Cholera Toxin (CT), Thermolabile Enterotoxin of E. Coli (LT), its B Subunit (LTB) and LT-R192G, but not The B Subunit of CT (CTB), induce the Decrease of Resting CD4+CD25+Foxp3+ T Cells in Vitro
Article Information
Fatou Thiam*, 1, 2, Cheikh Momar Nguer1, Claire Cachia2, John D. Clements3, Evelyne Kohli2 and Christelle Basset2
1Groupe de Recherche Biotechnologies Appliquées & Bioprocédés environnementaux (GRBA-BE), École Supérieure Polytechnique (ESP)–Université Cheikh Anta Diop, Dakar-Fann, Dakar 5085, Senegal
2Laboratoire des Interactions Muqueuses-Agents transmissibles (LIMA), UPR562, UFRs Médecine et Pharmacie, IFR Santé-STIC, Université de Bourgogne, Dijon, France
3Department of Microbiology and Immunology, Tulane University Health Sciences Center, New Orleans, LA 70112, USA
*Corresponding author: Fatou Thiam, Groupe de Recherche Biotechnologies Appliquées & Bioprocédés environnementaux (GRBA-BE), École Supérieure Polytechnique (ESP)–Université Cheikh Anta Diop, Dakar-Fann, Dakar 5085, Senegal.
Received: 20 September 2022; Accepted: 30 September 2022; Published: 21 October 2022
Citation:
Fatou Thiam, Cheikh Momar Nguer, Claire Cachia, John D. Clements, Evelyne Kohli and Christelle Basset. Cholera Toxin (CT), Thermolabile Enterotoxin of E. Coli (LT), its B Subunit (LTB) and LT-R192G, but not The B Subunit of CT (CTB), induce the Decrease of Resting CD4+CD25+Foxp3+ T Cells in Vitro. Archives of Microbiology and Immunology 6 (2022): 231-241.
Share at FacebookAbstract
Previously study showed that LT-R192G, a less toxic mutant of the thermolabile enterotoxin of E. coli (LT), decreased murine regulatory CD4+CD25+Foxp3+ T cells in vitro. To go further, we tested other molecules of the cholera toxin family, i.e. native LT, the cholera toxin (CT), and their B subunits devoid of enzymatic activity, LTB and CTB. Cells from mesenteric lymph nodes of BALB/c mice were incubated with these molecules, and CD4+CD25+Foxp3+ T cells were analyzed by cytometry after two days of culture. All molecules, except CTB, decreased CD4+CD25+Foxp3+ T cells. T cell apoptosis has already been reported with these molecules, preferential effect on CD8+ T cells. So, we analyzed CD3+CD8+ and CD4+CD25+/- T cell apoptosis in the presence of LT, LT-R192G, LTB, CT and CTB in our in vitro model to detect possible preferential apoptosis of Tregs. Unexpectedly, CTB did not affect CD8+ and CD4+ T cells. Whereas LT, LT-R192G and LTB induced rapid apoptosis of CD8+ T cells with a decrease in CD3 expression, CT induced less rapid apoptosis with no decrease in CD3 expression. We observed CD4 T cell apoptosis but did not find any preferential apoptosis of CD4+CD25+ T cells (containing Foxp3+ T cells) compared with CD4+CD25- T cells. In conclusion, the enzymatic activity seems to play a significant role in apoptosis induction by CT, as CTB has no effect. In contrast, receptor binding may be essential in the observed effects for LT. Moreover, these results show the complexity and heterogeneity in the impact of these molecules.
Keywords
thermolabile enterotoxin of E. coli, LT-R192G, cholera toxin, LTB, CTB, Foxp3, CD4, CD8, CD25, apoptosis
Article Details
1. Background
Mucosal immunization is an important goal of vaccine development to protect against pathogens that use mucosa as portals of entry. The use of non-replicating antigens requires the addition of adjuvants and cholera-like enterotoxins, cholera toxin (CT) from Vibrio cholerae and the heat-labile enterotoxin (LT) from toxinogenic strains of E. coli have been proposed as they are potent mucosal adjuvants of immunization eliciting mucosal and systemic responses against unrelated co-administered antigens in experimental models [1-4].
Both toxins comprise a single A subunit responsible for the ADP-ribosyl transferase activity and five identical B subunits accountable for cell binding [5, 6]. The A subunit consists of two chains, A1 and A2, joined by a proteolytically sensitive peptide (Arg192), subtended by a disulfide loop [7]. To overcome the enterotoxicity and use them as an adjuvant in humans, non-toxic mutants of the A subunit that retain adjuvant properties have been developed [8] and, in some cases, tested in clinical trials [9-11]. Among them, LT-R192G is an attenuated mutant of LT, containing a single amino acid substitution altering the site of proteolytic cleavage within the disulfide subtended region joining A1 and A2 [12, 13].
The B subunit of these toxins is devoid of toxicity and thus could also be used as adjuvants in humans. The B subunit of LT (LTB) is an efficient adjuvant when used by the intranasal route with co-administered antigen [14-16]. The adjuvant activity of the B subunit of CT (CTB) remains confusing. Although some studies have reported that CTB can enhance immune responses to co-administered antigens by the intranasal route of immunization [5, 8, 17-20], CTB, on the other hand, can promote tolerance to heterologous antigens, and its use has been proposed as a strategy to prevent auto-immune and allergic diseases [21].
Although extensively studied, the mechanisms of action of these toxins and mutants are very complex and not fully understood. This comes from the observations that these toxins have been tested in many models and conditions; they have different actions depending on the route of immunization, the dose, the animal strain and whether they are studied in vitro or vivo. Moreover, Th17 CD4 T cells have recently been implicated in their adjuvanticity, making their mechanism of action even more complex [22-25].
In previous studies, we have looked at the impact of LT-R192G on B and T cell responses against a co-administered antigen, rotavirus (RV) virus-like particles (2/6-VLP), after intranasal (IN) and intrarectal (IR) immunization of BALB/c mice [26-28]. We showed that LT-R192G induced a strong VLP-2/6 specific B cell response after a single immunisation. A substantial proportion of these cells expressed CD5 and were considered B1-a cells [26, 27]. Unexpectedly, we found that a second immunization in the same conditions did not increase the frequency of specific B cells, whereas a secondary systemic and mucosal antibody response was observed [28].
We hypothesized that the massive B cell expansion observed when the adjuvant was used for immunization was probably regulated during the second contact. Such modulation may be essential to avoid potential deleterious autoreactivity of CD5+ expressing B-1a cells and T cell-mediated inflammation. To explain this result, we looked at the regulatory T cell response induced after IR immunization with VLP-2/6 and LT-R192G. Our study showed that LT-R192G induced VLP-2/6 specific CD4+CD25+ helper T cells, and VLP-2/6 specific CD4+CD25+Foxp3+ regulatory T cells [28]. These regulatory T cells might be generated to control both effector B and T cells. The other unexpected result was the decrease of natural regulatory T cells from non-immunized mice after in vitro contact with LT-R192G. During the first contact with LT-R192G and antigen, this decrease in natural regulatory T cells could participate in the adjuvant effect [29]. This effect on natural Tregs has never been reported. However, it has already been reported that these toxins and their B subunits induce the death of both CD8+ and CD4+ T cells by a mechanism of apoptosis, with a preferential death of CD8+ T cells, although there are several contradictory studies [14, 30, 31].
The death of regulatory T cells also is probably caused by the same mechanism, and this death could be preferential among the CD4+ T cells. Several research groups have studied the effect of enterotoxins and their B subunits on T cells and the induction of apoptosis in these cells. Tamayo et al. [32-34] have looked at the effect of LT in vivo after systemic (footpad) and mucosal administrations (oral and intranasal). Their studies highlight differences between immunization routes, in vivo and in vitro contact, the status of differentiation and the subtype of T cells, and different apoptotic mechanisms. LT induces death of immature T and B cell progenitors, with preferential apoptosis of CD4+CD8+ thymocytes in vivo by systemic and oral administration, whereas in vitro, CD4-CD8+ thymocytes are more sensitive. By the intranasal route, the phenomenon was not observed. Moreover, the enzymatic activity of LT was necessary. Apoptosis occurs through the stimulation of endogenous glucocorticoids with intrinsic caspase-dependent elimination of immature lymphocytes and with intrinsic and extrinsic caspase-dependent elimination of mature peripheral lymphocytes, CD8+ T cells being the most susceptible to apoptosis in vitro.
The effect of LTB on mature T cells has also been studied but only in vitro. A research group [14, 35, 36] has reported the selective apoptosis of resting and antigen-activated CD8+ T cells from MLN. Another group [37] found that LTB can induce apoptosis of resting and antigen-activated CD8 and CD4 T cells from the spleen and mesenteric lymph nodes. Cholera toxin and its B subunit have also been reported to induce apoptosis of T cells. [38] found that in vivo, CT, given orally, causes a marked depletion of intestinal CD8+ intraepithelial lymphocytes. Previously, these authors showed that in vitro, both CT and CTB could inhibit the proliferative response of polyclonal activated T cells from the spleen. [39] looked more precisely at the effect of CT and recombinant CTB on CD4+ T cells and reported that CT, but not rCTB, induced apoptosis of anti-CD3 activated CD4+ T cells from PP. Wang et al. [40] found that rCTB only induces apoptosis of activated CD4+ and CD8+ T cells at doses above one µg/ml. In contrast, CTB directly stimulated KLH-primed CD4+ and CD8+ T cells below this concentration [41]. Found that recombinant CT induced only apoptosis of resting and activated CD8+ T cells from the spleen [42] reported that CT did not affect resting T cells, whereas CTB induced the preferential apoptosis of resting CD8+T cells. This preferential effect of CTB on resting CD8+ T cells was confirmed by [43] with recombinant CTB.
Overall, all toxins could induce apoptosis of T cells, although some studies are contradictory. This is due to different experimental methods, mouse strains, source and dose of toxins and organs used. More precisely, if we look at the effect of these molecules on resting T cells in vitro, LT and rLTB induce apoptosis of both CD4+ and CD8+ T cells, with a preference for CD8+ T cells and CT and rCTB induce apoptosis of resting T cells, with a preference for CD8+ T cells. In this work, we assessed the effects of these different molecules on T cells in our in vitro model. Precisely, (i) we looked at the effect of LT, LTB, CT and CTB on CD4+CD25+Foxp3+ to check whether they have the same effect as LT-R192G; (ii) we looked at the effect of cholera-like enterotoxins on CD8+ and CD4+ T cells in our in vitro animal model; (iii) we studied the induction of apoptosis of CD3+CD8+ and CD4+CD25+/- T cells by the toxins and their B subunits.
2. Materials and Methods
2.1 Mice
Pathogen-free, adult female BALB/c mice (6-8 weeks of age) were obtained from Iffa-Credo (L’Arbresle, France). Study protocols were approved by the local institutional animal care committee.
2.2 Cholera-like enterotoxins
The enterotoxins tested in this study were LT-R192G, the cholera toxin (CT) and its B subunits, CTB. CT, CTB and LTB were purchased from Sigma-Aldrich (France). CTB is purified from Vibrio cholerae and contains ≤0,5% of A subunit, according to the manufacturer’s information. LTB is recombinant and expressed in Pichia pastoris.
2.3 Sample collection and preparation of cells
Mice were killed, and the lymphoid tissues, mesenteric lymph nodes (MLN) and spleen were removed. Single-cell suspensions were prepared by mechanical dissociation, filtered on 40-µm-pore nylon meshes and washed with an incomplete medium (RPMI-1640 supplemented with 0.3% glucose, 100U penicillin per mL, and 100µg streptomycin per mL). The cells were resuspended at 4x106/mL in a complete medium (incomplete medium plus 10% heat-inactivated FCS, 2 mM L-glutamine, and 1 mM sodium pyruvate) for in vitro contact with the toxins.
2.4 In vitro contact with the toxins
Cells (4x105 cells/well) were cultured in 96-well plates in the presence of LT-R192G, CT, CTB (5µg/mL) or RPMI medium only, then incubated at 37°C with 5% CO2 and harvested on day 2 for flow cytometry analysis of CD4+CD25+Foxp3+ T cells or harvested at 2, 4, 12, 24 and 36 hours for flow cytometry analysis of apoptosis of CD4+CD25+/- T cells and CD3+CD8+/- T cells.
2.5 Flow cytometry analysis
2.6. a. Analysis of CD4+CD25+Foxp3+ T cells and CD4+CD25+Foxp3- T cells
CD4+CD25+Foxp3+ T cells and CD4+CD25+Foxp3- T cells were quantified using the Mouse Regulatory T cell Staining kit #2 (w/APC Foxp3 FJK-16s, FITC CD4, PE CD25; eBioscience, San Diego, USA). Briefly, pellets containing 4x105 cells in 100 µL PBS 1% BSA 0.1% sodium azide were incubated with a mixture of FITC-labelled anti-CD4 and PE-labelled anti-CD25 for 30 min in the dark at 4°C. The cells were washed and resuspended in 1 mL of freshly prepared fixation/permeabilization working solution and then incubated at 4°C for 30 min in the dark. The cells were washed with permeabilization buffer and then labelled with APC-labelled anti-Foxp3 for 30 min in the dark. After incubation, the cells were washed, resuspended in a buffer containing PBS 1% BSA 0.1% sodium azide, and analysed by flow cytometry. Approximately 5x104 cells were acquired. Lymphocytes were first identified by their light-scatter profile, and T cell subsets were then identified by CD4 expression. CD25+Foxp3+ and CD25+Foxp3- T cells were identified by binding CD25 and Foxp3 within CD4+ T cells. The mean fluorescence intensity (MFI) for PE and APC were analyzed to detect variations in CD25 and Foxp3 expression, respectively.
2.6. b. Analysis of CD4+CD25+/- T cells and CD4+CD8+/- T cells apoptosis
Cells were incubated for 15 min in PBS 5% BSA, washed with PBS 1% BSA and then incubated with a mixture of APC-labelled anti-CD4 and PE-labelled anti-CD25 (eBioscience) or APC-labelled anti-CD3 and PE-labelled anti-CD8 (BD Biosciences), for 30 min in the dark at 4°C. After one washing with cold PBS, 100 µL of 1X Annexin buffer (BD Biosciences), 5 µL of FITC-Annexin V (BD Biosciences) and 5 µL of 7-AAD (BD Biosciences) were added to the cell pellet. After 10-15 min at room temperature, each tube was completed with 300 µL of 1X Annexin buffer and the staining was immediately analysed by flow cytometry.
2.7 Statistical methods
For CD4+CD25+, Foxp3- and Foxp3+, T cell analysis, comparison of cell frequency, CD25 and Foxp3 MFI between stimulated and non-stimulated wells was done using the Wilcoxon paired non-parametric signed-rank test. P-values less than 0.05 were considered statistically significant.
3. Results
We have shown previously that LT-R192G decreased CD4+CD25+Foxp3+ T cells after in vitro contact with various tissues and organs (e.g. MLN, spleen) [44]. To go further, we first wished to check whether other cholera-like enterotoxins (LT, LTB, CT and CTB) had the same effect on this subpopulation of T cells. Secondly, in our in vitro model, we checked whether the toxins and their subunits induce the preferential apoptosis of CD8+ T cells, as previously reported. Third, we studied the induction of apoptosis in CD4+CD25+ T cells.
3.1 LT-R192G, LT, LTB and CT, but not CTB, decreased CD4+CD25+Foxp3+ T cells in vitro
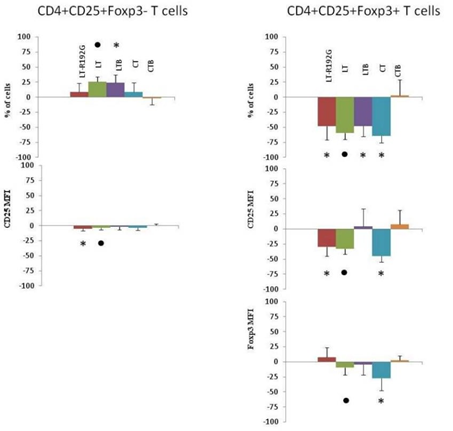
As shown in Figure 1, there was a decrease in the percentage of CD4+CD25+Foxp3+ T cells after two days of cell contact with LT, LT-R192G, LTB, and CT (p <0.01). LT, LT-R192G and CT (p< 0.01), but not LTB, decreased the expression of CD25. No decrease in cell frequency and MFI was seen with CTB. LT-R192G, CT and CTB did not affect CD4+CD25+Foxp3- T cells, whereas LTB and LT increased the frequency of these cells (p< 0.05).
Figure 1: In vitro effect of LT, LT-R192G, LTB, CT and CTB on murine mesenteric lymph nodes (MLN) CD4+CD25+Foxp3+/- T cells after in vitro contact. Cells from MLN (4 × 105 cells/well) were cultured in the presence of the cholera-like enterotoxin or their B subunit (5μ/mL) for two days. Percentage of CD4+CD25+Foxp3− and CD4+CD25+Foxp3+ T cells and both CD25 and Foxp3 MFI were analyzed by flow cytometry. Lymphocytes were first identified in a dotplot from light-scatter, and T cell subsets were then identified by expression of CD4. CD25+Foxp3+ and CD25+Foxp3− T cells were then identified by binding of anti-CD25 and anti-Foxp3 antibodies within CD4+ T cells. The histograms show the percentage of increase or decrease for each parameter compared to the control well (medium only). P-values were calculated using the Wilcoxon paired non-parametric signedrank test.* Data points statistically different between stimulated and medium wells (n=7-9).
: statistical analysis could not been performed as n<6.
3.2 LT-R192G, LT and CT, to a lesser degree LTB, but not CTB, induce the death (apoptosis) of cells from spleen and mesenteric lymph nodes in vitro
As CTB does not have any effect on CD4+CD25+Foxp3+ nor on CD4+CD25+Foxp3- T cells, we wanted to revisit the effect of the toxins and their subunits on T lymphocytes in our model. Indeed, it has been previously reported that CTB induced the preferential apoptosis of CD8+ T cells[42, 43]. Firstly, we analyzed the effect of the toxins and their subunits on the totality of spleen and mesenteric lymph nodes (MLN) cells (Figure 2) and secondly, we studied the induction of apoptosis (Figure 3).
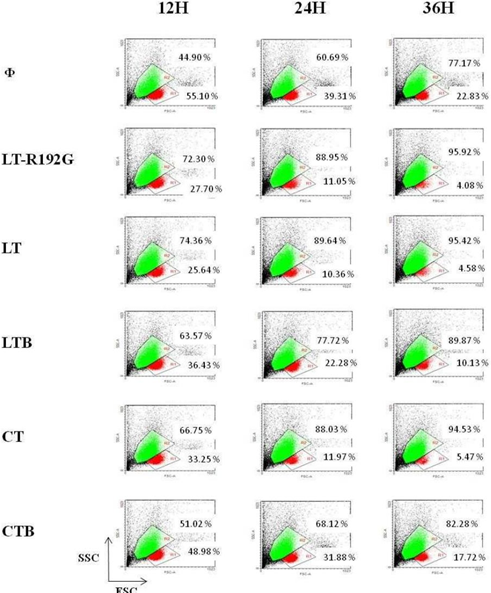
Figure 2: In vitro effect of LT, LT-R192G, LTB, CT and CTB on murine MLN cells. Cells from MLN (4 × 105 cells/well) were cultured in the presence of the cholera- like enterotoxin or their B subunit (5μg/mL) for 12, 24 and 36 hours. MLN cells were analyzed by cytometry, based on FSC/SSC. Two populations can be seen, with different size and granularity, labeled R1 and R2.
If we first analyze MLN cells from the control well, by cytometry, based on FSC/SSC, there are two populations, R1 and R2 (Figure 2). Staining with Annexin-V and 7-AAD demonstrated that the population in R1 contained mainly viable cells (Annexin-V-/7-AAD-) (Figure 3), 95.40% of the cells after 12 hours of culture. In R2, most cells were dead (Annexin-V+/7-AAD+) (data not shown). Over time (from 12 to 36 hours of culture), the percentage of viable cells decreased as expected, cells being not activated by any stimulus (from 55.10% at 12h to 22.83% at 36h) (Figure 2). Then, we focused on cells in the R1 region to study the effect of the toxins and their B subunits on MLN and spleen cells.
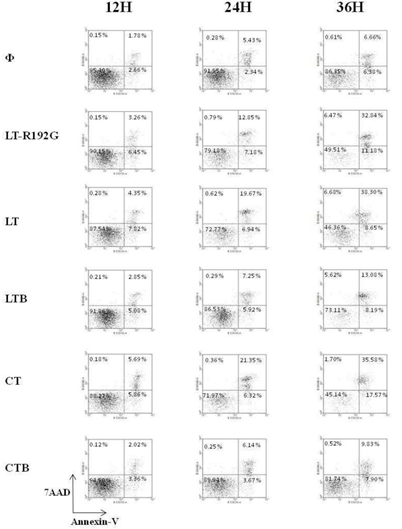
Figure 3: Apoptosis study of murine MLN cells after in vitro contact with LT, LTR192G, LTB, CT and CTB. Cells from MLN (4 × 105 cells/well) were cultured in the presence of the cholera- like enterotoxin or their B subunit (5μg/mL) for 12, 24 and 36 hours. MLN cells were stained with Annexin-V and 7-AAD and analyzed by flow cytometry. Population labeled R1 (See figure 2) was targeted.
LT-R192G, LT and CT induced a substantial decrease of MLN cells compared with the control, between 2 and 3 fold, depending on the toxin (Figure 2) at 12h, this difference being between 4 and 5 fold at 36h. LTB had a lower effect, whereas CTB had little effect. In terms of apoptosis induction, it is clear that LT-R192G, LT and CT induced apoptosis of cells from MLN as an increase of cells in early and late apoptosis can be seen from 12 to 36 hours compared with the control (Figure 3). This induction of apoptosis was less obvious in cells from the spleen, although, at 36 hours, cells in late apoptosis increased with the 3 toxins compared with the control (Data not shown). As expected, LTB induced weak apoptosis in MLN (Figure 3) and spleen (data not shown). With CTB, at 36 hours, a very weak increase of cells in late apoptosis was observed in MLN (Figure 3) and the spleen compared with the control (data not shown).
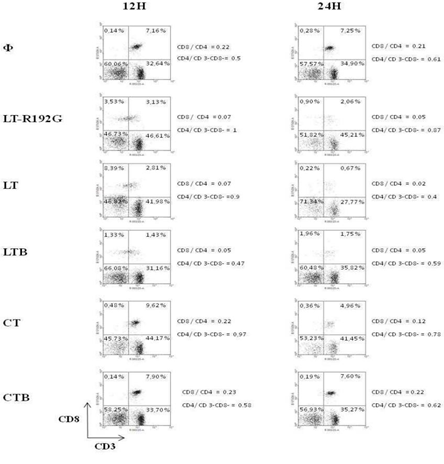
We then looked at the cells that underwent apoptosis, particularly CD8+ and CD4+ T cells, as they have been reported to be the main target of these toxins [31, 45]. LT-R192G, LT, LTB and CT, but not CTB, induce the preferential apoptosis of spleen CD8+ T cells in vitro. As expected, LT-R192G, LT, LTB and CT induced preferential death of CD8+ T cells, as seen in Figure 4, as soon as 12 hours for LT-R192G, LT and LTB, and at 24 hours for CT. Moreover, this death was associated with a decrease in CD8 expression. On the opposite of to, LT, its mutant and B subunit also decreased the expression of CD3 on CD8+ T cells, as seen with the increase of the percentage of CD3-CD8+ T cells. The death was due to the induction of apoptosis, as seen in Table 1.
Figure 4: In vitro effect of LT, LT-R192G, LTB, CT and CTB on murine MLN CD8+ and CD4+ T cells. Cells from spleen (4 × 105 cells/well) were cultured in the presence of the cholera-like enterotoxin or their B subunit (5μg/mL) for 12 and 24 hours. Cells were then stained with anti- CD3 and anti-CD8 and analyzed by flow cytometry. Population labeled R1 (See figure 2) was targeted.
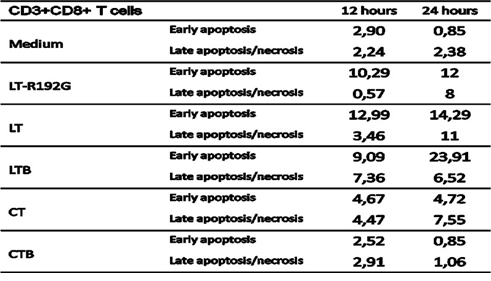
Table 1: Apoptosis study of spleen CD3+CD8+ T cells after in vitro incubation with LT, LT- R192G, LTB, CT and CTB. Cells from spleen (4 × 105 cells/well) were cultured in the presence of the cholera-like enterotoxin or their B subunit (5μg/mL) for 12 and 24 hours. Cells were then stained with anti-CD3, anti-CD8, 7-AAD and Annexin-V and analyzed by flow cytometry. The table shows the kinetics of early (Annexin-V+7-AAD–) and late (Annexin-V+7-AAD+) necrosis/apoptotic cells.
We also analyzed the ratio of CD4+ T cells/CD3-CD8- cells and noticed that LTB, opposite the three toxins, did not decrease CD3-CD8- cells (Figure 4). When we stained cells with anti-CD4 and anti-CD25 antibodies, we observed only LTB increased CD4-CD25+ T cells compared with the control (data not shown). This observation could explain the previous one, i.e. LTB activates cells in the CD3-CD8-population (due to CD25 expression), and then these cells could proliferate. These cells could be B cells, as previously reported [45]. By our previous observations, i.e. CTB did not induce the death of spleen cells, no variation in the % of CD3-CD8-, CD3+CD8+ and CD3+CD8- T cells was observed with CTB compared with the control (Figure 4).
LT-R192G, LT, LTB and CT do not induce preferential apoptosis of CD4+CD25+ T cells compared with CD4+CD25- T cells in vitro. To precise the mechanism by which the toxins and LTB decrease CD4+CD25+Foxp3+ T cells, we then looked at the induction of apoptosis of CD4+CD25+ T cells (among CD4+CD25+ T cells, there are CD4+CD25+Foxp3+ T cells) in comparison with CD4+CD25- T cells. Preferential apoptosis of CD4+CD25+ T cells, as for CD8+ T cells, could explain our previous data [28].
As already noticed and reported previously [28, 46], CD4+CD25+ T cells are more sensitive to cell death in vitro, and after 24 hours of culture, 11,93% of cells are dead compared with only 2,39% of CD4+CD25- T cells (Tables 2 and 3). In the presence of the toxins and the LTB, there was an increase of early apoptosis for CD4+CD25+ T cells, compared with the control, as soon as 12h, and CT had the most prominent effect. The same observation can be done for CD4+CD25- T cells but with a more pronounced effect at 24h. CTB did not induce apoptosis in CD4+CD25- T cells, as expected. Although we could notice an increase in early apoptosis in CD4+CD25+ T cells at 12h, this effect was not seen at 24h, and on the contrary, there was less early and late apoptosis when compared with the control.
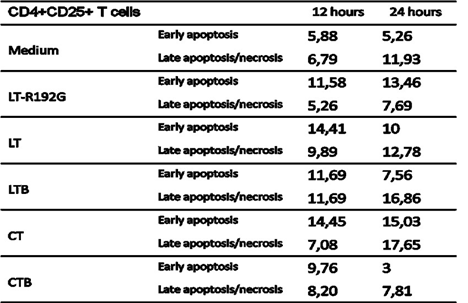
Table 2: Apoptosis study of MLN CD4+CD25+ T cells after in vitro incubation with LT, LT- R192G, LTB, CT and CTB. Cells from MLN (4 × 105 cells/well) were cultured in the presence of the cholera-like enterotoxin or their B subunit (5μg/mL) for 12 and 24 hours. Cells were then stained with anti-CD4, anti-CD25, 7-AAD and Annexin-V and analyzed by flow cytometry. The table shows the kinetics of early (Annexin-V+7-AAD–) and late (Annexin-V+7-AAD+) necrosis/apoptotic cells.
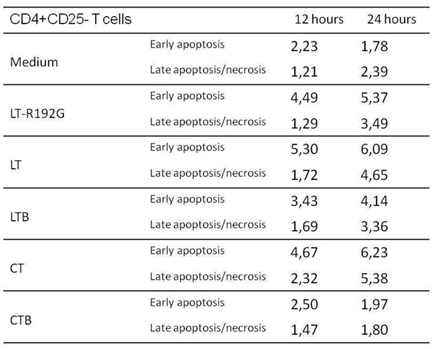
Table 3: Apoptosis study of MLN CD4+CD25- T cells after in vitro incubation with LT, LT- R192G, LTB, CT and CTB. Cells from MLN (4 × 105 cells/well) were cultured in the presence of the cholera-like enterotoxin or their B subunit (5μg/mL) for 12 and 24 hours. Cells were then stained with anti-CD4, anti-CD25, 7-AAD and Annexin-V and analyzed by flow cytometry. The table shows the kinetics of early (Annexin-V+7-AAD–) and late (Annexin-V+7-AAD+) necrosis/apoptotic cells.
4. Discussion
We have shown previously that LT-R192G decreased CD4+CD25+Foxp3+ T cells after in vitro contact with various tissues and organs (e.g. MLN, spleen) [28]. As this effect has never been reported, we wanted to check whether other cholera-like enterotoxins had the same effect. We tested native LT and its B subunit, LTB, and CT and its B subunit, CTB. All molecules except CTB decreased CD4+CD25+Foxp3+ T cells after two days of in vitro contact. Moreover, a decrease in CD25 expression was found with LT and CT but not with LTB. On the opposite, CTB did not have any effect. From this experiment, differences could be highlighted between the molecules. It seems that for LT, the ADP-ribosyl transferase activity is not necessary for the induction of Tregs decrease but this activity, even reduced (i.e. LT-R192G), modulates CD25 expression. In the case of CT, this activity is compulsory to induce a Treg decrease. Their different binding affinities could explain the difference between CTB and LTB.
Indeed, LTB, in addition to binding to the ganglioside GM1, binds to asialoGM1, lactosylceramide and some galactoproteins. Because of these differences and the precise mechanism by which the toxins and LTB decrease CD4+CD25+Foxp3+ T cells, we decided to revisit the global effect of cholera-like enterotoxins on T lymphocytes in our in vitro model. Globally, previous studies have reported the preferential apoptosis of CD8+ T cells after in vitro contact with LT, LTB and CTB, but not with CT. However, several studies are contradictory [14, 31, 36]. One explanation could be the dose of toxin. Indeed, we have previously shown a dose effect with LT-R192G [28]. Moreover, it has been reported that CTB from Vibrio cholerae and recombinant CTB can induce CD8+ and CD4+ T cells apoptosis [42, 43]; however, in our in vitro model and using CTB from Vibrio cholerae, we did not find any decrease of CD4+CD25+Foxp3+ T cells. There are two possibilities: 1. CTB induces apoptosis of T lymphocytes (CD8 and CD4) but saves up natural regulatory T cells 2. It does not induce in our in vitro model apoptosis of T lymphocytes. So, we decided to revisit the effect of the toxins and their subunits on T lymphocytes in our in vitro model. We first looked at the global impact of the molecules on cells from the spleen and mesenteric lymph nodes (MLN). LT, LT-R192G and CT depleted viable spleen and ML cells, LTB to a lesser degree, and CTB did not have any effect. This depletion was due to the induction of apoptosis in MLN. In the spleen, the induction of apoptosis was less pronounced, as we did not observe an increase in early apoptosis. In contrast, at 24 and 36h, there was more late apoptosis with the molecules compared with the control (data not shown). The sensitivity of the cells could be different from one organ to another.
When we looked more precisely at the targeted cellular populations, we found that LT, LT-R192G, LTB and to a lesser extent CT induced preferential apoptosis of resting CD8+ T cells. However, CD4+ T cells also come to die after contact with these molecules. Our data agree with previous studies reporting the preferential apoptosis of resting CD8+ T cells with LT and LTB [32, 37]. CT also induces CD8+ T cell apoptosis but was delayed compared with LT and LTB. This is in disagreement with previous studies [42]. In addition, LT, LT-R192G and LTB decreased CD3 expression on CD8+ T cells, contrary to CT. This result has never been reported. Moreover, we noticed some differences between LT/LT-R192G and LTB regarding the ratio of CD4+ T cells/CD3-CD8- T cells and the activation of CD4- cells, seen with the induction of CD25 expression (data not shown). These differences could be explained by the fact that LTB can activate B cells, as previously reported by Williams et al. [47], and not LT. This is an essential difference between the whole toxin and the B subunit and can explain that LTB conserves adjuvant properties.
Counter to [42] and [43]; we did not find any effect of CTB on T cells, although we used non-recombinant CTB. This absence of effect led us to control whether the CTB we used induced antibodies against VLP-2/6 when both were co-administered intrarectally in BALB/c mice, i.e. had adjuvant properties as previously reported [17, 18, 48, 49]. CTB, as LT-R192G and CT, induced antibodies against VLP-2/6 [28]. This result underlines a significant difference between CTB and LTB, which could partly be explained by the difference in their binding properties [8]. However, like LTB, CTB can activate B cells, probably by a different mechanism, as we did not observe any induction of CD25 expression on CD4- T Cells [50]. These new results highlight differences between CT and LT, their subunits, and their mechanism of action. Moreover, these results question again the role played by the A subunit, i.e. the toxicity, in the adjuvant effect of LT and CT. It is clear that CT and LT use different mechanisms when they interact with T cells.
We then looked at the subpopulations of CD4+ T cells using CD25 to study any preferential apoptosis of CD4+CD25+ T cells among CD4+ T cells and to explain the decrease of CD4+CD25+Foxp3+ T cells seen previously, after in vitro contact with LT-R192G, LT, LTB and CT. In culture, CD4+CD25+ T cells were more sensitive than other T cells (CD8+ and CD4+CD25-T cells) and died more quickly. This is seen in the control wells' early and late apoptosis levels. This observation was previously noticed [44] and reported elsewhere [46]. When we then analyzed the effect of LT, LT-R192G, LTB and CT, all four increased early apoptosis compared with the control as soon as 12h for CD4+CD25+ T cells, mainly from 24 hours for CD4+CD25- T cells. Regarding CTB, we concluded that it does not induce apoptosis of any CD4+ T cells. Conversely, CTB seems to increase the survival of CD4+CD25+ T cells, although we cannot be affirmative. There are, of course, limits to the method we have used. We analyzed CD4+CD25+ T cells. However, only a part of these cells are Foxp3+. We did not study the induction of apoptosis using a purified subpopulation of T cells. Finally, the staining of CD4+CD25+Foxp3+ T cells requires fixation and permeabilization of cells.
5. Conclusion
This study confirms that both toxins, LT and CT, and LT-R192G, a less toxic mutant of LT and LTB, induce the death of both CD4+ and CD8+ T cells in vitro. Unexpectedly, CTB had no effect. Whereas LT, LT-R192G and LTB induced rapid apoptosis of CD8+ T cells with a decrease in CD3 expression, CT induced less rapid apoptosis with no decrease in CD3 expression. On the other hand, they induced the depletion of CD4+CD25+Foxp3+ T cells. However, using the method, we could not show preferential apoptosis of these cells among the CD4+ T cells. While toxicity plays a significant role in CT, receptor binding may be essential in the observed effects of LT.
Finally, the results show the complexity and heterogeneity in the effects of these different molecules.
Competing interests
The authors declare that they have no competing interests.
Authors’ Contributions
FT and CMN conceived the study, the methodology and drafted the manuscript.
EK & CB coordinated this study, revised the manuscript, and approved the final version
CMN contributed to the correction and revision of the manuscript, and approved the final version.
CC conducted the statisticl analysis
JDC provide the virus like particles
All authors read and approved the final manuscript.
Acknowledgements
This work was supported by the Ministère de l’Enseignement Supérieur et de la Recherche. We thank Arlette Hammann and Anabelle Sequeira- Le Grand (Plateau technique de cytométrie, Inserm U866, IFR Santé-STIC, Dijon, France) for technical assistance with FCM.
References
- Freytag LC and JD. Clements, Mucosal adjuvants Vaccine 23 (2005): 1804-13.
- Cox E, et al. Adjuvants modulating mucosal immune responses or directing systemic responses towards the mucosa. Vet Res 37 (2006): 511-39.
- Sanchez J and Holmgren J. Cholera toxin structure, gene regulation and pathophysiological and immunological aspects. Cell Mol Life Sci 65 (2008): 1347-60.
- Kazemi R, et al. Immunogenic properties of trivalent recombinant protein composed of B-subunits of LT, STX-2, and CT toxins. Microbes Infect 18 (2016): 421-429.
- Holmgren J, et al. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunol Lett 97 (2005): 181-8.
- Basset C, et al. Cholera-like enterotoxins and Regulatory T cells. Toxins (Basel) 2 (2010): 1774-95.
- Lingwood C. Therapeutic Uses of Bacterial Subunit Toxins. Toxins (Basel) 13 (2021).
- Rappuoli R, et al. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol Today 20 (1999): 493-500.
- Kotloff KL, et al. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect Immun 69 (2001): 3581-90.
- Lapa JA, et al. Randomized clinical trial assessing the safety and immunogenicity of oral microencapsulated enterotoxigenic Escherichia coli surface antigen 6 with or without heat-labile enterotoxin with mutation R192G. Clin Vaccine Immunol 15 (2008): 1222-8.
- Lemere CA. Developing novel immunogens for a safe and effective Alzheimer's disease vaccine. Prog Brain Res 175 (2009): 83-93.
- Dickinson BL and JD. Clements, Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun 63 (1995): 1617-23.
- Bernstein DI, et al. A Phase 1 dose escalating study of double mutant heat-labile toxin LTR192G/L211A (dmLT) from Enterotoxigenic Escherichia coli (ETEC) by sublingual or oral immunization. Vaccine 37 (2019): 602-611.
- Nashar TO, et al. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin: receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc Natl Acad Sci USA 93 (1996): 226-30.
- Baldauf KJ, et al. Cholera toxin B: one subunit with many pharmaceutical applications. Toxins (Basel) 7 (2015): 974-96.
- Royal JM and Matoba N. Therapeutic Potential of Cholera Toxin B Subunit for the Treatment of Inflammatory Diseases of the Mucosa. Toxins (Basel) 9 (2017).
- Kim HJ, et al. Intranasal vaccination with peptides and cholera toxin subunit B as adjuvant to enhance mucosal and systemic immunity to respiratory syncytial virus. Arch Pharm Res 30 (2007): 366-71.
- Isaka M, et al. Recombinant cholera toxin B subunit (rCTB) as a mucosal adjuvant enhances induction of diphtheria and tetanus antitoxin antibodies in mice by intranasal administration with diphtheria-pertussis-tetanus (DPT) combination vaccine. Vaccine 22 (2004): 3061-8.
- Wu HY and Russell MW. Induction of mucosal and systemic immune responses by intranasal immunization using recombinant cholera toxin B subunit as an adjuvant. Vaccine 16 (1998): 286-92.
- Kim, M.Y., et al., Oral immunisation of mice with transgenic rice calli expressing cholera toxin B subunit fused to consensus dengue cEDIII antigen induces antibodies to all four dengue serotypes. Plant Mol Biol 92 (2016): 347-56.
- Sun JB, et al. Nasal administration of Schistosoma mansoni egg antigens-cholera toxin B subunit conjugate to infected mice reduces immunopathology and mortality. Adv Exp Med Biol 495 (2001): 305-9.
- Lee JB, et al. Intranasal delivery of cholera toxin induces th17-dominated T-cell response to bystander antigens. PLoS One 4 (2009): e5190.
- Datta SK, et al. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. Proc Natl Acad Sci USA 107 (2010): 10638-43.
- Meza-Sanchez D, et al. Intradermal immunization in the ear with cholera toxin and its non-toxic beta subunit promotes efficient Th1 and Th17 differentiation dependent on migrating DCs. Eur J Immunol 41 (2011): 2894-904.
- Brereton CF, et al. Escherichia coli heat-labile enterotoxin promotes protective Th17 responses against infection by driving innate IL-1 and IL-23 production. J Immunol 186 (2011): 5896-906.
- Ogier A, et al. Distribution and phenotype of murine rotavirus-specific B cells induced by intranasal immunization with 2/6 virus-like particles. Eur J Immunol 35 (2005): 2122-30.
- Sakaguchi S, et al. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med 161 (1985): 72-87.
- Sakaguchi S, Takahashi T and Nishizuka Y. Study on cellular events in postthymectomy autoimmune oophoritis in mice. I. Requirement of Lyt-1 effector cells for oocytes damage after adoptive transfer. J Exp Med 156 (1982): 1565-76.
- Sakaguchi S, Wing K and Miyara M. Regulatory T cells - a brief history and perspective. Eur J Immunol 37 (2007): S116-23.
- Salmond RJ, Luross JA and Williams NA. Immune modulation by the cholera-like enterotoxins. Expert Rev Mol Med 4 (2002): 1-16.
- Szabo SJ, et al. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med 185 (1997): 817-24.
- Tamayo E, et al. The Escherichia coli heat-labile enterotoxin induces apoptosis of immature lymphocytes in vivo via a glucocorticoid-dependent pathway. Eur J Immunol 35 (2005): 3505-15.
- Tamayo E, et al. Involvement of the intrinsic and extrinsic cell-death pathways in the induction of apoptosis of mature lymphocytes by the Escherichia coli heat-labile enterotoxin. Eur J Immunol 39 (2009): 439-46.
- Tamayo E, et al. GITR contributes to the systemic adjuvanticity of the Escherichia coli heat-labile enterotoxin. Eur J Immunol 40 (2010): 754-63.
- Soriani M, Williams NA and Hirst TR. Escherichia coli enterotoxin B subunit triggers apoptosis of CD8(+) T cells by activating transcription factor c-myc. Infect Immun 69 (2001): 4923-30.
- Salmond RJ, et al. CD8+ T cell apoptosis induced by Escherichia coli heat-labile enterotoxin B subunit occurs via a novel pathway involving NF-kappaB-dependent caspase activation. Eur J Immunol 32 (2002): 1737-47.
- Truitt RL, et al. Glycosphingolipids as novel targets for T-cell suppression by the B subunit of recombinant heat-labile enterotoxin. Infect Immun 66 (1998): 1299-308.
- Elson CO, et al. Morphologic and functional alterations of mucosal T cells by cholera toxin and its B subunit. J Immunol 154 (1995): 1032-40.
- Yamamoto M, et al. Direct effects on antigen-presenting cells and T lymphocytes explain the adjuvanticity of a nontoxic cholera toxin mutant. J Immunol 162 (1999): 7015-21.
- Wang M, Bregenholt S and Petersen JS. The cholera toxin B subunit directly costimulates antigen-primed CD4+ T cells ex vivo. Scand J Immunol 58 (2003): 342-9.
- Arce S, et al. Differential binding of Escherichia coli enterotoxins LT-IIa and LT-IIb and of cholera toxin elicits differences in apoptosis, proliferation, and activation of lymphoid cells. Infect Immun 73 (2005): 2718-27.
- Yankelevich B, et al. Differential induction of programmed cell death in CD8+ and CD4+ T cells by the B subunit of cholera toxin. Cell Immunol 168 (1996): 229-34.
- Aman AT, et al. A mutant cholera toxin B subunit that binds GM1- ganglioside but lacks immunomodulatory or toxic activity. Proc Natl Acad Sci USA 98 (2001): 8536-41.
- Thiam F, et al. Unexpected modulation of recall B and T cell responses after immunization with rotavirus-like particles in the presence of LT-R192G. Toxins (Basel) 2 (2010): 2007-27.
- Nashar TO, Williams NA and Hirst TR. Cross-linking of cell surface ganglioside GM1 induces the selective apoptosis of mature CD8+ T lymphocytes. Int Immunol 8 (1996): 731-6.
- Sakaguchi S, et al. Regulatory T cells and immune tolerance. Cell 133 (2008): 775-87.
- Williams NA, Hirst TR and Nashar TO. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol Today 20 (1999): 95-101.
- Lei H, et al. Evaluation of oral immunization with recombinant avian influenza virus HA1 displayed on the Lactococcus lactis surface and combined with the mucosal adjuvant cholera toxin subunit B. Clin Vaccine Immunol 18 (2011): 1046-51.
- Cho HJ, et al. Enhanced humoral and cellular immune responses after sublingual immunization against human papillomavirus 16 L1 protein with adjuvants. Vaccine 28 (2010): 2598-606.
- Francis ML, et al. Cyclic AMP-independent effects of cholera toxin on B cell activation. II. Binding of ganglioside GM1 induces B cell activation. J Immunol 148 (1992): 1999-2005.