedule was at one time described as the most effective chemotherapy regimen for CLL but failed to gain acceptance in the USA and West Europe and remains rarely used outside of centers that participated in the original trials. Toxicity of Clb, even when administered at 20 mg daily for weeks, is generally mild, mostly hematological [4]. Nausea and vomiting are more frequent in regimens where the drug is administered in very high doses every two or four weeks [5]. CLL cells are CD20 positive but combinations of rituximab and chemotherapy became standard treatment later than in lymphomas. This was due to two causes.
First, early experience was that cytokine release syndrome can be fatal in CLL patients with high circulating tumor burden. Second, rituximab monotherapy was substantially less effective in CLL than in follicular lymphoma. Since a study showed that efficacy of rituximab monotherapy can be improved by increasing its dose investigators from MD Anderson chose to modify rituximab dosing in their combination of fludarabine, cyclophosphamide and rituximab (FCR) that became the gold standard for treating fit CLL patients [6, 7]. This then became the standard schedule of rituximab dosage for CLL: 375 mg/m2 1st cycle, 500 mg/m2 cycles 2-6. Interestingly, the total dose of rituximab administered with this schedule is slightly less than in the standard lymphoma schedule of 375 mg/m2/cycle for 8 cycles (2875 mg/m2 vs. 3000 mg/m2) [8-10].
The addition of rituximab to Clb has been studied in two major randomized trials, the CLL 11 study run by the German CLL Study group and the British NCRI study [11-14]. Both showed a clear superiority of the combination without significantly increased toxicity. However, schedules of Clb in the two studies varied substantially, and so did the outcomes of patients receiving R-Clb. It remains unclear whether these two facts are related. Before the introduction of rituximab for this indication, two schedules of Clb were used in Croatia: the intermittent 7 days q 4 wk and the continuous high-dose schedule. When it became clear that rituximab improves outcomes of CLL patients, hematologists combined it with the Clb regimen they usually used. Due to reimbursement reasons, rituximab was administered at a dose of 375 mg/m2/cycle for 8 cycles. KroHem, the Croatian Cooperative Group for Hematologic Diseases performed this retrospective study to analyze the outcomes of patients with CLL who received different Clb and rituximab combinations as front-line treatment.
2. Patients and Methods
2.1 Patients
Data were collected by retrospective chart review. Patients were included in this analysis if they had CLL diagnosed by standard criteria, were in need of therapy, received Clb and rituximab with or without steroids for front-line treatment and had sufficient data available in their files for analysis, including CT scans for determination of lymph node and spleen size prior to and at the end of treatment.
2.2 Therapy
In the continuous high-dose Clb plus rituximab regimen (cHD-Clb-R), Clb was administered daily at a dose of 12-20 mg (6-10 mg/m2) until response or toxicity and rituximab at a dose of 375 mg/m2. If response occurred earlier, low-dose Clb was administered at a dose of 2-6 mg/day for a total duration of therapy of 3 months. In the intermittent Clb plus rituximab regimen (iClb-R), Clb was administered at a dose of 10-20 mg (6-10 mg/m2) daily for 7 days q 4 wk and rituximab at a dose of 375 mg/m2/cycle. Eight cycles of therapy were planned. With both regimens physicians sometimes chose to delay rituximab until the number of circulating lymphocytes was reduced below 50 x 109/l to reduce the risk of severe cytokine-release syndrome.
2.3 Outcomes and toxicity
Outcomes were defined according to standard IWCLL criteria [15]. Patients achieving at least partial remission (PR) were considered responders. Since this was not a clinical trial, bone marrow biopsies were not routinely performed at the end of treatment. We considered patients who had normal blood findings and were in CR by CT as having hematological CR (hCR). Overall survival (OS) was calculated from treatment start until last follow-up or death. Progression-free survival (PFS) was calculated from treatment start until last follow-up, progression (as defined by IWCLL), death or start of new treatment. Survival without new treatment (TTNT) was calculated from treatment start until last follow-up, death or start of new treatment. Toxicities were extracted from patient files and graded according to CTCAE 4.0 [16].
2.4 Data analysis
OS, PFS and TTNT were estimated using the method of Kaplan & Meier. Fisher’s exact test was used for 2x2 table analyses and log-rank test for survival comparisons. The assumed level of significance was 0.05.
2.5 Ethics
This is a non-interventional retrospective study of patient data performed with the approval of the Ethical Committee of the Medical School, University of Zagreb in accordance with pertinent Croatian, EU and international rules and regulations and the Declaration of Helsinki. Prior to treatment all patients gave informed consent for therapy.
3. Results
We identified 68 patients fulfilling these criteria, 24 women and 44 men whose median age was 66 years. Patients' characteristics are presented in Table 1. Seventeen patients had advanced stage disease (Binet C/Rai 3-4) and 5 patients had the presence of del(17p).
3.1 Treatment disposition
In the cHD-Clb-R group the median duration of high-dose Clb treatment was 36 days (range 14-134), dose of Clb 20 mg/day (range 10-20), and median total dose 880 mg. Median number of rituximab cycles was eight, total dose 5600 mg and time from treatment start until end of high-dose Clb or rituximab 4 months. In the iClb-R group patients recevied a median of 7 Clb and 8 rituximab cycles. Median dose of Clb was 112 mg per cycle, range 70-140. The median total dose of Clb administered was 784 mg and of rituximab 5600 mg. Median duration of treatment was 28 weeks.
3.2 Efficacy
Response rate was 86% in the cHD-Clb-R and 88% in the iClb-R group; 20 out of 36 and 16 out of 32 obtained hCR at time of end of treatment evaluation in each group respectively. After a median follow-up of survivors of 30 months 25 patients in the cHD-Clb-R group are alive, 17 of them without new treatment and 11 without progression. In the iClb-R group 28 patients are alive, 17 of them without new treatment patients and 17 without progression. There was no significant difference in efficacy between these two groups (cHD-Clb-R vs. iClb-R) regardless of the treatment schedule in all measured outcomes: OS at 30 months was 70% vs. 83%, PFS 49% vs. 55% and TTNT 67% vs. 75% respectively (Figure 1). Unfavorable prognostic factors were advanced stage (Binet C, Rai 3-4), presence of del 17p, high beta-2 microglobulin levels and age (data not shown).
3.3 Toxicities
The toxicity profile was relatively favorable. Most common adverse events (AE) were nausea and a mild skin rash, observed in 50% of patients. Gastrointestinal toxicity manifested by vomiting and nausea was more common in patients receiving intermittent Clb. Hematologic toxicity was similar in both groups. Severe granulocytopenia (grade 3 or 4) occurred in 14 patients while infections were observed in 7 patients. Most frequent infectious complications were upper respiratory tract infections and pneumonia, while one patient in the iClb-R group died of invasive pulmonary mycosis. One additional patient stopped treatment due to hepatotoxicity. Allergic reactions, mainly cutaneous, were seen in six patients, while one patient developed a serious infusion reaction related to rituximab administration.
|
Groups |
cHD-Clb-R |
iClb-R |
Total |
|
Age (median / range) |
67/41-80 |
67/44-78 |
66/41-80 |
|
Sex |
11 female |
13 female |
24 female |
|
25 male |
19 male |
44 male |
|
|
Advanced stage (Binet C / Rai 3-4) |
7 (19%) |
10 (31%) |
17 (25%) |
|
Del (17p) |
2 (6%) |
3 (9%) |
5 (7%) |
Table 1: Patients' characteristics.
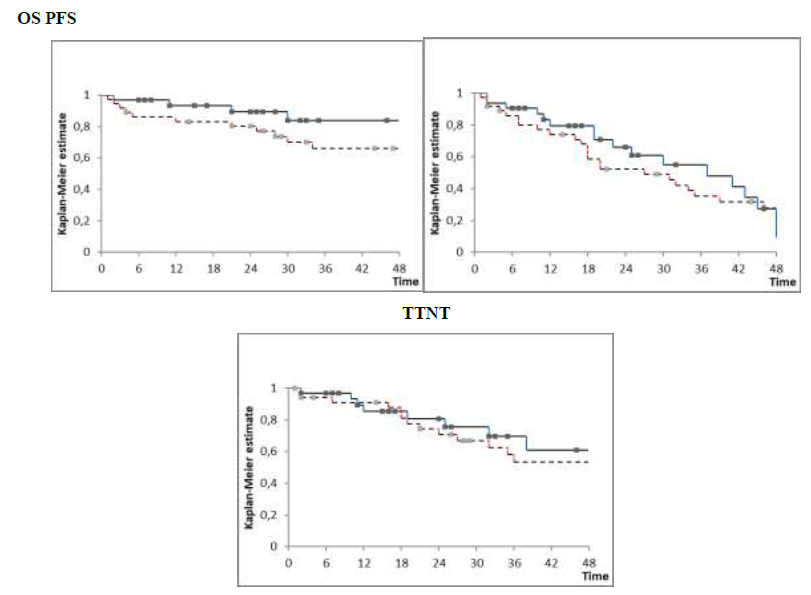
Figure 1: cHD-Clb-R vs. iClb-R overall survival (OS), progression-free survival (PFS) and survival without next treatment (TTNT) at 30 mo. 70% vs. 83%, 49% vs. 55% and 67% vs. 75% .
4. Discussion
Chlorambucil has been given in a variety of doses and schedules for the treatment of elderly patients with CLL and comorbidities. Jaksic and Brugiatelli first described the high-dose continuous chlorambucil regimen in 1988 and demonstrated the practicability of dose escalation of chlorambucil in treatment of CLL patients [17]. Studies have shown higher ORR for high-dose chlorambucil versus low-dose (ORR: 420 mg per 28-day cycle, 90% v 70 mg/m2 per 28-day cycle, 72%) [18, 19]. Although high-dose therapy is more effective and associated with better response, it comes at the expense of increased myelosuppression and incidence of infections, particularly in older and more fragile patients. Regardless, one of the advantages of chlorambucil is a well-established toxicity profile with good tolerance, even in this patient population. Another benefit is its low cost. One of the primary disadvantages of chlorambucil therapy is a low CR rate, even in treatment-naive patients. A pivotal phase 3 trial by the GCLLSG demonstrated that the addition of rituximab to chlorambucil in elderly patients with CLL prolongs PFS (16 months vs. 11 months) and doubles the overall response rate, without increasing the rates of infection [14]. Results from CLL11 study also confirm that the addition of anti-CD20 monoclonal antibody to Clb in this population of patients results in substantial TTNT and OS benefit compared with Clb alone [11-13].
Our results seem superior to the German CLL Study Group CLL11 study (ORR 87% vrs 65.9%, PFS 30 vrs 15.7 mo) and similar to the British NCRI study (ORR 84%, PFS 23.5 mo). This is most probably due to the low dose of chlorambucil used in the CLL11 study (0.5 mg/kg body weight p.o., day 1 and 15 in 6 cycles) [13-14]. For reimbursement reasons, we were unable to use the standard rituximab CLL schedule of 500 mg/m2 in cycles 2-6 but administered 8 instead of 6 cycles. Therefore our patients received a slightly higher total dose of rituximab (3000 mg/m2 vs. 2875 mg/m2). It seems that this change in schedule did not affect outcomes adversely. Frontline treatment for CLL has evolved in the last few years with rapid approvals of several novel targeted agents. Even though latest clinical trial results have established ibrutinib and venetoclax with or without anti-CD20 antibodies (rituximab, obinutuzumab) as the preferred first –line treatment for most CLL patients we think that our data is nonetheless interesting and shows that chlorambucil continues to be a valid alternative in unfit patients [20]. In conclusion, our study confirms that combinations of rituximab and high-dose chlorambucil are well tolerated and effective treatments for patients ineligible for fludarabine-based regimens. Outcomes seem to be related more to total drug doses than schedules.
Conflicts of Interest
SBK, IA, SOK, VP, IR and OJ have received honoraria and/or research support from Roche.
Acknowledgement
Presented in part at the16th iwCLL meeting, Sydney, Australia, 2015
References
- Eichhorst BF, Busch R, Stilgenbauer S, et al. The German CLL Study Group (GCLLSG); First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood 114 (2009): 3382-3391.
- Vidal L, Gurion R, Ram R, et al. Chlorambucil for the treatment of patients with chronic lymphocytic leukemia (CLL) - a systematic review and meta-analysis of randomized trials. Leuk Lymphoma 57 (2016): 2047-2057.
- https://www.just.edu.jo/DIC/AZLibrary/Chlorambucil.pdf
- Summerfield GP, Taylor PRA, Mounter PJ, et al. High dose chlorambucil for the treatment of chronic lymphocytic leukaemia and low grade non Hodgkin's lymphoma. British Journal of Haematology 116 (2002): 781-786.
- Knauf WU, Lissichkov T, Aldaoud A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol 27 (2009): 4378-4384.
- Hauptrock B, Hess G. Rituximab in the treatment of non-Hodgkin's lymphoma. Biologics 2 (2008): 619-633.
- O'Brien SM, Kantarjian H, Thomas DA, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol 19 (2001): 2165-2170.
- Hallek M, Fischer K, Fingerle-Rowson G. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 376 (2010): 1164-1174.
- Keating MJ, O’Brien S, Albitar M. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 23 (2005): 4079-4088.
- Li J, Zhi J, Wenger M, et al. Population pharmacokinetics of rituximab in patients with chronic lymphocytic leukemia. J Clin Pharmacol 52 (2012): 1918-1926.
- Goede V, Fisher K, Bosch F, et al. Updated Survival Analysis from the CLL11 Study: Obinutuzumab Versus Rituximab inChemoimmunotherapy-Treated Patients with Chronic Lymphocytic Leukemia. Blood 126 (2015): 1733.
- Goede V, Fisher K, Busch R, et al Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions. N Engl J Med 370 (2014): 1101-1110.
- Goede V, Fischer K, Busch R, et al. Chemoimmunotherapy with GA101 plus chlorambucil in patients with chronic lymphocytic leukemia and comorbidity: results of the CLL11 (BO21004) safety run-in. Leukemia 27 (2013): 1172-1174.
- Hillmen P, Gribben JG, Follows GA, et al. Rituximab plus chlorambucil as first-line treatment for chronic lymphocytic leukemia: Final analysis of an open-label phase II study. J Clin Oncol 32 (2014): 1236-1241.
- Hallek M, Bruce DC, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 131 (2018): 2745-2760.
- Cancer Therapy Evaluation Program. National Cancer Instituate (2020).
- Jaksic B, Brugiatelli M. High dose chlorambucil vs intermittent chlorambucil plus prednisone for treatment of BCLL: IGCI CLL01 trial. Nouvelle Revue Francaise d'6. Hematologie 30 (1988): 437-442.
- Catovsky D, Hamblin T, Richards SJ. Preliminary results of the UK MRC trial in chronic lymphocytic leukaemia: CLL3. Br J Haematol 102 (1998): 278.
- Jaksic B, Brugiatelli M, Krc I, et al. High dose chlorambucil versus Binet's modified cyclophosphamide, doxorubicin, vincristine, and prednisone regimen in the treatment of patients with advanced B-cell chronic lymphocytic leukemia: Results of an international multicenter randomized trial—International Society for Chemo-Immuno-therapy, Vienna. Cancer 79 (1997): 2107-2114.
- Rhodes JM, Barrientos JC. Chemotherapy-free frontline therapy for CLL: is it worth it?. Hematology Am Soc Hematol Educ Program 2020 (2020): 24-32.
