Clinical Significance of Anti-U1 Ribonucleoprotein Antibody in Anti-Topoisomerase I Antibody-Positive Systemic Sclerosis Patients: A Retrospective Observational Study
Article Information
Kazuki M Matsuda1, Ayumi Yoshizaki1*, Hirohito Kotani1, Ai Kuzumi1, Maiko Fukayama1, Takemichi Fukasawa1, Satoshi Ebata1, Asako Yoshizaki-Ogawa1, Yoshihide Asano1, Koji Oba2, Shinichi Sato1
1Department of Dermatology, The University of Tokyo Graduate School of Medicine, Tokyo, Japan
2Department of Biostatistics, School of Public Health, The University of Tokyo Graduate School of Medicine, Tokyo, Japan
*Corresponding author: Ayumi Yoshizaki, Department of Dermatology, The University of Tokyo Graduate School of Medicine, 7-3-1, Hongo, Bunkyo-ku, Tokyo, Japan
Received: 28 December 2020; Accepted: 06 January 2021; Published: 20 January 2021
Citation: Kazuki M Matsuda, Ayumi Yoshizaki, Hirohito Kotani, Ai Kuzumi, Maiko Fukayama, Takemichi Fukasawa, Satoshi Ebata, Asako Yoshizaki-Ogawa, Yoshihide Asano, Koji Oba, Shinichi Sato. Clinical Significance of Anti-U1 Ribonucleoprotein Antibody in Anti-Topoisomerase I Antibody-Positive Systemic Sclerosis Patients: A Retrospective Observational Study. Archives of Clinical and Biomedical Research 5 (2021): 76-84.
Share at FacebookAbstract
Coexistence of anti-topoisomerase (topo) I antibody (Ab) and anti-U1 ribonucleoprotein (RNP) Ab is occasionally observed among patients with systemic sclerosis (SSc). To clarify the clinical significance of anti-U1 RNP Ab in patients with anti-topo I Ab-positive SSc, we conducted a retrospective review of SSc patients initially arrived at our clinic since April 2011 until March 2020. Serum levels of anti-topo I Ab were examined by enzyme-linked immunosorbent assay, and anti-topo I Ab-positive patients were recruited into the study. Serum anti-U1 RNP Ab positivity was explored by chemiluminescent enzyme immunoassay. In total, 92 patients were enrolled into our study. Of these, 11 cases were anti-U1 RNP Ab-positive (anti-U1RNP+) and 81 patients were anti-U1 RNP Ab-negative (anti-U1RNP-). Age of SSc onset was significantly lower in anti-U1RNP+ group than in anti-U1RNP- group (median [25-75th percentiles], 26.8 [20.3-39.6] vs 45.1 [31.5-56.8] years, P < 0.01). Additionally, serum levels of anti-topo I Ab were significantly higher among anti-U1RNP+ patients compared to the others (166 [124-195] vs 135 [104-174] U/mL, P < 0.05). Moreover, concurrence of systemic lupus erythematosus and Sjögren’s syndrome was more common in anti-U1RNP+ group than in anti-U1RNP- group (45.5% vs 1.2%, P < 0.001; 54.5% vs 12.3%, P < 0.01). In conclusion, anti-U1 RNP Ab is associated with early disease onset, reduced vital capacity of the lung, and concurrence of systemic lupus erythematosus and Sjögren’s syndrome among SSc patients with anti-topo I Ab.
Keywords
Autoantibody; Systemic sclerosis; Ribonucleoprotein; Topoisomerase
Article Details
Abbreviations:
%DLco: The percentage of diffusing capacity for carbon monoxide; %VC: The percentage of predicted vital capacity; Ab: Antibody; APS: Anti-phospholipid antibody syndrome; CTD: Aonnective tissue disease; dcSSc: Diffuse cutaneous systemic sclerosis; DM: Dermatomyositis; dsDNA: Double stranded DNA; ILD: Interstitial lung disease; mRSS: Modified Rodnan total skin thickness score; PM: Polymyositis; RNP: Ribonucleoprotein; SjS: Sjögren’s syndrome; SLE: Systemic lupus erythematosus; SSc: Systemic sclerosis; Topo: Topoisomerase
1. Introduction
Systemic sclerosis (SSc) is a connective tissue disease (CTD) characterized by organ fibrosis and vasculopathy with an autoimmune background [1]. The hallmark of immunological abnormality in SSc patients is autoantibodies detected from their serum; anti-nuclear antibody (Ab) is detected from more than 90% of the patients [2]. The Abs targeting some of the intranuclear autoantigens are highly specific to SSc, each of which represents a clinical subtype of SSc with distinct features. For instance, the appearance of anti-topoisomerase (topo) I Ab is associated with diffuse skin sclerosis and severe lung fibrosis [3].
U1 ribonucleoprotein (RNP) is one of the RNA-protein complexes that participate in pre-messenger RNA processing in the nucleus. Anti-U1 RNP Ab is generally known as a serologic marker of mixed connective tissue disease, and also detected from the sera of patients with other CTDs. The prevalence of anti-U1 RNP Ab in the serum of Japanese patients with SSc has been reported to be around 10% [4].
Some of the patients with SSc have multiple autoantibodies in their serum at the same time. In particular, coexistence of anti-topo I Ab and anti-U1 RNP Ab is relatively common; the frequency of anti-U1 RNP Ab detection among anti-topo I Ab-positive SSc was reported to be 24% in a cohort consisting of Caucasians, Latins, and African Americans [5]. Although the combination of anti-topo I Ab and anti-U1 RNP Ab has also been reported in Japanese population [6] to its clinical significance has not yet been fully elucidated. Herein, we conducted a retrospective observational study to clarify the impact of anti-U1 RNP Ab among anti-topo I Ab-positive Japanese patients with SSc.
2. Materials & Methods
2.1 Patient enrollment and autoantibody profiling
We recruited Japanese SSc patients initially arrived at our clinic since April 2011 until March 2020, all of whom fulfilled the classification criteria established by the American College of Rheumatology and European League Against Rheumatism in 2013 [7]. Serum levels of anti-topo I Ab, anti-centromere Ab, anti-RNA polymerase III Ab, and anti-double stranded DNA (dsDNA) Ab were examined by enzyme-linked immunosorbent assays. Anti-topo I Ab-positive patients were recruited into the study, while anti-centromere Ab-positive or anti-RNA polymerase III Ab-positive patients were excluded. Pediatric patients were also ruled out. Serum anti-U1 RNP Ab, anti-Sm Ab, anti-SSA Ab, and anti-SSB Ab positivity was explored by chemiluminescent enzyme immunoassays. The whole study was approved by the ethical committee of The University of Tokyo Hospital, and the study complied with the Declaration of Helsinki guidelines. Informed consent was obtained from the participants.
2.2 Clinical assessment
Clinical data of the patients were gathered by retrospective review of electric medical records. Demographic information, laboratory results, and examination findings were obtained at the time closest to the first arrival to our clinic. Onset of the disease was defined as the first clinical event that was a clear manifestation of SSc other than Raynaud’s phenomenon. Disease duration was defined as the interval between the onset of the disease and the time of the initial visit to our clinic. Patients were categorized by LeRoy’s classification rule into diffuse cutaneous SSc (dcSSc) or limited cutaneous SSc [1]. Skin thickness was semi-quantitatively examined by the modified Rodnan total skin thickness score (mRSS). Interstitial lung disease (ILD) was defined as bibasilar interstitial fibrosis on high-resolution computer tomography. Pulmonary hypertension was defined as mean pulmonary artery pressure higher than 25 mmHg on right heart catheterization. Scleroderma renal crisis was framed as malignant hypertension and/or rapidly progressive renal dysfunction. Reflux esophagitis was defined as Grade M or more on Los Angeles classification on the basis of gastrointestinal endoscopy [8]. Concurrence of systemic lupus erythematosus (SLE), Sjögren’s syndrome (SjS), polymyositis (PM)/derma-tomyositis (DM), and anti-phospholipid Ab syndr-ome (APS) was determined in compliance with their classification criteria previously established [9-12].
2.3 Statistical analysis
Statistical analysis was performed by Mann-Whitney’s U-test for continuous variables, and Fisher’s exact test for categorical variables. All the analyses were performed using Stata/IC 15 (StataCorp LLC, TX, USA).
3. Results
3.1 Anti-U1 RNP Ab was associated with earlier disease onset and higher serum titers of anti-topo I Ab
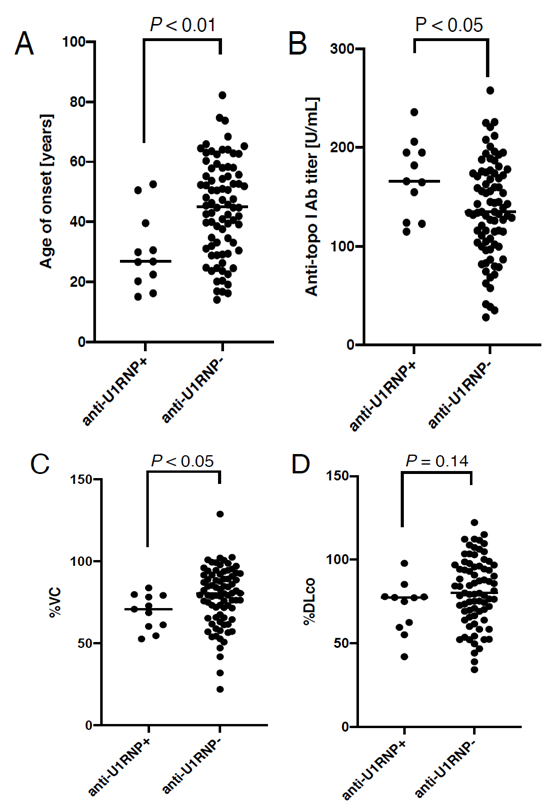
In total, 92 patients were enrolled into our study. Of these, 11 cases were anti-U1 RNP Ab-positive (anti-U1RNP+, Table 1) and 81 patients were anti-U1 RNP Ab-negative (anti-U1RNP-). Their clinical features stratified by anti-U1 RNP Ab positivity are described in Table 2. Sex ratio and disease duration was balanced between the two groups. On the contrary, the age of disease onset was significantly lower in anti-U1RNP+ group than in anti-U1RNP- group (median [25-75th percentiles], 26.8 [20.3-39.6] vs 45.1 [31.5-56.8] years, P < 0.01; Figure 1A). In addition, the serum levels of anti-topo I Ab were significantly higher in anti-U1RNP+ group (166 [124-195] vs 135 [104-174] U/mL, P < 0.05; Figure 1B).
3.2 Vital capacity of the lung was significantly reduced in anti-U1RNP+ group than in anti-U1RNP- group
The proportion of dcSSc and the extension of skin sclerosis measured by mRSS were comparable bet-ween anti-U1RNP+ group and anti-U1RNP- group. In addition, there was no significant difference in the prevalence of cutaneous vascular symptoms, inclu-ding Raynaud’s phenomenon, nail fold bleeding, or telangiectasia, between the two groups. With regard to lung involvement, the percentage of predicted vital capacity was significantly lower in anti-U1RNP+ group than in anti-U1RNP- group (70.9% [60.4-79.3%] vs 80.4% [65.9-91.7%], P < 0.05; Figure 1C). Although not statistically significant, the percentage of diffusing capacity for carbon monoxide was also lower in anti-U1RNP Ab+ group than in anti-U1RNP- group (77.3% [59.6-78.0%] vs 80.2% [69.1-96.2%], P = 0.14; Figure 1D). The prevalence of other visceral involvements such as pulmonary hypertension, scleroderma renal crisis, and reflux esophagitis was comparable between the two groups.
3.3 Anti-dsDNA Ab positivity, anti-SSA Ab positivity, and concurrence of SLE and SjS was more common in anti-U1RNP+ group than in anti-U1RNP- group
The proportion of anti-dsDNA Ab positivity was significantly higher among anti-U1RNP+ group compared to the other (27.3% vs 2.5%, P < 0.05). In addition, anti-SSA Ab positivity was more common in anti-U1RNP+ group than in anti-U1RNP- group (63.6% vs 30.9%, P < 0.05). Moreover, the prevalence of SLE and SjS was significantly higher among anti-U1RNP+ group than in anti-U1RNP- group (45.5% vs 1.2%, P < 0.001; 54.5% vs 12.3%, P < 0.01). The prevalence of anti-Sm Ab and anti-SSB Ab positivity, PM/DM, and APS was comparable between the two groups.
|
No |
Sex |
Age of disease onset |
ILD |
%VC |
%DLco |
SLE |
SjS |
PM/DM |
APS |
|
1 |
M |
23 |
+ |
54.6 |
55.2 |
+ |
- |
- |
+ |
|
2 |
F |
15 |
+ |
85.0 |
77.5 |
- |
+ |
- |
- |
|
3 |
F |
16 |
+ |
88.3 |
77.3 |
+ |
+ |
- |
- |
|
4 |
F |
20 |
+ |
58.7 |
42.1 |
+ |
+ |
- |
- |
|
5 |
F |
27 |
+ |
80.9 |
85.3 |
- |
- |
+ |
- |
|
6 |
F |
27 |
- |
68.8 |
79.5 |
- |
- |
- |
- |
|
7 |
F |
31 |
- |
93.4 |
97.9 |
+ |
- |
- |
- |
|
8 |
F |
40 |
+ |
77.2 |
77.8 |
- |
+ |
- |
- |
|
9 |
F |
40 |
+ |
83.4 |
59.6 |
+ |
+ |
- |
- |
|
10 |
F |
51 |
+ |
66.4 |
62.5 |
- |
+ |
- |
- |
|
11 |
F |
53 |
+ |
67.1 |
75.0 |
- |
- |
- |
- |
No: the number of patients; M: male; F: female; ILD: interstitial lung disease, %VC: the percentage of predicted vital capacity, %DLco: the percentage of diffusing capacity for carbon monoxide. SLE: systemic lupus erythematosus, SjS: Sjögren’s syndrome, PM: polymyositis, DM: dermatomyositis, APS: anti-phospholipid antibody syndrome.
Table 1: Clinical characteristics of systemic sclerosis cases with both anti-topoisomerase I antibody and anti-U1 ribonucleoprotein antibody.
|
Anti-U1RNP+ (N = 11) |
Anti-U1RNP- (N = 81) |
P value |
|
|
Demographics |
|||
|
Male/Female (number) |
1/10 |
13/68 |
1.00 |
|
Age of onset, years (median) |
26.8 |
45.1 |
< 0.01 |
|
Disease duration, years (median) |
2.4 |
2.2 |
0.44 |
|
Serum anti-topo I Ab levels, U/mL (median) |
166 |
135 |
< 0.05 |
|
Skin involvement |
|||
|
dcSSc |
63.6 |
59.3 |
1.00 |
|
mRSS (median) |
12.5 |
12.0 |
0.67 |
|
Cutaneous vascular symptoms |
|||
|
Raynaud’s phenomenon |
81.8 |
92.6 |
0.52 |
|
Neil fold bleeding |
72.7 |
67.9 |
1.00 |
|
Telangiectasia |
45.5 |
21.0 |
0.09 |
|
Lung involvement |
|||
|
ILD |
81.8 |
77.8 |
1.00 |
|
%VC (median) |
70.9 |
80.4 |
< 0.05 |
|
%DLco (median) |
77.3 |
80.2 |
0.14 |
|
Other visceral involvements |
|||
|
Pulmonary hypertension |
0 |
0 |
- |
|
Scleroderma renal crisis |
0 |
3.7 |
1.00 |
|
Reflux esophagitis |
27.3 |
38.3 |
0.73 |
|
Autoantibody profiles |
|||
|
Anti-dsDNA Ab |
27.3 |
2.6 |
< 0.05 |
|
Anti-Sm Ab |
9.1 |
1.2 |
0.22 |
|
Anti-SSA Ab |
63.6 |
30.9 |
< 0.05 |
|
Anti-SSB Ab |
9.1 |
6.2 |
0.55 |
|
Concurrent diseases |
|||
|
SLE |
45.5 |
1.2 |
< 0.001 |
|
SjS |
54.5 |
12.3 |
< 0.01 |
|
PM/DM |
9.1 |
0 |
0.12 |
|
APS |
9.1 |
2.5 |
0.32 |
The values are percentages otherwise noted. Statistical analysis was carried out by Mann-Whitney’s U-test for continuous variables and Fisher’s exact test for categorical variables. SSc: systemic sclerosis, N: number, dcSSc: diffuse cutaneous SSc, mRSS: modified Rodnan total skin thickness score. ILD: interstitial lung disease, %VC: the percentage of predicted vital capacity, %DLco: the percentage of diffusing capacity for carbon monoxide. dsDNA: double stranded DNA, SLE: systemic lupus erythematosus, SjS: Sjögren’s syndrome, PM: polymyositis, DM: dermatomyositis, APS: anti-phospholipid antibody syndrome.
Table 2: Clinical features of systemic sclerosis stratified by anti-U1 ribonucleoprotein (RNP) antibody (Ab) positivity.
Figure 1: Comparison between anti-U1 RNP antibody (Ab)-positive (anti-U1RNP+) group and anti-U1 RNP Ab-negative (anti-U1RNP-) group in SSc patients with anti-topoisomerase I Ab. The dot graphs show the age of disease onset (A), the serum levels of anti-topo I Ab (B), the percentage of predicted vital capacity (%VC) (C), and the percentage of diffusing capacity for carbon monoxide (%DLco) (D). P values were calculated by Mann-Whitney’s U-test. The horizontal line in each column shows the median.
4. Discussion
Our retrospective observation of Japanese patients with SSc revealed that anti-U1 RNP Ab was associated with early onset of SSc, lower vital capacity of the lung, and higher serum levels of anti-topo I Ab. Moreover, concurrence of SLE and SjS was more common in anti-U1RNP+ group than in anti-U1RNP- group, in line with a higher prevalence of anti-dsDNA Ab and anti-SSA Ab in connection with anti-U1 RNP Ab positivity. These data suggested the clinical significance of anti-U1 RNP Ab in anti-topo I Ab-positive SSc.
Our results are consistent with previous studies that suggested the potential impact of anti-U1 RNP Ab on the clinical features of SSc. Retrospective review of 223 patients with SSc revealed that decreased vital capacity was associated with anti-U1 RNP Ab detected from their serum by double immunodiffusion method [4]. Another examination of sera collected from 119 patients with SSc by immunoprecipitation method demonstrated that overlap with SLE was more common in patients with both anti-topo I Ab and anti-U1 RNP Ab than in those with anti-topo I Ab alone [5].
It is generally believed that CTDs share, to some extent, their immunologic background. This speculation derives from the fact that CTDs frequently overlap each other [13]. In addition, the early onset of SSc associated with the presence of anti-U1 RNP Ab found in the current study may reflect that SSc patients with concurrent anti-U1 RNP and anti-topo I Abs have a congenital vulnerability to CTDs, such as the specific human leukocyte antigen haplotypes [6].
We propose two hypotheses to explain why anti-U1 RNP Ab was associated with decreased vital capacity in SSc patients with anti-topo I Ab. First, the reduction in vital capacity possibly resulted from stronger disease activity of SSc. This hypothesis stands on our insight that higher serum levels of anti-topo I Ab were observed in anti-U1RNP+ group. Because topo I antigens are nuclear antigens, it is unlikely that anti-topo I Abs present in the blood enter the cell directly and exert their pathogenicity. However, the appearance of anti-topo I Ab has been strongly suggested in clinical practice to be associated with severe symptoms, which makes it plausible that immune abnormalities against autoantigens may play an important role in the development of SSc. Thus, the fact that anti-topo I Ab levels were higher in anti-U1RNP+ patients may reflect their robust immune response to self and may have been associated with severe ILD as a result. Another hypothesis is that anti-U1 RNP Ab reflects etiologies invading the lung other than SSc. This theory is based on the fact that concurrence of SLE and SjS were more common in anti-U1RNP+ group than in anti-U1RNP1- group. Both hypotheses might be true because immunizing with either topo I or U1 RNP antigens can induce ILD in mice, [14, 15] although the mechanism of how autoantibodies contribute to the CTD development still remains to be investigated.
The major limitation of this study is its retrospective design. In addition, the clinical significance of anti-U1 RNP Ab positivity among patients with other SSc-specific Abs, such as anti-centromere Ab and anti-RNA polymerase III Ab, should be examined in the future.
5. Conclusions
Retrospective observation revealed that anti-U1 RNP Ab was associated with early disease onset, reduced vital capacity of the lung, and concurrence of systemic lupus erythematosus and Sjögren’s syndrome among SSc patients with anti-topo I Ab in Japanese population.
References
- LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 15 (1988): 202-205.
- Okano Y. Antinuclear antibody in systemic sclerosis (scleroderma). Rheum Dis Clin North Am 22 (1996): 709-735.
- Sato S, Hamaguchi Y, Hasegawa M TK. Clinical significance of anti-topoisomerase I antibody levels determined by ELISA in systemic sclerosis. Rheumatol (Oxford) 40 (2001): 1135-1140.
- Ihn H, Yamane K, Yazawa N, Kubo M, Fujimoto M, Sato S, et al. Distribution and antigen specificity of anti-U1RNP antibodies in patients with systemic sclerosis. Clin Exp Immunol 117 (1999): 383-387.
- Satoh M, Krzyszczak ME, Li Y, Ceribelli A, Ross SJ, Chan EKL, et al. Frequent coexistence of anti-topoisomerase I and anti-U1RNP autoantibodies in African American patients associated with mild skin involvement: A retrospective clinical study. Arthritis Res Ther 13 (2011): R73.
- Kuwana M, Kaburaki J, Arnett FC, Howard RF, Medsger TA, Wright TM. Influence of ethnic background on clinical and serologic features in patients with systemic sclerosis and anti-dna topoisomerase I antibody. Arthritis Rheum 42 (1999): 465-474.
- Van Den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum 72 (2013): 1747-1755.
- Armstrong D, Bennett JR, Blum AL, Dent J, De Dombal FT, Galmiche P, et al. The endoscopic assessment of esophagitis: A progress report on observer agreement. Gastroenterology 111 (1996): 85-92.
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40 (1997): 1725.
- Fujibayashi T, Sugai S, Miyasaka N, Hayashi Y, Tsubota K. Revised Japanese criteria for Sjögren’s syndrome (1999): Availability and validity. Modern Rheumatology 6 (2004): 425-34.
- Bohan A PJ. Polymyositis and dermatomyositis. N Engl J Med 272 (1975): 344-347.
- Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost (2006).
- Iaccarino L, Gatto M, Bettio S, Caso F, Rampudda M, Zen M, et al. Overlap connective tissue disease syndromes. Autoimmun Rev 12 (2013): 363-373.
- Yoshizaki A, Yanaba K, Ogawa A, Asano Y, Kadono T, Sato S. Immunization with DNA topoisomerase I and Freund’s complete adjuvant induces skin and lung fibrosis and autoimmunity via interleukin-6 signaling. Arthritis Rheumatol 63 (2011): 3575-3585.
- Greidinger EL, Zang Y, Jaimes K, Hogenmiller S, Nassiri M, Bejarano P, et al. A murine model of mixed connective tissue disease induced with U1 small nuclear RNP autoantigen. Arthritis Rheum 54 (2006): 661-669.