Clinical Outcome of Stealth Cranioplasty for Adult Chiari Malformation 1: An Encouraging Experience
Article Information
Asifur RahmanORCID ID1*, Nazmin AhmedORCID iD2, Abu Saleh Mohammad Abu ObaidaORCID iD1, Abu Naim Wakil UddinORCID iD1, Bipin ChaurasiaORCID iD3, Md Atikur RahmanORCID iD1, Md Shamsul AlamORCID iD1, Md Moududul HaqueORCID iD1, Marco Maria FontanellaORCID iD4, Edoardo AgostiORCID iD4
1Department of Neurosurgery, Bangabandhu Sheikh Mujib Medical University, Kazi Nazrul Islam Avenue
Shahbag, Dhaka 1000, Bangladesh
2Department of Neurosurgery, Ibrahim Cardiac Hospital & Research Institute, Kazi Nazrul Islam Avenue
Shahbag, Dhaka-1000, Bangladesh
3Consultant neurosurgeon, Neurosurgery clinic, Birgunj 44300, Nepal
4Division of Neurosurgery, Department of Medical and Surgical Specialties, Radiological Sciences and Public Health, University of Brescia, Piazza Spedali Civili 1, 25123, Brescia, Italy
*Corresponding Author: Asifur Rahman, Department of Neurosurgery, Bangabandhu Sheikh Mujib Medical University, Kazi Nazrul Islam Avenue Shahbag, Dhaka 1000, Bangladesh;
Received: 23 January 2024; Accepted: 29 January 2024; Published: 29 February 2024
Citation: Asifur Rahman, Nazmin Ahmed, Abu Saleh Mohammad Abu Obaida, Abu Naim Wakil Uddin, Bipin Chaurasia, Md Atikur Rahman, Md Shamsul Alam, Md Moududul Haque, Marco Maria Fontanella, Edoardo Agosti. Clinical Outcome of Stealth Cranioplasty for Adult Chiari Malformation 1: An Encouraging Experience. Journal of Surgery and Research. 7 (2024): 87-95.
Share at FacebookAbstract
Objectives: Regardless of the surgical techniques, outcomes of symptomatic adult Chiari malformation 1 (CM1) with or without syrinx (SX) are debatable. This study aimed to assess and compare the clinical outcomes of 2 surgical procedures: posterior fossa decompression with or without duraplasty (PFD±DP) and Stealth cranioplasty (SC).
Methodology: This comparative cross-sectional study was conducted on adult CM1 patients who underwent PFD±DP and SC, from June 2019 to May 2021. Clinical outcomes were evaluated and compared by changes in clinical symptoms and signs, the Chicago Chiari Outcome Scale (CCOS) scoring, and the rate of complications.
Results: This study was conducted on 53 symptomatic adult CM1 patients of an average age of 30.35 ± 7.49 years, with or without SX. 30 patients (56.6%) underwent PFD±DP and 23 (43.4%) underwent SC. No significant statistical difference in postoperative changes in symptoms or neurological findings was found between the 2 groups. The mean CCOS score of the SC group (13.00 ± 1.78) was significantly better (p 0.001) than the PFD±DP (11.46 ± 1.25) group. Categorizing outcomes into groups based on CCOS was also significantly better (p 0.001) in the SC group. 11 patients (36.7%) had complications in the PFD±DP group while none of the patients in the SC group had complications which was statistically significant (p 0.003).
Conclusion: The SC group fared better than the PFD±DP group concerning outcomes based on CCOS and rate of complications. Thus, SC can be a good option in managing symptomatic adult Chiari malformation 1 with or without syrinx.
Keywords
Chiari malformation 1, Syrinx, Stealth Cranioplasty, Posterior Fossa Decompression
Article Details
Highlights
- Chiari malformation 1 (CM1) is still as enigmatic an entity as it was since its first mention.
- The clinical outcomes are perplexing despite endless endeavors with innovations of numerous surgical techniques and the management still formidable.
- Stealth cranioplasty (SC) is a good surgical technique with good clinical outcomes.
- The postoperative complication rate is remarkably low in Stealth cranioplasty than in traditional procedures.
Abbreviations list
C1: First cervical vertebra
CCOS: Chicago Chiari Outcome Scale
CM1: Chiari malformation 1
CSF: Cerebrospinal fluid
CVJ: Craniovertebral junction
FM: foramen magnum
PFD±DP: Posterior fossa decompression with or without duraplasty
SC: Stealth cranioplasty
SX: Syrinx
Introduction
From the very beginning of its discovery, Chiari malformation 1 (CM1) remains as enigmatic as ever. Its pathophysiology, diagnosis, management, and prognosis are still baffling. The outcomes vary a lot depending on the duration of symptoms, presentation, and management protocol. Often the accompanying syringomyelia (SM) with CM1 makes the management more complicated and the outcomes also vary.
CM1, the mildest form of the Chiari spectrum, is defined as more than 5 mm of caudal displacement of the tonsils below the foramen magnum in adults [1-6]. However, other than tonsillar ectopia combinations of different clinical and radiological findings are essential in decision-making for diagnosis and treatment [3]. CM1 has generally been recognized as a disorder of the craniovertebral junction (CVJ) resulting from a small posterior fossa with overcrowding of the neural structures. Disruption of cerebrospinal fluid (CSF) flow resulting in altered dynamics around the CVJ owing to impediment around the foramen magnum (FM) by the herniated tonsils often leads to associated syringomyelia [1,4-9].
The surgery for CM1, associated with or without SX, is primarily directed to decompress the neural elements around the CVJ and restore CSF flow and dynamics as much as possible [1,10,11]. Posterior fossa decompression with or without duraplasty (PFD±DP) along with the removal of the posterior arch of the first cervical vertebra (C1) is the most commonly performed and widely accepted surgical technique for CM1 and associated SX. Various combinations of surgical procedures have evolved to achieve improvement in symptoms and signs as well as to minimize complications [4,9-11].
Generally, current surgical strategies can achieve satisfactory success in providing a favorable outcome [4]. However, the heterogeneous array of the population, small cohorts, and retrospective nature of most of the studies make the outcomes widely variable. Different factors like duration of symptoms, presence of SM, and discrepancy in outcomes pose difficulty in coming to a concrete decision about any definitive surgical protocol [9,10,12]. With advanced technologies and research, the pathophysiology of CM1 and SX are gradually unfolding but at the same time, newer dimensions of the complex nature are emerging. With all the odds, a definitive surgical protocol appears to be a remote possibility in these circumstances. Many modifications centering on this basic idea have been introduced and each has its own merits and demerits.
After a long way of trial and error, we have introduced our technique, Stealth cranioplasty (SC). This study compared the clinical outcomes between SC and the traditional commonly practiced procedure, the PFD±DP with the removal of the C1 posterior arch in regard to changes in pre and postoperative symptoms, neurological signs, the Chicago Chiari Outcome Scale (CCOS) score, or rate of complications. From the results of this study, SC appears to be a good surgical technique considering its outcome.
Materials and Methods
Patient selection
After obtaining approval from the institutional review board (IRB) of Bangabandhu Sheikh Mujib Medical University (BSMMU) (NO. BSMMU/2019/3941), Dhaka, Bangladesh, patients were recruited for this cross-sectional observational study between June 2019 to May 2021. All consecutive adult symptomatic Chiari malformation patients with or without syringomyelia with postoperative follow-up of a minimum of 3 months were evaluated. All the patients undergoing SC or PFD±DP met the same inclusion and exclusion criteria with no history of any previous cranial surgery or comorbidity, and it was the surgeon’s choice to select the procedure.
Inclusion criteria
Patients in both groups were recruited for the study if they: 1. had a preoperative diagnosis of Chiari malformation 1 confirmed by tonsillar descent >5 millimeters with or without syringomyelia. 2. were aged >18 years. 3. had symptoms and signs related to CM1 and/or SM. 4. underwent any of the 2 practiced surgical procedures, posterior fossa decompression with or without duraplasty (PFD±DP) and stealth cranioplasty (SC).
Exclusion criteria
Patients were excluded from the study if they: 1. had associated anomalies other than CM1 like basilar invagination, atlanto-axial dislocation, or hydrocephalus. 2. did not have a minimum of 3 months of follow-up clinically. 3. refused to give consent to take part in the study.
Procedural details
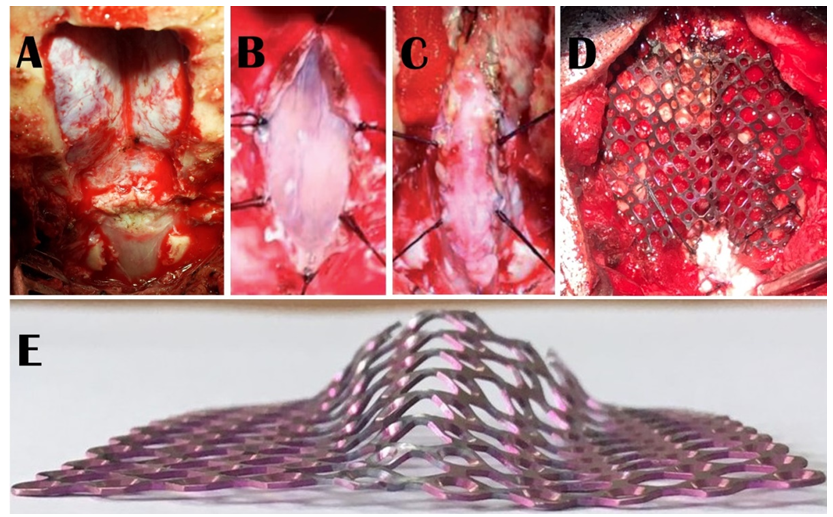
For surgery, informed written consent was obtained from the patients or their legal guardians for one of the two procedures. Posterior fossa decompression with or without duraplasty included suboccipital craniectomy (3 cm X 3 cm approx.) and removal of the posterior arch of the C1 with or without the best possible watertight duraplasty with pericranium or fascia Lata following “Y” shaped dural opening depending on the surgeon’s choice. During the dural opening, the arachnoid was not opened except for accidental breaches where watertight dural closure was ensured by the application of glue. On the other hand, the Stealth cranioplasty, which we described earlier in detail (13-15), comprised of 3 cm X 3 cm suboccipital craniectomy with removal of the posterior arch of the C1 (Figure 1A), midline linear arachnoid preserving durotomy (Figure 1B), duraplasty with a strip of the superficial layer of the deep cervical fascia (Figure 1C), and finally followed by cranioplasty with a pre-shaped 5 cm X 5 cm titanium mesh which resembles the cockpit of a Stealth bomber with flat wings (Figure 1E) fixed with screws around the margin of the craniectomy and tacking of the duraplasty with the titanium mesh (Figure 1D). Arachnoid was not breached in any of the cases of Stealth cranioplasty as it is one of the prime philosophies of the procedure.
All the patients were evaluated at 3 months postoperatively for changes in symptoms like occipital headache, neck pain, arm pain, limb weakness, wasting, paraesthesia, and gait disturbance as well as changes in neurological signs like weakness of limbs, wasting, dissociated sensory loss, gait disturbance, and cerebellar signs. Patients were evaluated by the Chicago Chiari outcome scale score to measure the outcome. Complications were assessed by observing infection, cerebellar sagging, CSF leak, hydrocephalus, or pseudomeningocele.
Figure 1: Steps of Stealth cranioplasty which comprised of 3 cm X 3 cm suboccipital craniectomy with removal of the posterior arch of the C1 (1A); a midline linear arachnoid preserving durotomy (1B); and duraplasty with strip of the superficial layer of the deep cervical fascia (1C). Cranioplasty is done with a 5 cm X 5 cm titanium mesh that is pre-shaped like the Stealth bomber cockpit with flat wings (1E). The cranioplasty is fixed with screws and tacking of the duraplasty with the titanium mesh is done (1D).
Statistical analysis
The parametric data like the CCOS scores were expressed as mean ± SD and were compared via unpaired t-test. Non-parametric data like the number of patients having different changes in Symptoms / Complaints and neurological findings, the number of patients in different categories of the CCOS, and the number of patients having complications were expressed as medians and compared via Chi-square test. Adjustment of confounding variables was not needed. All statistical analysis was performed using SPSS 23 software. A p-value <.05 was considered to be statistically significant.
Results
Overview
This study was conducted on 53 adult symptomatic CM1 patients with or without SM, 30 and 23 of whom underwent, PFD±DP and SC respectively. The average age of the 37 males and 16 females enrolled in the study was 30.35 ± 7.49 years ranging from 18 to 47 years. All the patients were assessed preoperatively and were followed up postoperatively to evaluate outcomes clinically with history and neurological examination as well as observing the complications.
Preoperative data
Regarding preoperative symptoms, out of 53 patients altogether in 2 groups, 29 (54.7%) patients had occipital headache, 45 (84.9%) had neck pain, 9 (17.0%) had arm pain, 48 (90.6%) had paraesthesia, 46 (86.8%) had weakness of limbs, and 11 (20.8%) had gait disturbance. (Table 1) Preoperative neurological signs comprised weakness of limbs in 46 (86.79%), dissociated sensory loss in 47 (88.68%), gait disturbance in 12 (22.64%), wasting in 35 (66.04%), and cerebellar signs in 3 (5.66%) out of 53 patients. (Table 2) There was no significant difference in the patients’ preoperative clinical status between the 2 groups regarding symptoms and findings as well as radiological findings like syringomyelia, which was absent only in 2 patients in the PFD±DP group.
Postoperative data
Postoperatively, combined in both the groups, occipital headache in 22 (75.9%), neck pain in 28 (62.2%), arm pain in 4 (44.4%), paraesthesia in 13 (27.1%), weakness of limbs in 19 (41.3%) and gait disturbance in 3 (27.3%) patients improved in those who had these symptoms. Among the groups, only the weakness of the limb improved significantly (p 0.001) in the SC group than in the PFD±DP group. (Table 1)
Postoperative neurological examination of all 53 patients revealed improvement of weakness of limbs in 14 (30.4%), dissociated sensory loss in 12 (25.5%), and gait disturbance in 2 (16.7%) while none of the 35 patients having wasting or 3 patients having cerebellar sign improved. (Table 2)
CCOS scores were significantly better in the SC group than in the PFD±DP group both category-wise (p 0.001) and while measuring mean CCOS scores (p 0.001). (Table 3)
Postoperative complications were very high in the PFD±DP group. Most of the complications were CSF-related and cerebellar sagging. There was no complication in any form in the SC group and this was significantly better (p 0.003) in the SC group than in the PFD±DP groups. (Table 4)
|
Surgery |
Symptoms / Complaints |
p-value |
|
|
↑ |
= |
||
|
Occipital Headache |
|||
|
PFD±DP |
13/19 (68.4%) |
6/19 (31.6%) |
0.367 |
|
STEALTH |
9/10 (90.0%) |
1/10 (10.0%) |
|
|
Neck pain |
|||
|
PFD±DP |
16/27 (59.3%) |
11/27 (40.7%) |
0.616 |
|
STEALTH |
12/18 (66.7%) |
6/18 (33.3%) |
|
|
Arm pain |
|||
|
PFD±DP |
3/7 (42.9%) |
4/7 (57.1%) |
1 |
|
STEALTH |
1/2 (50.0%) |
1/2 (50.0%) |
|
|
Paresthesia |
|||
|
PFD±DP |
5/25 (20.0%) |
20/25 (80.0%) |
0.25 |
|
STEALTH |
8/23 (34.8%) |
15/23 (65.2%) |
|
|
Weakness |
|||
|
PFD±DP |
5/25 (20.0%) |
20/25 (80.0%) |
0.001 |
|
STEALTH |
14/21 (66.7%) |
7/21 (33.3%) |
|
|
Gait disturbance |
|||
|
PFD±DP |
1/6 (16.7%) |
5/6 (83.3%) |
0.545 |
|
STEALTH |
2/5 (40.0%) |
3/5 (60.0%) |
|
Chi-square test was done
Same =, Improved ↑
Table 1: Postoperative Symptoms / Complaints Change
|
Surgery |
Symptoms / Complaints |
p-value |
|
|
↑ |
= |
||
|
Weakness |
|||
|
PFD±DP |
4/25 (16.0%) |
21/25 (84.0%) |
0.0274 |
|
STEALTH |
10/21 (47.6%) |
11/21 (52.4%) |
|
|
Dissociated Sensory loss |
|||
|
PFD±DP |
5/25 (20.0%) |
20/25 (80.0%) |
0.354 |
|
STEALTH |
7/22 (31.8%) |
15/22 (68.2%) |
|
|
Gait disturbance |
|||
|
PFD±DP |
0/6 (0.0%) |
6/6 (100.0%) |
0.455 |
|
STEALTH |
2/6 (33.3%) |
4/6 (66.7%) |
|
|
Wasting |
|||
|
PFD±DP |
19/19 (100.0%) |
||
|
STEALTH |
16/16 (100.0%) |
||
|
Cerebellar Signs |
|||
|
PFD±DP |
1/1 (100.0%) |
||
|
STEALTH |
2/2 (100.0%) |
||
Chi-square test was done
Same =, Improved ↑
Table 2: Post-Operative change in neurological finding.
|
Surgery |
CCOS [n (%)] |
CCOS |
|||||
|
4 |
5-8 |
9-12 |
13-16 |
p-value |
Mean ± SD |
p-value |
|
|
PFD±DP |
- |
- |
24/30 (80.0%) |
6/30 (20.0%) |
|
11.46 ± 1.25 |
|
|
STEALTH |
- |
- |
8/23 (34.8%) |
15/23 (65.2%) |
a0.001 |
13.00 ± 1.78 |
b0.001 |
aChi-Square test and bUnpaired t test was done
4 = Incapacitated outcome; 5 – 8 = Impaired outcome; 9 – 12 = Functional outcome; 13 – 16 = Excellent outcome
Table 3: Post-operative CCOS.
|
Surgery |
Infection |
Cerebellar sagging |
CSF leak |
HCP |
Pseudomeningocele |
Total |
P-value |
|
PFD±DP |
1/30 (3.3%) |
5/30 (16.7%) |
3/30 (10.0%) |
1/30 (3.3%) |
1/30 (3.3%) |
11/30 (36.7%) |
0.003 |
|
STEALTH |
0/23 (0%) |
0/23 (0%) |
0/23 (0%) |
0/23 (0%) |
0/23 (0%) |
0/23 (0%) |
Chi-Square test was done
Table 4: Complications in different types of surgery.
Discussion
Chiari malformation 1 is defined as caudal ectopia of the cerebellar tonsils more than 5 mm beyond the foramen magnum in adults. However, blending both the clinical and radiological findings is essential for the definitive diagnosis and more significantly, for decision-making in management and evaluating the outcomes [3,5,6].
CM1 is generally a congenital disorder where the anomaly occurs around the craniovertebral junction. Shallow and crowded posterior fossa and disparity of CSF flow and dynamics between the cranial and spinal compartments lead to tonsillar herniation, which consequently often leads to the formation of syringomyelia [4,5,7,16].
CM1 patients may present with a myriad of symptoms, which progress gradually in response to the tonsillar herniation leading to compression of the neural structures around the CVJ. Most of the symptoms arise from the involvement of the cerebellum, brainstem, or spinal cord, and the subsequent and frequent formation of SX. Patients commonly present with Valsalva maneuver-related occipital headache along with numerous other symptoms. Occasionally, patients may even be asymptomatic and diagnosed by incidental image findings only [1,4,7,16].
Surgical management aims to decompress the crowded posterior fossa and the CVJ as well as to reestablish the CSF flow and dynamics. The well-accepted and the most practiced surgery for both CM1 and associated SX is the posterior fossa bony decompression with or without duraplasty and removal of the posterior arch of C1. Combinations of different surgical techniques with this have evolved and each has its arguable advantages and disadvantages. All the measures eventually aim to improve symptoms and signs and alleviate the complications and prevent recurrences [4,6,9-11]. Our procedure aims to alleviate the pathology at the CVJ to get relief of the Chiari-related symptoms. We do not do anything additional for SM as we believe and have seen from our experience that once the CVJ is decompressed and CSF flow and dynamics are restored, the SM automatically tends to resolve.
The selection of patients of CM1 for surgery and the need for tailoring surgery accordingly need to be considered for every individual patient. Although the present surgical techniques yield better results than before, outcomes of different modalities of surgery for CM1 are still varied. Defining any absolute scale to measure outcomes is also difficult and is a big reason for diverse results. In this study, we compared the clinical outcomes between SC and commonly practiced procedure, PFD±DP and we feel the technique of Stealth cranioplasty, that we introduced, is a good one considering its outcomes.
Most of the adult CM1 patients are affected in their 3rd and 4th decades, and females outnumber males in most of the series [2,9-11,17,18]. Contrary to the findings in the literature, most of our patients were in the age group of 18-30 years (52.8%) and most of the patients were males (69.8%). The variations of the dimensions of the posterior fossa as well as the diameter of the foramen magnum resulting from racial variations may have some role in the relatively early manifestations in the patients. And the male dominance may have resulted from the fact that males in our country start to work at an early age and most of them are manual heavy workers which possibly affects the existing problems to manifest more and earlier also in them.
The mean duration of symptoms in our series was 36.24 ± 20.28 months. Although many patients (n=22, 41.5%) presented with symptoms within 0-24 months, most of the patients (n=31, 58.5%) presented after more than 2 years of having symptoms, and even as late as after 8 years. This late presentation reflects illiteracy, negligence, and lack of awareness about the condition, in addition to financial constraints sometimes. In most of the literature, the duration of symptoms is about 1 year, albeit, more than 3 years of duration of symptoms has also been described in some series [4,18,19].
Duration of symptoms has a profound influence over the outcome as works of literature show that the longer the duration of symptoms the worse the prognosis. This stresses the urgency to diagnose and treat at the earliest to prevent or even reverse the deficits in some cases [3,20,21]. We also agree with the importance of earlier detection and urgency of treatment to give a better outcome. However, there was no significant difference in the duration of symptoms between the 2 groups and we did not find any correlation between the outcome and duration of symptoms as seen in many series.
CM1 patients usually present with a myriad of symptoms, especially when they have associated SM, and the heterogeneity of presentations continually poses a mystery. Symptoms and signs typically arise from impairment of functions alone or in combination involving the cerebellum, the brain stem, and the spinal cord particularly when associated syringomyelia is present to different extents. In CM1 patients, most studies labeled short-lived Valsalva-type maneuvers provoked headache, neck pain, arm pain, paraesthesia, numbness, muscle weakness, and gait disturbance as the most common symptoms. Among the neurological findings, weakness of limbs, dissociated sensory loss, gait disturbance, muscle wasting, and cerebellar signs are the common ones [3,16,22-26].
Many other symptoms and signs are also described. However, most of those are not very common. The commonest symptoms in our study in descending order were paraesthesia, weakness of limbs, neck pain, occipital headache, and gait disturbance while the most frequent neurological findings were dissociated sensory loss, weakness of limbs, muscle wasting, and cerebellar signs.
Generally, reported postoperative improvement of symptoms and signs ranges from 73.6% to 88.6% [2,11,27]. Postoperatively, we found that almost all the symptoms and signs improved noticeably, albeit, the improvements were not statistically significant between the 2 groups. However, the clinical symptoms, both preoperatively and postoperatively, were evaluated based on the complaints of the patients.Many patients in this study had symptoms related to spinal cord involvement which can be associated with the presence of SX. It is recognized and the findings of this study also confirm that the postoperative changes in SX, restoration of CSF spaces around the CVJ, particularly, the appearance of Cisterna magna do not coincide with the clinical outcome always. However, a direct comparison of surgical outcomes with previous studies would not be judicious as the parameters of clinical evaluation, types of surgeries, and time of follow-ups are diverse.
Chicago Chiari Outcome Scale was introduced in 2012 for the postoperative evaluation of CM1 patients and was validated later as a good outcome assessment tool [28,29]. Outcome assessment of CM1 patients with CCOS is used widely and it has become a reliable tool to assess outcomes [4,6,17-19,21]. Postoperative outcomes can be measured with CCOS in 2 ways; by assessment of improvement with an overall score and by categorizing the patients into outcome groups based on that score.
Most of the studies on adult CM1 patients show that the average CCOS that could be achieved ranges between 13 to 14.5 [6,17-19,21]. Maximum of the patients in different series succeeded in achieving excellent outcomes on average with good CCOS. Interestingly, the average score or the groups that the patients fall in is varied depending on several factors like the duration of symptoms, variability of symptoms, diversity of surgical techniques, or duration of follow-up periods [6,17,19,21,37].
Unlike the other series, altogether we found excellent outcomes in only about 40% of patients while the rest of the patients fell in the functional outcome group. However, about 65% of patients in the SC group were in the excellent outcome group and the average CCOS score was also better in the SC group than in the PFD±DP group. In most of the series, the outcome measured with CCOS improved gradually with time and varied noticeably among sets of patients depending on different factors. The overall surprisingly low number of excellent outcomes in our patients can be attributed to the short period of follow-up. The outcomes as a whole probably could have been better if there would be longer follow-ups. The relatively better outcome in the SC group than the PFD±DP might be due to the absence of any complications as well as the better formation and maintenance of more space in the posterior fossa in this group.
Complications are common following surgery for CM1 as in any other surgery. The rates vary from center to center and depend on many factors including the surgical procedures and some surgical procedures may have some definite sets of complications. The complication rate varies from zero complication to as high as 33.3% in different series [4,6, 11,18,19,27,30-32].
Common complications include cerebrospinal fluid leak, pseudomeningocele, meningitis, wound infection, cerebellar slump or ptosis, hydrocephalus, subdural collections, and many more sporadic ones [4,6,10,11,16,24,33-36]. Our study complies with the literature, although, the complications were at the higher end of the percentage of complications compared to other series in the PFD±DP group. CSF-related complications like CSF leakage, pseudomeningocele, and hydrocephalus comprised almost half of the complications in the PFD±DP group. The CSF leaks resulted from the unintended arachnoid breach during the procedure of dural opening or duraplasty which ultimately resulted in these complications. The other bulk of the complications in this group was cerebellar sagging where the cerebellum descends through the craniectomy defect resulting from extravagant craniectomy with the thought of giving more relief. On the other hand, the craniectomy is generally small in stealth cranioplasty. However, in cases of inadvertent larger craniectomies, it has the advantage of correcting it by contouring the craniectomy gap. It also helps prevent cerebellar sagging by supporting the cerebellum with the flatter upper part of Titanium mesh.
The cerebellar sagging is characterized clinically by headache unrelated to the Valsalva maneuver and radiologically by obstruction of CSF flow at the CVJ which often needs re-exploration with cranioplasty. The SC group did not have any complications, which clearly marks the superiority of this technique over the PFD±DP group regarding complications. We developed our technique initially to evade recurrence and complications and it seems, this works well. As we perform arachnoid preserving duraplasty, the chances of CSF-related complications are automatically eliminated.
With the smaller craniectomy augmented by the measured cranioplasty, the chance of cerebellar slump is refuted as well. This study was conducted on 53 adult symptomatic CM1 patients with or without SM, 30 and 23 of whom underwent, PFD±DP and SC respectively. The average age of the 37 males and 16 females enrolled in the study was 30.35 ± 7.49 years ranging from 18 to 47 years. All the patients were assessed preoperatively and were followed up postoperatively to evaluate outcomes clinically with history and neurological examination as well as observing the complications. Although the SC fared better than the PFD±DP regarding CCOS and the number of complications, the changes in clinical symptoms and the neurological findings were not different significantly, and thus it cannot be concluded in general that the SC is far better than the other surgical procedures. However, the results surely make the SC a good procedure to be practiced. Nevertheless, the short follow-up period makes the overall inference weaker in terms of long-term outcomes.
Conclusions
Substantial variations exist in the surgical management of CM1 with or without SX and a common management algorithm that would be a “gold standard” or “one-size-fits-all” with a uniform outcome is yet to be devised and seems to be a faraway dream. However, Stealth cranioplasty for adult CM1 can be a better option compared to the traditional commonly practiced procedures.
Strength
The strength of this study is that it was the first of its kind conducted in our university and also the first ever to compare the clinical outcomes of Stealth cranioplasty with other traditional methods.
Limitations
The study has several limitations though. Due to the COVID-19 pandemic, the study population was smaller than anticipated and was not randomized which obviously raise the possibility of some bias. Although the SC was done by a single surgeon, the other surgeries (PFD±DP) were done by multiple surgeons which may have influenced the outcome in those. Follow up period was short which is a major limitation of this study. Anticipating the difficulties of long follow-ups in our context, we opted for shorter follow-ups which was further jeopardized by the COVID-19 pandemic.
Recommendations
As longer follow-ups could have yielded different findings and insights, multi-center randomized controlled trials with larger population with longer follow-ups are advocated to see the real benefits of SC.
Disclosure of conflict of interest
None.
Acknowledgement
We are grateful to our nursing and other staff and the patients.
Funding
This study was partially funded by the research grant of Bangabandhu Sheikh Mujib Medical University (BSMMU).
Credit author statement
Asifur Rahman: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing - original draft, Writing - review & editing
Nazmin Ahmed: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - review & editing
Abu Saleh Mohammad Abu Obaida: Conceptualization, Data curation, Investigation, Writing - review & editing
Abu Naim Wakil Uddin: Conceptualization, Data curation, Investigation, Writing - review & editing
Bipin Chaurasia: Conceptualization, Data curation, Investigation, Writing - review & editing
Md Atikur Rahman: Conceptualization, Data curation, Investigation, Writing - review & editing
Md Shamsul Alam: Conceptualization, Data curation, Investigation, Writing - review & editing
Md Moududul Haque: Conceptualization, Data curation, Investigation, Writing - review & editing
Marco Maria Fontanella: Writing - review & editing
Edoardo Agosti: Writing - review & editing
References
- Hale AT, Adelson PD, Albert GW, et al. Factors associated with syringomyelia size in pediatric patients treated for Chiari malformation type I and syringomyelia: a study from the Park-Reeves Syringomyelia Research Consortium. Journal of Neurosurgery: Pediatrics 25 (2020): 629-639.
- Jussila MP, Nissilä J, Vakkuri M, et al. Preoperative measurements on MRI in Chiari 1 patients fail to predict outcome after decompressive surgery. Acta Neurochirurgica 163 (2021): 2005-2014.
- McClugage SG, Oakes WJ. The Chiari I malformation: JNSPG 75th anniversary invited review article. Journal of Neurosurgery: Pediatrics 24 (2019): 217-226.
- Rangari K, Das KK, Singh S, et al. Type I Chiari malformation without concomitant bony instability: assessment of different surgical procedures and outcomes in 73 patients. Neurospine 18 (2021): 126.
- Tosi U, Lara-Reyna J, Chae J, et al. Persistent syringomyelia after posterior fossa decompression for chiari malformation. World neurosurgery 136 (2020): 454-461.
- Walker-Palmer TK, Cochrane DD, Singhal A, et al. Outcomes and complications for individual neurosurgeons for the treatment of Chiari I malformation at a children’s hospital. Child's Nervous System 35 (2019): 1895-1904.
- Luciano MG, Batzdorf U, Kula RW, et al. Development of common data elements for use in Chiari malformation type I clinical research: an NIH/NINDS project. Neurosurgery 85 (2019): 854-860.
- Sadler B, Kuensting T, Strahle J, et al. Prevalence and impact of underlying diagnosis and comorbidities on Chiari 1 malformation. Pediatric neurology 106 (2020): 32-37.
- Takeshima Y, Matsuda R, Nishimura F, et al. Sequential enlargement of posterior fossa after duraplasty for Chiari malformation type 1. World Neurosurgery 2 (2019): 100004.
- Perrini P, Anania Y, Cagnazzo F, et al. Radiological outcome after surgical treatment of syringomyelia-Chiari I complex in adults: a systematic review and meta-analysis. Neurosurgical review 44 (2021): 177-187.
- Zhao JL, Li MH, Wang CL, et al. A systematic review of Chiari I malformation: techniques and outcomes. World neurosurgery 88 (2016): 7-14.
- Soleman J, Roth J, Constantini S. Direct syringomyelia drainage in patients with Chiari I malformation. Child's Nervous System 35 (2019): 1863-1868.
- Rahman A. “Stealth Cranioplasty” for Adult Chiari Malformation Type 1: A Philosophical Journey of Innovation, Adaptation, and Evolution. Neurosurgical Procedures-Innovative Approaches: IntechOpen (2019).
- Rahman A. Role of Cranioplasty in Management of Chiari Malformation. Neurosurgical Procedures-Innovative Approaches: IntechOpen (2020).
- Rahman A, Rana M, Bhandari P, et al. " Stealth cranioplasty:" A novel endeavor for symptomatic adult Chiari I patients with syringomyelia: Technical note, appraisal, and philosophical considerations. Journal of Craniovertebral Junction & Spine 8 (2017): 243-252.
- Nikoobakht M, Shojaei H, Gerszten PC, et al. Craniometrical imaging and clinical findings of adult Chiari malformation type 1 before and after posterior fossa decompression surgery with duraplasty. British Journal of Neurosurgery 33(2019): 481-485.
- Gilmer HS, Xi M, Young SH. Surgical decompression for Chiari malformation type I: an age-based outcomes study based on the Chicago Chiari Outcome Scale. World neurosurgery 107 (2017): 285-290.
- Lei Z, Wu S, Zhang Z, et al. Clinical characteristics, imaging findings and surgical outcomes of Chiari malformation type I in pediatric and adult patients. Current Medical Science 38 (2018): 289-295.
- Feghali J, Marinaro E, Xie Y, et al. Family History in Chiari Malformation Type I: Presentation and Outcome. World neurosurgery 142 (2020): e350-e356.
- Cheng JS, Nash J, Meyer GA. Chiari type I malformation revisited: diagnosis and treatment. The Neurologist 8 (2002): 357-362.
- Koechlin NO, Abuhusain HJ, Gunawardena M, et al. Symptomatic outcome after bone-only suboccipital decompression in adult patients with Chiari type I malformations in the absence of hydromyelia or hydrocephalus. Journal of Neurological Surgery Part A: Central European Neurosurgery 78 (2017): 344-349.
- Batzdorf U. Clinical presentation and alternative diagnoses in the adult population. Neurosurgery Clinics 26 (2015): 515-517.
- Fischbein R, Saling JR, Marty P, et al. Patient-reported Chiari malformation type I symptoms and diagnostic experiences: a report from the national Conquer Chiari Patient Registry database. Neurological sciences 36 (2015): 1617-1624.
- Holly LT, Batzdorf U. Chiari malformation and syringomyelia: JNSPG 75th Anniversary Invited Review Article. Journal of Neurosurgery: Spine 31 (2019): 619-628.
- Olszewski AM, Proctor MR. Headache, Chiari I malformation and foramen magnum decompression. Current Opinion in Pediatrics 30 (2018): 786-790.
- Pindrik J, Johnston JM. Clinical presentation of Chiari I malformation and syringomyelia in children. Neurosurgery Clinics 26 (2015): 509-514.
- Klekamp J. Surgical treatment of Chiari I malformation- analysis of intraoperative findings, complications, and outcome for 371 foramen magnum decompressions. Neurosurgery 71 (2012): 365-380.
- Aliaga L, Hekman KE, Yassari R, et al. A novel scoring system for assessing Chiari malformation type I treatment outcomes. Neurosurgery 70 (2012): 656-665.
- Yarbrough CK, Greenberg JK, Smyth MD, et al. External validation of the Chicago Chiari outcome scale. Journal of Neurosurgery: Pediatrics 13 (2014): 679-684.
- Beecher JS, Liu Y, Qi X, et al. Minimally invasive subpial tonsillectomy for Chiari I decompression. Acta Neurochirurgica 158 (2016): 1807-1811.
- Duddy JC, Allcutt D, Crimmins D, et al. Foramen magnum decompression for Chiari I malformation: a procedure not to be underestimated. British Journal of Neurosurgery 28 (2014): 330-334.
- Geng LY, Liu X, Zhang YS, et al. Dura-splitting versus a combined technique for Chiari malformation type I complicated with syringomyelia. British Journal of Neurosurgery 32 (2018): 479-483.
- Assina R, Meleis AM, Cohen MA, et al. Titanium mesh-assisted dural tenting for an expansile suboccipital cranioplasty in the treatment of Chiari 1 malformation. Journal of clinical neuroscience 21 (2014): 1641-1646.
- Holly LT, Batzdorf U. Management of cerebellar ptosis following craniovertebral decompression for Chiari I malformation. Journal of neurosurgery 94 (2001): 21-26.
- Kumar A, Pruthi N, Devi BI, et al. Response of syringomyelia associated with Chiari I malformation to posterior fossa decompression with or without duraplasty and correlation with functional outcome: a prospective study of 22 patients. Journal of Neurosciences in Rural Practice 9 (2018): 587-592.
- Shetty J, Kandasamy J, Sokol D, et al. Clinical deterioration despite syringomyelia resolution after successful foramen magnum decompression for Chiari malformation-case series. European Journal of Paediatric Neurology 23 (2019): 333-337.
- Encarnacion D, Chmutin G, Chaurasia B, et al. Hundred Pediatric Cases Treated for Chiari Type II Malformation with Hydrocephalus and Myelomeningocele. Asian Journal of Neurosurgery 18 (2023): 258-264.