Comparative Effects of Human Chorionic Gonadotropin and Recombinant Luteinizing Hormone Supplementation during the Late Follicular Phase
Article Information
Le Duc Thang1*, Hoang Bao Long2, Tham Chi Dung1, Vu Thi Mai Anh1, Nguyen Thi Thuy Linh1, Nguyen Phuc Hieu1, Giap Thi Mai Phuong1, Nguyen Thi Lien Huong1, Le Hoang1, Jean-Noël Hugues3
1 Tam Anh General Hospital, 108 Hoang Nhu Tiep street, Bo De ward, Long Bien district, Hanoi, Vietnam
2 Institute of Gastroenterology and Hepatology, 9 Dao Duy Anh street, Dong Da district, Hanoi, Vietnam
3 Department of Obstetrics, Gynecology and Reproductive Medecine, Hôpitaux Universitaires Paris Seine Saint-Denis, Assistance Publique-Hôpitaux de Paris, Bondy, France. Université Paris 13, UFR SMBH, Bobigny, France
*Corresponding Author: Le Duc Thang, MD, Tam Anh General Hospital, 108 Hoang Nhu Tiep street, Bo De ward, Long Bien district, Hanoi, Vietnam.
Received: 22 April 2023; Accepted: 26 April 2023; Published: 08 May 2023
Citation: Le Duc Thang, MD, Hoang Bao Long, MD, MPH, Tham Chi Dung, MD, PhD, Vu Thi Mai Anh, MD, Nguyen Thi Thuy Linh, MD, Nguyen Phuc Hieu, MD, Giap Thi Mai Phuong, B.Sc, Nguyen Thi Lien Huong,MD, PhD, Le Hoang, MD, PhD, Assoc. Prof, and Jean-Noël Hugues, MD, PhD, Prof. Comparative effects of human chorionic gonadotropin and recombinant luteinizing hormone supplementation during the late follicular phase. Obstetrics and Gynecology Research. 6 (2023): 143-151.
Share at FacebookAbstract
Background:
In selected patients, addition of LH activity can improve the outcome of ovarian stimulation. Two common LH activity supplementation strategies are recombinant human LH (rLH) and human chorionic gonadotropin (hCG). The respective effectiveness of hCG versus rLH using various daily dosages and modes of administration is still discussed. In this study, we compared the outcomes of hCG versus rLH supplementation among patients with slow response to recombinant follicle stimulating hormone (rFSH).
Methods:
A retrospective study was conducted among infertile women with slow response to rFSH, who were given either rLH or hCG on day 6 of ovarian stimulation. The treatment effect of hCG versus rLH was adjusted using propensity score matching.
Results:
The study included 772 patients, of which 275 received rLH and 497 received hCG. In the unadjusted analysis, the number of oocytes retrieved, MII oocytes, cleavage embryos, and blastocyst embryos were higher in the hCG group than in the rLH group (mean ratios: 1.38 (95% CI 1.25, 1.52), 1.47 (95%CI 1.31, 1.65), 1.41 (95%CI 1.25, 1.58), 1.41 (95%CI 1.23, 1.61), respectively). In the adjusted analysis, the mean differences decreased to 1.19 (95%CI 1.01, 1.40), 1.23 (95%CI 1.00, 1.52), 1.14 (95%CI 0.94, 1.38), and 1.22 (95%CI 0.98, 1.53), respectively. Among the 121 patients in the rLH group and 302 in the hCG group who underwent embryo transfer, no significant differences could be demonstrated in endometrial preparation, endometrial thickness, number or type of embryos, or pregnancy outcomes.
Conclusions:
In infertile women displaying a slow response to rFSH, hCG supplementation was associated with a modest improvement in oocyterelated outcomes but comparable effects in terms of embryo-related and pregnancy outcomes as compared to rLH. hCG could be a potential alternative to rLH when addition of LH activity is required.
Keywords
Recombinant Luteinizing Hormone; Human Chorionic Gonadotrophin; Ovarian Stimulation; Ovarian Hyporesponse
Recombinant Luteinizing Hormone articles Recombinant Luteinizing Hormone Research articles Recombinant Luteinizing Hormone review articles Recombinant Luteinizing Hormone PubMed articles Recombinant Luteinizing Hormone PubMed Central articles Recombinant Luteinizing Hormone 2023 articles Recombinant Luteinizing Hormone 2024 articles Recombinant Luteinizing Hormone Scopus articles Recombinant Luteinizing Hormone impact factor journals Recombinant Luteinizing Hormone Scopus journals Recombinant Luteinizing Hormone PubMed journals Recombinant Luteinizing Hormone medical journals Recombinant Luteinizing Hormone free journals Recombinant Luteinizing Hormone best journals Recombinant Luteinizing Hormone top journals Recombinant Luteinizing Hormone free medical journals Recombinant Luteinizing Hormone famous journals Recombinant Luteinizing Hormone Google Scholar indexed journals Human Chorionic Gonadotrophin articles Human Chorionic Gonadotrophin Research articles Human Chorionic Gonadotrophin review articles Human Chorionic Gonadotrophin PubMed articles Human Chorionic Gonadotrophin PubMed Central articles Human Chorionic Gonadotrophin 2023 articles Human Chorionic Gonadotrophin 2024 articles Human Chorionic Gonadotrophin Scopus articles Human Chorionic Gonadotrophin impact factor journals Human Chorionic Gonadotrophin Scopus journals Human Chorionic Gonadotrophin PubMed journals Human Chorionic Gonadotrophin medical journals Human Chorionic Gonadotrophin free journals Human Chorionic Gonadotrophin best journals Human Chorionic Gonadotrophin top journals Human Chorionic Gonadotrophin free medical journals Human Chorionic Gonadotrophin famous journals Human Chorionic Gonadotrophin Google Scholar indexed journals Ovarian Stimulation articles Ovarian Stimulation Research articles Ovarian Stimulation review articles Ovarian Stimulation PubMed articles Ovarian Stimulation PubMed Central articles Ovarian Stimulation 2023 articles Ovarian Stimulation 2024 articles Ovarian Stimulation Scopus articles Ovarian Stimulation impact factor journals Ovarian Stimulation Scopus journals Ovarian Stimulation PubMed journals Ovarian Stimulation medical journals Ovarian Stimulation free journals Ovarian Stimulation best journals Ovarian Stimulation top journals Ovarian Stimulation free medical journals Ovarian Stimulation famous journals Ovarian Stimulation Google Scholar indexed journals Ovarian Hyporesponse articles Ovarian Hyporesponse Research articles Ovarian Hyporesponse review articles Ovarian Hyporesponse PubMed articles Ovarian Hyporesponse PubMed Central articles Ovarian Hyporesponse 2023 articles Ovarian Hyporesponse 2024 articles Ovarian Hyporesponse Scopus articles Ovarian Hyporesponse impact factor journals Ovarian Hyporesponse Scopus journals Ovarian Hyporesponse PubMed journals Ovarian Hyporesponse medical journals Ovarian Hyporesponse free journals Ovarian Hyporesponse best journals Ovarian Hyporesponse top journals Ovarian Hyporesponse free medical journals Ovarian Hyporesponse famous journals Ovarian Hyporesponse Google Scholar indexed journals in vitro fertilization articles in vitro fertilization Research articles in vitro fertilization review articles in vitro fertilization PubMed articles in vitro fertilization PubMed Central articles in vitro fertilization 2023 articles in vitro fertilization 2024 articles in vitro fertilization Scopus articles in vitro fertilization impact factor journals in vitro fertilization Scopus journals in vitro fertilization PubMed journals in vitro fertilization medical journals in vitro fertilization free journals in vitro fertilization best journals in vitro fertilization top journals in vitro fertilization free medical journals in vitro fertilization famous journals in vitro fertilization Google Scholar indexed journals estrogen levels articles estrogen levels Research articles estrogen levels review articles estrogen levels PubMed articles estrogen levels PubMed Central articles estrogen levels 2023 articles estrogen levels 2024 articles estrogen levels Scopus articles estrogen levels impact factor journals estrogen levels Scopus journals estrogen levels PubMed journals estrogen levels medical journals estrogen levels free journals estrogen levels best journals estrogen levels top journals estrogen levels free medical journals estrogen levels famous journals estrogen levels Google Scholar indexed journals Steroid hormone synthesis articles Steroid hormone synthesis Research articles Steroid hormone synthesis review articles Steroid hormone synthesis PubMed articles Steroid hormone synthesis PubMed Central articles Steroid hormone synthesis 2023 articles Steroid hormone synthesis 2024 articles Steroid hormone synthesis Scopus articles Steroid hormone synthesis impact factor journals Steroid hormone synthesis Scopus journals Steroid hormone synthesis PubMed journals Steroid hormone synthesis medical journals Steroid hormone synthesis free journals Steroid hormone synthesis best journals Steroid hormone synthesis top journals Steroid hormone synthesis free medical journals Steroid hormone synthesis famous journals Steroid hormone synthesis Google Scholar indexed journals FSH articles FSH Research articles FSH review articles FSH PubMed articles FSH PubMed Central articles FSH 2023 articles FSH 2024 articles FSH Scopus articles FSH impact factor journals FSH Scopus journals FSH PubMed journals FSH medical journals FSH free journals FSH best journals FSH top journals FSH free medical journals FSH famous journals FSH Google Scholar indexed journals Assisted Reproduction articles Assisted Reproduction Research articles Assisted Reproduction review articles Assisted Reproduction PubMed articles Assisted Reproduction PubMed Central articles Assisted Reproduction 2023 articles Assisted Reproduction 2024 articles Assisted Reproduction Scopus articles Assisted Reproduction impact factor journals Assisted Reproduction Scopus journals Assisted Reproduction PubMed journals Assisted Reproduction medical journals Assisted Reproduction free journals Assisted Reproduction best journals Assisted Reproduction top journals Assisted Reproduction free medical journals Assisted Reproduction famous journals Assisted Reproduction Google Scholar indexed journals progesterone levels articles progesterone levels Research articles progesterone levels review articles progesterone levels PubMed articles progesterone levels PubMed Central articles progesterone levels 2023 articles progesterone levels 2024 articles progesterone levels Scopus articles progesterone levels impact factor journals progesterone levels Scopus journals progesterone levels PubMed journals progesterone levels medical journals progesterone levels free journals progesterone levels best journals progesterone levels top journals progesterone levels free medical journals progesterone levels famous journals progesterone levels Google Scholar indexed journals
Article Details
INTRODUCTION
Ovarian stimulation is a cornerstone of in vitro fertilization (IVF), which allows the recruitment of an optimum number of oocytes to maximize fertilization success in the safest possible way. Nowadays, instead of using a conventional “one-size-fits-all” protocol, individualization of ovarian stimulation provides every single woman the optimal treatment matched to her unique characteristics, therefore enhancing patient compliance and improving clinical practice. However, individualizing protocol of stimulation is not that simple due to the large variety of medications, such as gonadotropin-releasing hormone (GnRH) analogs, gonadotrophin preparations, and other adjuvant therapies, and because of the lack of a clear evidence-based therapeutic strategy for various subgroups of patients[1]. The positive effect of exogenous luteinizing hormone (LH) in ovarian stimulation is not demonstrated in the general population A systematic review of 30 trials in normogonadotropic women undergoing IVF showed that two subgroups could benefit from recombinant LH (rLH) supplementation— women 36-39 years of age and those with adequate pre-stimulation ovarian reserve parameters but unexpected hyporesponse to recombinant FSH (rFSH) monotherapy[2].
Different criteria of hyporesponse to ovarian stimulation have been proposed, including a slow response to rFSH, slowly rising estrogen levels, or the requirement of a gonadotropin dose higher than usual for ovarian stimulation[3]. The reasons for hyporesponse to FSH during ovarian stimulation are not still clearly defined. The most common hypothesis focuses on genetic mutations or single nucleotide polymorphisms (SNPs) of gonadotropins or their receptors that can impair ovarian sensitivity to exogenous gonadotropins. For example, LH beta chain variant carriers may exhibit ovarian resistance to exogenous gonadotropin and require a higher dose of recombinant FSH during ovarian stimulation[4]. Hyporesponse to FSH is more prevalent in carriers of the serine variant in position 680 of FSH-receptor than in wild-type haplotypes[5]. Therefore, FSH receptor variant N680S and LH/hCG receptor variant N312S could predict the outcome of ovarian stimulation in women undergoing IVF[6]. In women with these genetic variants, exogenous LH activity supplementation might overcome the ovarian resistance to FSH stimulation[2].
In addition to LH supplementation using recombinant LH, several studies have used human chorionic gonadotrophin (hCG), a hormone with LH activity. Although previously considered to have overlapping functions based on similar molecular structures and a shared binding site—the LH/hCG receptor, the two hormones have been shown to interact differently with this receptor, producing distinct intracellular signaling and steroidogenesis[7]. In particular, their beta subunits differ in length and confer structural uniqueness and specificity of pharmacodynamics and bioactivities. Consequently, LH has a significant shorter half-life (30-60 minutes) while hCG has a half-life of about 37 hours. LH binding results in a more robust stimulation of the proliferative and anti-apoptotic ERK1/2 and AKT pathways, while hCG has greater efficacy for activating the steroidogenic cAMP pathways. Steroid hormone synthesis also differs between hCG and LH. Whereas LH and hCG both ultimately promote testosterone synthesis, LH only partly promotes progesterone production, with about a half of the potency of hCG[7]. A study by Casarini et al. (2016) has provided valuable insights into the differential effects of LH and hCG in the presence of FSH. This study demonstrated that, in human granulosa-lutein cells, FSH co-treatment potentiated different LH- and hCG-dependent responses, measured in terms of cAMP, phospho-CREB, -ERK1/2, and -AKT activation, gene expression, and progesterone and estradiol production[8]. Therefore, as rLH and hCG have different pharmacodynamic and bioactive properties, their respective effect on ovarian stimulation outcomes might differ.
Some studies have compared the effects of hCG versus rLH using various daily dosages and modes of administration; however, unclear conclusions have been reached in terms of superiority[9]. Such a comparison has not been yet performed in patients with hyporesponse to FSH. Therefore, our study aimed to compare the effects of hCG versus rLH supplementation during the late follicular phase in patients with an initial slow response to rFSH.
Materials and methods
Study design
We conducted a retrospective analysis of data collected among women who underwent IVF at the Assisted Reproduction Center from January 1st, 2020 to November 7th, 2021 and had either rLH or hCG added during the late follicular phase due to a slow response to rFSH.
A slow response to rFSH was defined by the presence of dominant follicle of <12 mm in size and >50% of the follicles of <10 mm in size on day 6 of ovarian stimulation. Data were extracted from the electronic database at the Assisted Reproduction Center. Collected data included baseline characteristics, treatment given on day 6, and treatment outcomes. Inclusion criteria the following : normal ovarian function regarding baseline serum FSH, LH, estradiol, and progesterone levels, the use of antagonist protocol, addition of either rLH or hCG on day 6 of ovarian stimulation. Women with history of polycystic ovary syndrome, endometriosis, or history of severe ovarian hyperstimulation syndrome were excluded from the study. Because the majority (98%) of women in the study received the initial rFSH dose of 300 IU, we also excluded those who were given other doses.
Treatment protocol of ovarian stimulation
Stimulation was initiated on day 2 of the menstrual cycle with rFSH (Follitrope, LG Chem, South Korea) at a daily dose of 300 IU. On day 6 of ovarian stimulation, pituitary gonadotrophin suppression was started with daily administration of a GnRH antagonist (ganirelix, Orgalutran®, MSD, Australia; or cetrorelix, Cetrotide®, Merck, Netherlands) at a dose of 0.25 mg, to inhibit premature LH surge. For patients diagnosed with slow response to rFSH, either hCG 100 IU per day (IVF-C, LG Chem, South Korea) or rLH 75 IU per day (Pergoveris, Merck Serono, United Kingdom) was added in order to rescue the ongoing cycle[2]. The choice ot the product depended on physician’s preferences and on patient’s characteristics, among which the patient’s financial capacity was a vital consideration. If Pergoveris was prescribed, the initial dose of rFSH should be decreased by 150 units as Pergoveris already contains 150 units of rFSH. Pergoveris was used because it is the only medication containing rLH available in Vietnam , following the withdrawal of the other rLH-containing drug, Luveris,. The hCG dose of 100 IU/day was used because it is as effective as 150 IU/day while reducing the risk of ovarian hyperstimulation syndrome (OHSS) [10].
As soon as three follicles of 17 mm diameter were detected, triggering of final oocyte maturation was induced with 5000 IU of hCG (Pregnyl®, MSD, United States). After 36 hours, oocyte retrieval was performed and the oocyte cumulus complexes were harvested by transvaginal aspiration.
Laboratory protocol
Following follicle aspiration, the oocyte cumulus complexes were transferred into a dish containing a culture medium of G-IVF (Vitrolife) and continued incubating in benchtop incubators (Origio), which allowed oocytes to achieve their fully mature status. After 2 hours of incubation, oocyte-cumulus complex denuding took place. The denuded oocytes were examined under inverted microscope to assess the nuclear maturation stage. Only oocytes that reached the MII stage, which had the first polar body extruded and homogenous size were suitable for intracytoplasmic sperm injection. Otherwise, oocytes were discarded if they were a giant cell, severe abnormal shape, or degenerated. Intracytoplasmic sperm injection was performed 3-4 hours after oocyte retrieval by one of the experienced embryologists. The oocytes then were transferred into the post-intracytoplasmic sperm injection dishes for further culturing with continuous single culture media (Fujifilm Irvine Scientific) in the tri-gas incubator of 37 oC, 5% oxygen, and 6% carbon dioxide. Fertilized oocytes should present 2 pronuclei inside the ooplasm. Those with 3 pronuclei or more were considered multinuclei and eliminated. The quality of cleavage embryos was assessed at 67 to 69 hours post-insemination following the Istanbul consensus and classified into good, fair, or poor grade depending on the embryos number of cells, cell fragmentation, multinucleation, and cell size[11]. After a thorough discussion with the embryologist, the couple will decide whether to continue culture their embryos to blastocysts or cryopreservation. Embryonic development stage (compression phase, early blastocyst, complete blastocyst, hatching blastocyst), trophoblast, and inner cell mass morphology were used to classify obtained blastocysts according to Gardner and Schoolcraft classification. AA, AB, BA, and BB grades indicated a good blastocyst.
Endometrial Preparation for transfer of frozen embryos
According to our hormonal therapy protocol, a daily dose of 6-8 mg estrogen is given to promote endometrial growth, which is monitored by transvaginal ultrasound. Progesterone is added when the endometrial thickness reaches 7 mm. When natural cycle or mild stimulation protocols are used, patients receive a daily intramuscular injection of HMG of 75 IU from day 2 of the menstrual cycle transvaginal ultrasound is performed to assess follicular and endometrial development. Once the endometrial thickness reaches 7 mm and the diameter of the dominant follicle reaches 18 mm, 5000 IU of hCG (Pregnyl®, MSD, United States) is administered to induce ovulation. Progesterone is then added and the timing of embryo transfer is scheduled.
Outcomes
Outcomes of interest included oocyte-related parameters (total number of oocytes, number of MII oocytes), embryo-related parameters (total number of cleavage embryos, number of good cleavage embryos, number of blastocyst embryos, number of good blastocyst embryos), dynamic follicular growth (follicular output rate (FORT), follicle-to-oocyte index (FOI)), incidence of OHSS, and pregnancy outcomes.
The total number of oocytes was counted immediately after denuding the surrounding cumulus cells. The number of MII oocytes was then counted adapting the characteristics for intracytoplasmic injection mentioned in laboratory protocol. FORT was defined as the ratio of pre-ovulatory follicle (16-22 mm in diameter) count on hCG day to small antral follicle (3-8 mm in diameter) count at baseline[12]. FOI was defined as the ratio of the total number of oocytes collected at the end of ovarian stimulation to the number of antral follicles available at the start of stimulation[3].
OHSS was categorized according to the Practice Committee of the American Society for Reproductive Medicine[13]. Clinical symptoms and laboratory parameters (if any) were recorded on the day of oocyte retrieval, day 3 following oocyte retrieval, and anytime the patient was re-examined for symptoms related to OHSS.
The pregnancy outcomes after the first transfer of embryos from the two treatment regimens include: the pregnancy, which was determined based on the presence of beta hCG levels greater than or equal to 5 mIU/mL on the 12th day after embryo transfer; the biochemical pregnancy was determined based on serum beta hCG levels greater than 5 IU/L on the 12th day after embryo transfer, followed by a negative result and no visualization of a gestational sac on ultrasound; the clinical pregnancy was determined based on the visualization of a gestational sac on ultrasound on the 21st day after embryo transfer; the on-going pregnancy was defined as the number of patients with pregnancies continuing beyond 12 weeks; the multiple pregnancy was determined based on the observation of at least two gestational sacs on ultrasound on the 21st day after embryo transfer. The implantation rate was calculated by dividing the number of gestational sacs by the number of transferred embryos.
Statistical analysis
All analyses were performed using the R language version 4.1.1. Baseline characteristics and outcomes were compared between participants who received rLH and hCG, with categorical variables presented as number (percentage) and quantitative variables presented as means (standard deviation) or medians [interquartile range (IQR)]. Differences between two groups were tested using Chi-square for categorical variables and t-test for continuous variables.
To estimate the treatment effect of using hCG compared to rLH, we used propensity score matching since our data were retrospective in nature, which makes the analysis of treatment effect subject to confounding bias. Propensity score methods are causal inference methods used to emulate the design of randomized clinical trials[14]. Matching acts as a method to balance the differences in baseline covariates between two treatment groups, making them similar to those generated by randomization.
Propensity scores were estimated using the MatchIt package[15]. We fitted a multivariable logistic regression on the baseline characteristics of the participants, including age groups (<35, 35 to 38, and >38 years), BMI, basal FSH level, basal LH level, AMH level, antral follicle counts (AFC), infertility factor, and >2 previous IVF attempts. Various spline terms of the aforementioned variables were added; the cut points for these terms were decided by visual inspection using locally estimated scatterplot smoothing (LOESS) plot. In this analysis, we examined both 1:1 matching and full matching. In addition to matching using the propensity scores, we performed exact matching on the age group and fertility factor. A Love plot was created to examine the standardized mean differences (SMD) of the covariates before and after adjustment by propensity score matching. If the SMDs of all covariates decreased substantially after adjustment and were <0.1, balance was considered to have been achieved.
In this study, full matching provided better balance; therefore, we chose the average treatment effect (ATE) as our estimand of interest[16]. ATE answers the question “What would the average treatment effect have been had everyone in the target population been given hCG versus had everyone in the population been given rLH?” We performed weighted regression to estimate the ATE of six outcomes: total number of oocytes, number of MII oocytes, total number of cleavage embryos, number of good cleavage embryos, FOI, and FORT. Weighted Poisson regression was fitted for the outcomes. Variance in the weighted regression models was estimated using heteroscedasticity robust variance estimation.
For pregnancy outcomes, we did not perform adjusted analysis. We simply describe the characteristics of patients who underwent the first embryo transfer and the pregnancy outcomes, including the proportion of biochemical pregnancy, clinical pregnancy, ongoing pregnancy, implantation, and multiple pregnancy.
Results
Patient and ovarian stimulation characteristics
A total of 772 participants were included in the study, 275 receiving rLH and 497 hCG. Before propensity score matching, participants who received rLH appeared to be older and more overweight / obese than participants who received hCG. The proportion of participants who had more than 2 previous failed IVF attempts was also higher in the rLH group. Participants in the rLH group had lower LH and AMH levels, as well as lower AFC. Nearly two-thirds of the participants had secondary infertility. Patients in the rLH group had more previous IVF attempts. After matching, baseline characteristics were similar between the two treatment groups (p >0.05 for all comparisons, except mean number of previous IVF attempts). Full matching appeared to provide good balance between the two treatment groups (Figure S1).
For ovarian stimulation characteristics, the total FSH dose was the most notable difference between the hCG and rLH groups. Before matching, a significant difference was observed in the total FSH dose: 2672 ±143 IU for the hCG group and 3039 ±191 IU for the rLH group p<0.001). This difference remained significant after matching: 2672 ±143 IU for the hCG group and 3056 ± 164 IU for the rLH group (p < 0.001). On the other hand, stimulation duration and progesterone levels on the trigger day consistently showed no significant differences between the groups, both before and after matching. In contrast, estradiol levels on the trigger day initially exhibited a significant difference before matching. However, this difference became not significant after matching : 3601 ± 2536 pg/mL for the hCG group and 3158 ± 2261 pg/mL for the rLH group.
|
Variable |
Before matching |
After matching |
||||
|
hCG |
rLH |
p |
hCG |
rLH |
p |
|
|
Age (mean (SD)) |
35.73 (4.58) |
37.66 (4.50) |
<0.001 |
35.73 (4.58) |
36.15 (4.06) |
0.335 |
|
Age group (%) |
|
|
<0.001 |
|
|
1 |
|
<35 |
171 (34.4%) |
60 (21.8%) |
|
34.40% |
34.40% |
|
|
35-38 |
192 (38.6%) |
88 (32.0%) |
|
38.60% |
38.60% |
|
|
39-42 |
100 (20.1%) |
84 (30.5%) |
|
20.10% |
20.10% |
|
|
43+ |
34 (6.8%) |
43 (15.6%) |
|
6.80% |
6.80% |
|
|
BMI (mean (SD)) |
21.50 (2.18) |
22.06 (2.40) |
0.001 |
21.50 (2.18) |
21.41 (1.93) |
0.685 |
|
Basal FSH (mIU/mL) (mean (SD)) |
7.93 (2.82) |
8.06 (2.70) |
0.532 |
7.93 (2.82) |
7.95 (2.65) |
0.945 |
|
Basal LH (mIU/mL) (mean (SD)) |
5.64 (1.81) |
4.66 (1.96) |
<0.001 |
5.64 (1.81) |
5.72 (1.95) |
0.687 |
|
AMH (ng/mL) (mean (SD)) |
2.24 (1.57) |
1.89 (1.59) |
0.003 |
2.24 (1.57) |
2.10 (1.47) |
0.413 |
|
AFC (mean (SD)) |
12.02 (6.59) |
10.12 (6.78) |
<0.001 |
12.02 (6.59) |
11.52 (6.65) |
0.557 |
|
Infertility types (%) |
|
|
0.209 |
|
|
0.503 |
|
Primary |
194 (39.0%) |
94 (34.2%) |
|
39.00% |
35.20% |
|
|
Secondary |
303 (61.0%) |
181 (65.8%) |
|
61.00% |
64.80% |
|
|
Infertility factor (%) |
|
|
0.002 |
|
|
1 |
|
Female factor |
277 (55.7%) |
187 (68.0%) |
|
55.70% |
55.70% |
|
|
Male factor |
27 (5.4%) |
15 (5.5%) |
|
5.40% |
5.40% |
|
|
Both |
60 (12.1%) |
31 (11.3%) |
|
12.10% |
12.10% |
|
|
Unexplained |
133 (26.8%) |
42 (15.3%) |
|
26.80% |
26.80% |
|
|
Previous IVF attempts (mean (SD)) |
0.69 (1.10) |
1.29 (1.52) |
<0.001 |
0.69 (1.10) |
1.09 (1.09) |
<0.001 |
|
>2 previous IVF attempts (%) |
29 (5.8%) |
44 (16.0%) |
<0.001 |
5.80% |
7.40% |
0.446 |
|
Length of stimulation (days) (mean (SD)) |
10.21 (0.64) |
10.13 (0.64) |
0.095 |
10.21 (0.64) |
10.19 (0.55) |
0.702 |
|
Total FSH dosage (IU) (mean (SD)) |
2672 (143) |
3039 (191) |
<0.001 |
2672 (143) |
3056 (164) |
<0.001 |
|
Total hCG dosage (IU) (mean (SD)) |
521(64) |
NA |
NA |
NA |
NA |
NA |
|
Total LH dosage (IU) (mean (SD)) |
NA |
385 (48) |
NA |
NA |
NA |
NA |
|
Progesterone level on trigger day (ng/mL) (mean (SD)) |
0.92 (0.67) |
0.84 (0.69) |
0.085 |
0.92 (0.67) |
1.05 (0.86) |
0.24 |
|
Estradiol level on trigger day (pg/mL) (mean (SD)) |
3601 (2536) |
2675 (2113) |
<0.001 |
3601 (2536) |
3158 (2261) |
0.116 |
AFC, antral follicle count; AMH, anti-Mullerian hormone; BMI, body mass index; FSH, follicle-stimulating hormone; hCG, human chorionic gonadotropin; IVF, in vitro fertilization; LH, lutenizing hormone.
Table 1: Patient and ovarian stimulation characteristics
Oocyte-related, and embryo-related outcomes
In the unadjusted analysis, compared to the rLH group, patients in the hCG group had higher total number of oocytes and number of MII oocytes, and higher number of embyros, all difference being statistically significant. After adjusting for confounding using full matching of propensity scores, the estimates of treatment effect decreased. The average number of oocytes and MII oocytes in the hCG group was significantly higher: 1.19-times (95%CI 1.01, 1.40) and 1.23-times (95%CI 1.00, 1.52) respectively. The average number of cleavage embryos, good cleavage embryos, blastocyst embryos, and good blastocyst embryos in the hCG group was higher without achieving significance :1.14-times (95%CI 0.94, 1.38), 1.09-times (95%CI 0.81, 1.38), 1.22-times (95%CI 0.98, 1.53), and 1.09-times (95%CI 0.80, 1.49) respectively. The mean ratios of FOI and FORT were similar between the unadjusted and adjusted analysis (Table 2). Three patients in the hCG group and none in the rLH group had OHSS.
|
Outcome |
hCG |
rLH |
Unadjusted treatment effect |
Adjusted treatment effect |
|
Total number of oocytes |
8.97 (5.74) |
6.52 (4.48) |
1.38 (1.25, 1.52) |
1.19 (1.01, 1.40) |
|
Number of MII oocytes |
6.79 (5.00) |
4.63 (3.76) |
1.47 (1.31, 1.65) |
1.23 (1.00, 1.52) |
|
Number of cleavage embryos |
5.72 (4.35) |
4.07 (3.27) |
1.41 (1.25, 1.58) |
1.14 (0.94, 1.38) |
|
Number of good cleavage embryos |
3.87 (3.45) |
2.69 (2.62) |
1.44 (1.25, 1.65) |
1.09 (0.84, 1.40) |
|
Number of blastocyst embryos |
7.26 (4.49) |
5.15 (3.41) |
1.41 (1.23, 1.61) |
1.22 (0.98, 1.53) |
|
Number of good blastocyst embryos |
2.96 (2.74) |
2.18 (2.19) |
1.36 (1.11, 1.66) |
1.09 (0.80, 1.49) |
|
Follicular output rate (FORT) |
0.78 (0.38) |
0.71 (0.40) |
1.11 (1.02, 1.20) |
1.10 (0.97, 1.24) |
|
Follicle-to-oocyte index (FOI) |
0.70 (0.31) |
0.69 (0.34) |
1.01 (0.94, 1.08) |
1.00 (0.91, 1.10) |
Outcomes are expressed as mean (SD), treatment effect is difference in mean ratio (95%CI)
Table 2: Comparison of outcomes between hCG and rLH
Pregnancy outcomes
A total of 121 patients from the rLH group and 302 patients from the hCG group underwent the first embryo transfer. There were no significant differences between the groups in terms of endometrial preparation, endometrial thickness, number or type of embryos. Most patients in both groups had a single embryo transferred (63.9% for hCG and 58.7% for rLH). The two groups showed no significant difference in the percentage of cycles resulting in pregnancy (47.8% for hCG and 47.1% for rLH), biochemical pregnancy (7.8% for hCG and 4.2% for rLH), clinical pregnancy (39.9% for hCG and 42.5% for rLH), ongoing pregnancy (32.7% for hCG and 35.1% for rLH), and implantation (39.7% for hCG and 41.7% for rLH) (Table 3).
|
Characteristics |
hCG (n=302) |
rLH (n=121) |
p |
|
Endometrial preparation (%) |
|
|
0.415 |
|
Hormone therapy |
228 (75.5) |
92 (76.0) |
|
|
Natural cycle |
40 (13.2) |
13 (10.7) |
|
|
Mild stimulation |
2 (0.7) |
3 (2.5) |
|
|
Fresh embryo transfer (ET) |
32 (10.6) |
13 (10.7) |
|
|
Endometrial thickness (mm) (mean (SD)) |
9.55 (1.29) |
9.61 (1.39) |
0.694 |
|
Embyros transfer (%) |
|
|
0.297 |
|
Single embryo |
193 (63.9) |
71 (58.7) |
|
|
Double embryo |
105 (34.8) |
46 (38.0) |
|
|
Triple embryo |
4 (1.3) |
4 (3.3) |
|
|
Type of embryos (%) |
|
|
0.327 |
|
Cleavage stage |
129 (48.9) |
59 (55.1) |
|
|
Blastocyst stage |
135 (51.1) |
48 (44.9) |
|
|
Pregnancy (%) |
141 (47.8) |
56 (47.1) |
0.978 |
|
Biochemical pregnancy (%) |
23 (7.8) |
5 (4.2) |
0.262 |
|
Clinical pregnancy (%) |
118 (39.9) |
51 (42.5) |
0.7 |
|
Ongoing pregnancy (%) |
91 (32.7) |
39 (35.1) |
0.738 |
|
Implantation (%) |
117 (39.7) |
50 (41.7) |
0.789 |
|
Multiple pregnancy (%) |
11 (3.7) |
8 (6.7) |
0.299 |
Table 3: Pregnancy outcomes after the first transfer of embryos originating from the two regimens
Discussion
In this study, the effects of rLH and hCG supplementation during the late follicular phase were compared in patients with slow response to rFSH. Results showed that hCG supplementation at a dosage of 100 IU/day was associated with significant better oocyte-related outcomes compared to rLH supplementation, but the differences in embryo-related outcomes was not statistically significant.
A slow response to rFSH is a relatively common phenomenon encountered in clinical practice. One of the main difficulties arises from the lack of a standardized definition and the limited availability of evidence-based interventions. When a slow response to rFSH is observed during ovarian stimulation, two potential approaches have been proposed: (1) to increase the dose of FSH, and/or (2) to supplement with luteinizing hormone (LH) activity products[17]. In the context of this study, all patients received an initial FSH dosage of 300 IU/day, which is the optimal dose, as previously suggested [18]. Pharmaceutical restrictions and cost considerations led us to use 75 IU rLH available within Pergoveris or 100 IU hCG as therapeutic interventions. Meanwhile, the ratio of bioactivity between hCG and LH is 6:1, this means that 6 IU of LH supplementation is expected to be as effective as 1 IU of hCG[19] .Therefore, the effects of 100 IU/day hCG would have been equivalent to 600 IU of LH which was not conceivable for cost reasons. We would like to emphasize that the primary objective of our study was not to compare the potency of LH activity between these two treatment strategies, but rather to evaluate their respective effectiveness in enhancing ovarian response.
Although oocyte-related outcomes were improved following hCG supplementation, we did not observe any significant improvement in terms of embryo-related outcomes. The concept of “the more oocytes, the better” in ovarian stimulation has been controversial for a long time. While a robust stimulation is able to increase the number of retrieved oocytes and to reduce cycle cancellations, the chances of a live birth also depends on woman’s prognostic profile including age and genetic quality of the oocytes[20]. In our study, patients with a slow response to rFSH monotherapy were older than normo-responders and a smaller number of oocytes could be collected. Therefore, adding LH-activity products is unlikely to improve embryo-related parameters and pregnancy outcomes.
In this study, we included FOI and FORT, parameters used to assess treatment outcomes. Both are indicators of the dynamic follicular growth during ovarian stimulation, with low values attesting for a suboptimal ovarian response to gonadotropins[3]. In our experience, delayed follicular development at day 6 of ovarian stimulation is usually associated with low FOI and FORT values, as commonly observed in POSEIDON Group 2 patients. Inspite of good ovarian reserve, a suboptimal follicular development is observed in these patients and results in a low yield of collected oocytes. Chen et al. demonstrated that POSEIDON Group 2 patients had the lowest FOI and FORT values (0.62 and 0.68, respectively) compared to other POSEIDON groups[21]. In these women, a supplementation with LH activity products has been proposed to increase the number of retrieved oocytes abd therefore to improve FOI or FORT. In our study, the mean ratios of both FOI and FORT were similar between the unadjusted and adjusted analyses, suggesting that both products have comparable effects on the dynamic follicular growth.
An important aspect when considering treatment options is the issue of cost effectiveness. Patients with a slow response to rFSH frequently require multiple stimulation cycles, receive large doses of gonadotropins, and are frequently supplemented with LH activity products. In our study, the total FSH dose required in the hCG group was significantly lower compared to those used in the rLH group. In addition, rLH is relatively expensive, while the cost of hCG is considerably lower, resulting in a marked difference in expenses related to ovarian stimulation medications. Consequently, the regimen using low-dose hCG supplementation becomes an attractive choice[22], particularly for developing nations, where IVF treatments may not be qualified for insurance, thus preventing access for couples with limited financial capacity. A cost effectiveness study is required to confirm these data on a large-scale clinical trial before new general recommendations can be made.
While performed on a large sample size selected population, this retrospective study is at risk of confounding bias due to the lack of randomization. Therefore, differences observed in baseline characteristics between the two treatment groups might have hedged our conclusion. For that reason, we have done our best to remove this confounding effect by using propensity score matching in order to provide a less biased treatment effect estimate. Nevertheless, some unmeasured confounding fac tors might exist such as the patient economic status[23] which was not recorded in our archived dataset. To overcome these drawbacks, large-scale prospective cohort studies or randomized clinical trials are needed.
In conclusion, in women with slow response to rFSH, hCG supplementation at a dosage of 100 IU/day was associated with a significant better oocyte-related outcome compared to rLH supplementation, but the differences in embryo-related outcomes were not significant. Addition of the inexpensive dose of hCG 100 IU/day to r.FSH is a potential effective alternative treatment in this selected group of patients. More research should be done to better determine the clinical interest of this therapeutic association and its cost effectiveness as well.
Supplementary Information
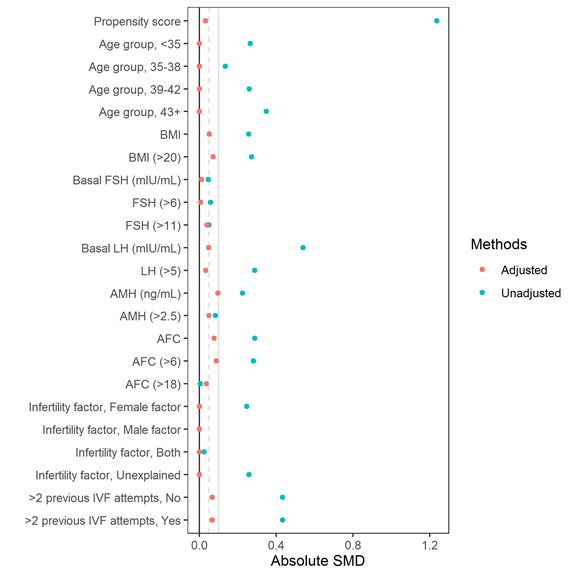
Figure S1: Love plot comparing standardized mean differences before and after full matching.
Declarations
Ethics approval and consent to participate:
The research was approved under the Decision No. IRB.TAHN.024 by the Institutional Review Board dated March 30th, 2023, before initiating any research activities.
Consent for publication:
Not applicable.
Availability of data and materials:
Deidentified data can be made upon requests with the approval of the principal investigators and the permission of the hospital. Requests may be subject to review by the local Institutional Review Board.
Competing interests:
The authors declare that they have no competing interests.
Funding:
No funding was received to assist with the preparation of this manuscript.
Authors' contributions:
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Hoang Bao Long, Vu Thi Mai Anh, Nguyen Thi Thuy Linh, Nguyen Phuc Hieu and Giap Thi Mai Phuong. The first draft of the manuscript was written by Le Duc Thang and Hoang Bao Long, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Acknowledgements:
The authors thank all the staff at the Assisted Reproduction Center, Tam Anh General Hospital for making this study possible.
REFERENCES
- La Marca A and Sunkara S K. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update, (2014); 20(1); 124-140.
- Alviggi C, Conforti A, Esteves S C, et al. Recombinant luteinizing hormone supplementation in assisted reproductive technology: a systematic review. Fertil Steril, (2018); 109(4); 644-664.
- Alviggi C, Conforti A, Esteves S C, et al. Understanding Ovarian Hypo-Response to Exogenous Gonadotropin in Ovarian Stimulation and Its New Proposed Marker—The Follicle-To-Oocyte (FOI) Index. Front Endocrinol, (2018); 9; 589.
- Alviggi C, Pettersson K, Longobardi S, et al. A common polymorphic allele of the LH beta-subunit gene is associated with higher exogenous FSH consumption during controlled ovarian stimulation for assisted reproductive technology. Reprod Biol Endocrinol RBE, (2013); 11; 51.
- Alviggi C, Conforti A, Caprio F, et al. In Estimated Good Prognosis Patients Could Unexpected “Hyporesponse” to Controlled Ovarian Stimulation be Related to Genetic Polymorphisms of FSH Receptor? Reprod Sci Thousand Oaks Calif, (2016); 23(8); 1103-1108.
- Lindgren I, Bååth M, Uvebrant K, et al. Combined assessment of polymorphisms in the LHCGR and FSHR genes predict chance of pregnancy after in vitro fertilization. Hum Reprod Oxf Engl, (2016); 31(3); 672-683.
- Smitz J and Platteau P. Influence of human chorionic gonadotrophin during ovarian stimulation: an overview. Reprod Biol Endocrinol, (2020); 18(1); 80.
- Casarini L, Riccetti L, De Pascali F, et al. Follicle-stimulating hormone potentiates the steroidogenic activity of chorionic gonadotropin and the anti-apoptotic activity of luteinizing hormone in human granulosa-lutein cells in vitro. Mol Cell Endocrinol, (2016); 422; 103-114.
- Orvieto R. HMG versus recombinant FSH plus recombinant LH in ovarian stimulation for IVF: does the source of LH preparation matter?. Reprod Biomed Online, (2019); 39(6); 1001-1006.
- Thuesen L L, Smitz J, Loft A, et al. Endocrine effects of hCG supplementation to recombinant FSH throughout controlled ovarian stimulation for IVF: a dose-response study. Clin Endocrinol (Oxf), (2013); 79(5); 708-715.
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod Oxf Engl, (2011); 26(6); 1270-1283.
- Genro V K, Grynberg M, Scheffer J B, et al. Serum anti-Müllerian hormone levels are negatively related to Follicular Output RaTe (FORT) in normo-cycling women undergoing controlled ovarian hyperstimulation. Hum Reprod Oxf Engl, (2011); 26(3); 671-677.
- Pfeifer S, Butts S, Dumesic D, et al. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril, (2016); 106(7), 1634-1647.
- Rubin D B. Using Propensity Scores to Help Design Observational Studies: Application to the Tobacco Litigation. Health Serv Outcomes Res Methodol, (2001); 2(3), 169-188.
- Ho D, Imai K, King G, et al. (2011). MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J Stat Softw, (2011); 42; 1-28.
- Greifer N and Stuart E A. Choosing the Estimand When Matching or Weighting in Observational Studies. ArXiv210610577 Stat, (2021).
- Conforti A, Esteves S C, Cimadomo D, et al. Management of Women With an Unexpected Low Ovarian Response to Gonadotropin. Front Endocrinol, (2019); 10; 387.
- Ovarian Stimulation T.E.G.G.O., Bosch E., Broer S., et al. ESHRE guideline: ovarian stimulation for IVF/ICSI†. Hum Reprod Open, (2020); 2020(2); hoaa009.
- Alsbjerg B, Elbaek H O, Laursen R J, et al. Bio-equivalent doses of recombinant HCG and recombinant LH during ovarian stimulation result in similar oestradiol output: a randomized controlled study. Reprod Biomed Online, (2017); 35(2), 232-238.
- Leijdekkers J A, Torrance H L, Schouten N E, et al. Individualized ovarian stimulation in IVF/ICSI treatment: it is time to stop using high FSH doses in predicted low responders. Hum Reprod, (2020); 35(9); 1954-1963.
- Chen L, Wang H, Zhou H, et al. Follicular Output Rate and Follicle-to-Oocyte Index of Low Prognosis Patients According to POSEIDON Criteria: A Retrospective Cohort Study of 32,128 Treatment Cycles. Front Endocrinol, (2020); 11; 181.
- Iaconelli C A R, Setti A S, Braga D P A F, et al. Concomitant use of FSH and low-dose recombinant hCG during the late follicular phase versus conventional controlled ovarian stimulation for intracytoplasmic sperm injection cycles. Hum Fertil Camb Engl, (2017); 20(4); 285-292.
- Disparities in access to effective treatment for infertility in the United States: an Ethics Committee opinion. Fertil Steril, (2021); 116(1); 54-63.
