Changes in the Maternal Risk Factors of Congenital Heart Disease in Chinese Population: A Meta-analysis
Article Information
Di Jiang, Chenxiao Bai, Liwen Wang, Ou Chen*
School of Nursing, Shandong University, Jinan, Shandong, 250012, China
*Corresponding author: Ou Chen, School of Nursing, Shandong University, Jinan, Shandong, 250012, China
Received: 22 May 2019; Accepted: 01 June 2019; Published: 3 June 2019
Citation: Di Jiang, Chenxiao Bai, Liwen Wang, Ou Chen. Changes in the Maternal Risk Factors of Congenital Heart Disease in Chinese Population: A Meta-analysis. Cardiology and Cardiovascular Medicine 3 (2019): 090-107.
Share at FacebookAbstract
Objective: To explore whether there were any changes in the maternal risk factors of congenital heart disease (CHD) in Chinese population between the ten years before 2008 (1999-2008) and the ten years after 2008 (2009-2018).
Methods: Databases including PubMed, Web of Science, Wanfang Data, China National Knowledge Infrastructure, Chinese Scientific and Technological Journal Database and China Biology Medicine disc were retrieved and reference lists of related articles were checked to collect relevant studies. Meta-analysis was conducted to calculate the pooled OR and 95%CI. Subgroup analyses, meta regression, sensitivity analysis and publication bias were further performed.
Results: A total of 44 studies were included in the meta-analysis. In the ten years before 2008, 6 risk factors including colds, infections, and medication use during pregnancy, exposure to chemical toxic substances during pregnancy, negative life events during pregnancy, and abnormal childbearing history were identified. While in the ten years after 2008, 12 factors were identified. The 6 new factors were periconceptional folic acid supplementation, vitamin supplementation, living near major traffic roads, living or working near factories, living in newly renovated rooms, and passive smoking during pregnancy.
Conclusion: The maternal risk factors of CHD in Chinese population has changed greatly between the ten years before 2008 and the ten years after 2008. People’s concerns on living environments, lifestyles, and pregnancy care have been greatly increased in recent years. Notably, environmental pollution has become an important issue endangering maternal and fetal health in the ten years after 2008 in China.
Keywords
Congenital Heart Disease; Risk Factors; Maternal; Changes; Meta-Analysis
Article Details
Introduction
Congenital heart disease (CHD) is one of the most common birth defects and one of the leading causes of infant death worldwide[1-3]. Although great advances in the surgical and medical treatment of CHD have led to a remarkable improvement in the survival rate of CHD neonates, due to the existence of complications such as heart failure, arrhythmia and pulmonary arterial hypertension, the life quality of CHD patients is still poorer and the mortality is much higher compared to general population, which exerts huge medical expenditures for both family and society [4, 5]. Obviously, the best solution towards this situation is to prevent CHD from happening. Therefore, to identify the risk factors of CHD, especially the modifiable factors such as maternal risk factors, and take corresponding measures is of vital importance. With the rapid development of China's modernization and industrialization in recent years, people’s living standards and lifestyles have undergone dramatic changes, people’s health care consciousness has been greatly enhanced, and more importantly, environmental pollution and ecological deterioration have been much aggravated, which may lead to changes in the maternal risk factors of CHD in Chinese population. Whether new maternal risk factors have emerged or whether the previous maternal risk factors have changed in recent years is unknown. Therefore, in the present meta-analysis, we made a comparison of the maternal risk factors of CHD in Chinese population between the ten years before 2008 (1999-2008) and the ten years after 2008 (2009-2018) to find whether there are any changes in the maternal risk factors of CHD, and to determine the current risk factors of CHD in Chinese population.
Methods
Literature selection
Literature selection was conducted in PubMed, Web of Science, China National Knowledge Infrastructure, Chinese Scientific and Technological Journal Database, China Biology Medicine disc and Wanfang Data to collect all Chinese and English relevant studies published in the ten years before 2008 and the ten years after 2008. The search strategy was as follows: (“congenital heart disease*” OR “congenital heart defect*” OR “heart abnormalit*” OR “malformation of heart” OR “congenital cardiovascular disease*” OR “CHD”) AND (“risk” OR“etiology” OR “onset” OR “association” OR “connection” OR “relationship”) AND (“Chinese” OR “China”). In addition, reference lists of the related original and review articles were checked manually to find additional studies that may meet the eligibility criteria. Literature selection was performed by two authors independently.
Eligibility Criteria
Eligible studies had to meet all of the following criteria: (1) relevant to the maternal risk factors of CHD in Chinese population; (2) were original epidemiological studies (i.e., case–control, cohort or RCT); (3) reported effect estimates and their 95% confidence intervals (CI), or provided sufficient data from which these measures could be calculated; (4) published in English or Chinese language. Studies was excluded if they: (1) were animal studies; (2) were of poor quality; (3) contained overlapped data; (4) reported incomplete data that cannot be obtained after contact with the author.
Titles and abstracts of retrieved studies were screened according to the above criteria by two authors independently, then full text of any potential relevant articles were assessed rigorously. Disagreements were resolved by discussion with the third author.
Data extraction
Data extraction was performed by two authors separately, and discrepancies were resolved by discussion with the third author. The following data were extracted from each eligible study: first author, geographical region, year of publication, study design, sample size, maternal risk factors and their adjusted estimates and corresponding 95% CI. If no effect estimate was provided in a given study, odds ratio (OR) or risk ratio (RR) and 95% CI would be calculated from the raw data presented in the study. And the authors would be contacted for further information if necessary.
Quality assessment
The quality of the eligible studies were assessed by the 9-star Newcastle-Ottawa Scale (NOS), which evaluates a study based on three major aspects: selection, comparability, and exposure or outcome [6]. Quality assessment was performed by two authors independently. If differences of opinion arose, these would be settled by discussion with the third author.
Statistical analysis
Where maternal risk factors had consistent definitions across studies (at least 3 studies) and effect estimates were presented in a similar fashion, meta-analysis would be performed to calculate the pooled OR and corresponding 95% CI. Cochran’s Q and I2 statistics were calculated to test for the heterogeneity among studies. If no evidence of heterogeneity was presented (p>0.1, I2<50%), a fixed effect model would be used. Otherwise, a random effect model would be adopted, and meta regression analyses would be further conducted based on year of publication, quality of study, geographical region and number of cases to explore the source of heterogeneity. Meta regression analyses would be conducted only when there were no fewer than 10 studies. Then subgroup analyses were performed based on the potential sources of heterogeneity. Sensitivity analysis was performed by omitting one study at a time to determine whether the results of meta-analysis were stable. Publication bias would be conducted only when there were no fewer than 10 studies, via Egger’s linear regression method. Probability value P<0.05 was considered statistically significant. All statistical analyses were performed using STATA software (version 14.0; Stata Corporation, College Station, Texas, USA).
Results
Literature selection
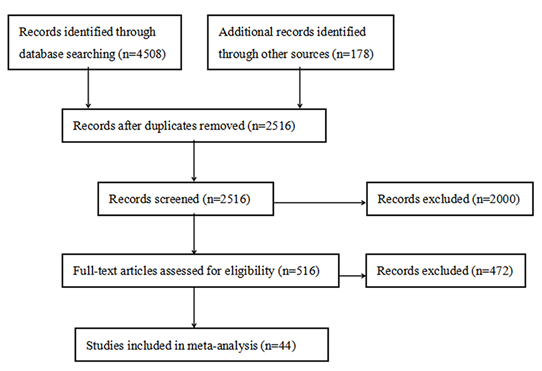
A total of 4686 studies were obtained initially, of which 4508 studies were obtained through database searching, 178 studies were obtained by reference lists checking. After the duplicate studies were removed, titles and abstracts of the remaining 2516 studies were further screened for eligibility. Full text of the 516 potentially relevant studies were assessed rigorously, and a total of 472 studies were then excluded. Finally, 44 studies were included in the meta-analysis (Figure 1).
Study characteristics and quality evaluation
A total of 44 case-control studies, covering 19 provinces (or municipalities) nationwide, were included in this meta-analysis. Of these studies, 12 [7-18] were published during the ten years before 2008, involving 3436 cases and 4157 controls; 32 studies [19-50] were published during the ten years after 2008, involving 10341 cases and 14289 controls. All of these studies were published in Chinese except for 2 studies published in English. After quality assessment by the Newcastle-Ottawa Scale, 15 studies got a score of 5, 21 studies got a score of 6, and 7 studies got a score of 7, 1 study got a score of 8. The main characteristics of the included studies are showed in Table 1.
|
First author (year) |
Region |
Study design |
No. of cases/controls |
Quality |
|
Qiu (1999) |
Guangxi, China |
Case-control |
1286 / 1286 |
5 |
|
Zhang (1999) |
Zhejiang, China |
Case-control |
351 / 351 |
6 |
|
Lai (2001) |
Chongqing, China |
Case-control |
761 / 761 |
6 |
|
Liu (2004) |
Shandong, China |
Case-control |
72 / 144 |
5 |
|
Gao (2005) |
Shandong, China |
Case-control |
73 / 146 |
6 |
|
Tan (2006) |
Hunan, China |
Case-control |
230 / 230 |
5 |
|
Yuan (2006) |
Beijing, China |
Case-control |
63 / 126 |
7 |
|
Hou (2007) |
Shanghai, China |
Case-control |
207 / 414 |
5 |
|
Zhong (2007) |
Guangxi, China |
Case-control |
132 / 132 |
5 |
|
Wang (2007) |
Shandong, China |
Case-control |
100 / 200 |
5 |
|
Wu (2008) |
Zhejiang, China |
Case-control |
45 / 135 |
7 |
|
Liu (2008) |
Anhui, China |
Case-control |
116 / 232 |
6 |
|
Gong (2009) |
Shaanxi, China |
Case-control |
80 / 80 |
5 |
|
Li (2009) |
Ningxia, China |
Case-control |
113 / 113 |
5 |
|
Mi (2010) |
Anhui, China |
Case-control |
86 / 86 |
7 |
|
Mei (2010) |
Zhejiang, China |
Case-control |
459 / 977 |
6 |
|
Chen (2010) |
Anhui, China |
Case-control |
65 / 130 |
7 |
|
Chen (2011) |
Fujian, China |
Case-control |
76 / 152 |
6 |
|
Ou (2011) |
Hunan, China |
Case-control |
123 / 246 |
6 |
|
Guo (2011) |
Shaanxi, China |
Case-control |
98 / 196 |
6 |
|
Yang (2012) |
Shaanxi, China |
Case-control |
42 / 221 |
5 |
|
Zhu (2013) |
Zhejiang, China |
Case-control |
108 / 108 |
6 |
|
Liu (2013) |
Jiangsu, China |
Case-control |
70 / 140 |
6 |
|
Zhang (2013) |
Shanxi, China |
Case-control |
203 / 406 |
5 |
|
Li (2013) |
Guangdong, China |
Case-control |
358 / 422 |
6 |
|
Chen (2014) |
Hebei, China |
Case-control |
110 / 297 |
6 |
|
Lin (2014) |
Fujian, China |
Case-control |
69 / 69 |
6 |
|
Dong (2014) |
Hebei, China |
Case-control |
815 / 1630 |
7 |
|
Hu (2014) |
Shenzhen, China |
Case-control |
364 / 364 |
6 |
|
Ou (2015) |
Guangdong, China |
Case-control |
4034 / 4034 |
6 |
|
Yang(2016) |
Beijing, China |
Case-control |
117 / 117 |
6 |
|
Bing (2016) |
Jiangsu, China |
Case-control |
48 / 48 |
7 |
|
Lv (2016) |
Fujian, China |
Case-control |
149 / 149 |
5 |
|
Zhang (2016) |
Henan, China |
Case-control |
728 / 728 |
6 |
|
Jiang (2016) |
Guangdong, China |
Case-control |
30 / 30 |
5 |
|
Xiang (2017) |
Zhejiang, China |
Case-control |
500 / 500 |
6 |
|
Li (2017) |
Fujian, China |
Case-control |
61 / 61 |
6 |
|
Li (2017) |
Fujian, China |
Case-control |
187 / 374 |
5 |
|
Zhang (2017) |
Shaanxi, China |
Case-control |
270 / 1633 |
5 |
|
Li (2017) |
Shandong, China |
Case-control |
160 / 160 |
5 |
|
Li (2017) |
Fujian, China |
Case-control |
72 / 72 |
6 |
|
Chen (2017) |
Sichuan, China |
Case-control |
250 / 250 |
6 |
|
Zhang (2017) |
Henan, China |
Case-control |
165 / 165 |
8 |
|
Xu (2018) |
Qinghai, China |
Case-control |
331 / 331 |
7 |
Table1: The main characteristics of the included studies
The major maternal risk factors in the ten years before 2008 (1999-2008)
- Colds during pregnancy
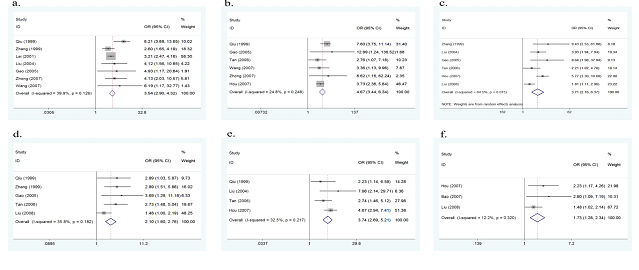
Seven of the 12 studies investigated colds during pregnancy as a potential risk factor for the onset of CHD. No evidence of heterogeneity was found among the 7 studies (p=0.126, I2=39.9%), therefore a fixed effect model was performed. The overall results of this meta-analysis showed that colds during pregnancy significantly increased the risk of CHD (OR=3.54, 95%CI:2.90-4.32, p<0.001, Figure 2a). After sequentially omitting each included study in sensitivity analysis, the result remained largely unchanged, indicating the results were stable.
- Exposure to chemical toxic substances during pregnancy
Six studies assessed exposure to chemical toxic substances (such as pesticides, organic solvents, etc.) during pregnancy as a possible risk factor. No evidence of heterogeneity was found (p=0.248, I2=24.8%), thus a fixed effect model was conducted. The overall results of this meta-analysis showed that exposure to chemical toxic substances during pregnancy significantly increased the risk of CHD (OR=4.67, 95%CI: 3.44-6.34, p<0.001, Figure 2b). No obvious change was found in sensitivity analysis, suggesting the results were stable.
- Negative life events during pregnancy
Six studies explored the association between negative life events during pregnancy and the risk of CHD. As there was heterogeneity between the 6 studies (p=0.015, I2=64.5%), a random effect model was used. The overall results of this meta-analysis showed that negative life events during pregnancy significantly increased the risk of CHD (OR=3.71, 95%CI: 2.16-6.37, p<0.001, Figure 2c). No significant change was found in sensitivity analysis, showing the results were stable.
- Medication use during pregnancy
Five studies reported the association between medication use during pregnancy and the risk of CHD. No evidence of heterogeneity was found (p=0.182, I2=35.8%), therefore a fixed effect model was used. The overall results of this meta-analysis showed that medication use during pregnancy significantly increased the risk of CHD (OR=2.10, 95%CI: 1.60-2.76, p<0.001, Figure 2d). No obvious change was found in sensitivity analysis, indicating the results were stable.
- Infection during pregnancy
Four studies assessed the association between infection during pregnancy and the risk of CHD. No evidence of heterogeneity was found (p=0.217, I2=32.5%), therefore a fixed effect model was used. The overall results of this meta-analysis showed that infection during pregnancy significantly increased the risk of CHD (OR=3.74, 95%CI: 2.69-5.21, p<0.001, Figure 2e). No significant change was found in sensitivity analysis, suggesting the results were stable.
- Abnormal childbearing history
Three studies assessed the association between abnormal childbearing history (such as abortion, stillbirth, etc.) and the risk of CHD. No evidence of heterogeneity was found (p=0.320, I2=12.2%), therefore a fixed effect model was used. The overall results of this meta-analysis showed that abnormal childbearing history significantly increased the risk of CHD (OR=1.73, 95%CI: 1.28-2.34, p<0.001, Figure 2f). No significant change was found in sensitivity analysis, suggesting the results were stable.
Figure 2a: forest plot for the association between colds during pregnancy and CHD risk.
Figure 2b: forest plot for the association between exposure to chemical toxic substances during pregnancy and CHD risk.
Figure 2c: forest plot for the association between negative life events during pregnancy and CHD risk.
Figure 2d: forest plot for the association between medication use during pregnancy and CHD risk.
Figure 2e: forest plot for the association between infection during pregnancy and CHD risk.
Figure 2f: forest plot for the association between abnormal childbearing history and CHD risk.
The major maternal risk factors in the ten years after 2008 (2009-2018)
The same risk factors as ten years before 2008
- Colds during pregnancy
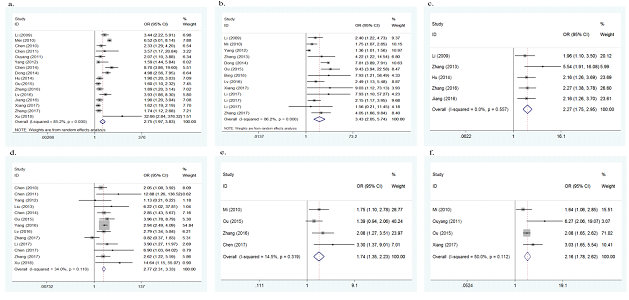
Sixteen of the 32 studies investigated colds during pregnancy as a potential risk factor for the onset of CHD. As there was heterogeneity between the 16 studies (p<0.001, I2=85.2%), a random effect model was adopted. The overall results of this meta-analysis showed that colds during pregnancy significantly increased the risk of CHD (OR=2.75, 95%CI: 1.97-3.83, p<0.001, Figure 3a). To explore the source of heterogeneity, meta regression analyses were conducted based on year of publication, quality of study, geographical region and number of cases. However, no significant results were derived. No significant change was found in sensitivity analysis, suggesting the results were stable. No publication bias was found in this meta-analysis (Egger's test p=0.870).
- Exposure to chemical toxic substances during pregnancy
Thirteen studies assessed exposure to chemical toxic substances during pregnancy as a possible risk factor. As there was heterogeneity between the 13 studies (p<0.001, I2=86.2%), a random effect model was adopted. The overall results of this meta-analysis showed that exposure to chemical toxic substances during pregnancy significantly increased the risk of CHD (OR=3.43, 95% CI: 2.05-5.74, p<0.001, Figure 3b). To explore the source of heterogeneity, meta regression analyses were conducted and number of cases was found to be the possible source (p=0.018). Subgroup analysis by number of cases was further performed, in the subgroup of cases ≤ 200, the pooled OR was 2.13 (95% CI: 1.53-2.94), with no evidence of heterogeneity (p=0.061, I2=46.3%); in the subgroup of cases > 200, the pooled OR was 7.72 (95% CI: 5.64-10.56), with no evidence of heterogeneity either (p=0.766, I2=0.0%). No significant change was found in sensitivity analysis, suggesting the results were stable. No publication bias was found in this meta-analysis (Egger's test p=0.152).
- Negative life events during pregnancy
Five studies explored the association between negative life events during pregnancy and the risk of CHD. No evidence of heterogeneity was found (p=0.557, I2=0.0%), therefore a fixed effect model was used. The overall results of this meta-analysis showed that negative life events during pregnancy significantly increased the risk of CHD (OR=2.27, 95%CI: 1.75-2.95, p<0.001, Figure 3c). No significant change was found in sensitivity analysis, showing the results were stable.
- Medication use during pregnancy
Thirteen studies reported the association between medication use during pregnancy and the risk of CHD. No evidence of heterogeneity was found (p=0.110, I2=34.0%), therefore a fixed effect model was used. The overall results of this meta-analysis showed that medication use during pregnancy significantly increased the risk of CHD (OR=2.77, 95%CI: 2.31-3.33, p<0.001, Figure 3d). No publication bias was found in this meta-analysis (Egger's test p=0.557). No obvious change was found in sensitivity analysis, indicating the results were stable.
- Infection during pregnancy
Four studies assessed the association between infection during pregnancy and the risk of CHD. No evidence of heterogeneity was found (p=0.319, I2=14.5%), therefore a fixed effect model was used. The overall results of this meta-analysis showed that infection during pregnancy significantly increased the risk of CHD (OR=1.74, 95%CI: 1.35-2.23, p<0.001, Figure 3e). No significant change was found in sensitivity analysis, suggesting the results were stable.
- Abnormal childbearing history
Four studies assessed the association between abnormal childbearing history and the risk of CHD. No evidence of heterogeneity was found (p=0.112, I2=50.0%), therefore a fixed effect model was used. The overall results of this meta-analysis showed that abnormal childbearing history significantly increased the risk of CHD (OR=2.16, 95%CI: 1.78-2.62, p<0.001, Figure 3f). No significant change was found in sensitivity analysis, suggesting the results were stable.
Figure 3a: forest plot for the association between colds during pregnancy and CHD risk.
Figure 3b: forest plot for the association between exposure to chemical toxic substances during pregnancy and CHD risk.
Figure 3c: forest plot for the association between negative life events during pregnancy and CHD risk.
Figure 3d: forest plot for the association between medication use during pregnancy and CHD risk.
Figure 3e: forest plot for the association between infection during pregnancy and CHD risk.
Figure 3f: forest plot for the association between abnormal childbearing history and CHD risk.
New risk factors in the ten years after 2008
- Passive smoking during pregnancy
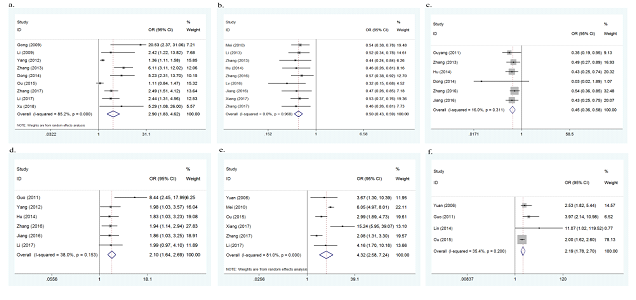
Nine studies investigated passive smoking during pregnancy as a potential risk factor for the onset of CHD. As there was heterogeneity between the 9 studies (p<0.001, I2=85.2%), a random effect model was adopted. The overall results of this meta-analysis showed that passive smoking during pregnancy significantly increased the risk of CHD (OR=2.90, 95%CI: 1.83-4.62, p<0.001, Figure 4a). No significant change was found in sensitivity analysis, suggesting the results were stable.
- Periconceptional folic acid supplementation
Nine studies assessed the association between periconceptional folic acid supplementation and the risk of CHD. No evidence of heterogeneity was found (p=0.968, I2=0.0%), therefore a fixed effect model was used. The overall results of this meta-analysis showed that periconceptional folic acid supplementation significantly decreased the risk of CHD (OR=0.50, 95%CI: 0.43-0.59, p<0.001, Figure 4b). No significant change was found in sensitivity analysis, suggesting the results were stable.
- Periconceptional vitamin supplementation
Six studies assessed the association between periconceptional vitamin supplementation and the risk of CHD. No evidence of heterogeneity was found (p=0.311, I2=16.0%), therefore a fixed effect model was used. The overall results of this meta-analysis showed that periconceptional vitamin supplementation significantly decreased the risk of CHD (OR=0.45, 95%CI: 0.36-0.58, p<0.001, Figure 4c). No significant change was found in sensitivity analysis, indicating the results were stable.
- Living or working near factories during pregnancy
Six studies assessed the association between living or working near factories during pregnancy and the risk of CHD. No evidence of heterogeneity was found (p=0.153, I2=38.0%), therefore a fixed effect model was adopted. The overall results of this meta-analysis showed that living or working near factories during pregnancy significantly increased the risk of CHD (OR=2.10, 95%CI: 1.64-2.69, p<0.001, Figure 4d). No significant change was found in sensitivity analysis, indicating the results were stable.
- Living in newly renovated rooms during pregnancy
Six studies explored the association between living in newly renovated rooms during pregnancy and the risk of CHD. As there was heterogeneity between the 6 studies (p<0.001, I2=81.0%), a random effect model was used. The overall results of this meta-analysis showed that living in newly renovated rooms during pregnancy significantly increased the risk of CHD (OR=4.32, 95%CI: 2.58-7.24, p<0.001, Figure 4e). No significant change was found in sensitivity analysis, showing the results were stable.
- Living near major traffic roads (<50m) during pregnancy
Four studies reported the association between living near major traffic roads during pregnancy and the risk of CHD. No evidence of heterogeneity was found (p=0.200, I2=35.4%), therefore a fixed effect model was adopted. The overall results of this meta-analysis showed that living near major traffic roads during pregnancy significantly increased the risk of CHD (OR=2.19, 95%CI: 1.78-2.70, p<0.001, Figure 4f). No obvious change was found in sensitivity analysis, indicating the results were stable.
Figure 4a: forest plot for the association between passive smoking during pregnancy and CHD risk.
Figure 4b: forest plot for the association between periconceptional folic acid supplementation and CHD risk.
Figure 4c: forest plot for the association between periconceptional vitamin supplementation and CHD risk.
Figure 4d. forest plot for the association between living or working near factories during pregnancy and CHD risk.
Figure 4e: forest plot for the association between living in newly renovated rooms during pregnancy and CHD risk.
Figure 4f: forest plot for the association between living near major traffic roads during pregnancy and CHD risk.
Discussion
According to the present meta-analysis, the major maternal risk factors of CHD in Chinese population has changed greatly, and a total of 12 risk factors were identified as the current risk factors of CHD in Chinese population. In the ten years before 2008, 6 major risk factors were identified. Exposure to chemical toxic substances during pregnancy was found to have the highest risk for the onset of CHD, with a 4.67-fold increase in risk. While in the ten years after 2008 (2009-2018), the number of studies exploring the risk factors of CHD has greatly increased, and a total of 12 major risk factors were identified. Apart from the 6 risk factors identified ten years ago, 6 newly emerged risk factors concerning the living environments, lifestyles and pregnancy care were further identified. Living in newly renovated rooms during pregnancy was observed to have the highest risk for the onset of CHD (OR=4.32, 95%CI: 2.58-7.24).
Among the 6 same risk factors, the OR value of infection during pregnancy has changed significantly from 3.74 (95%CI: 2.69-5.21) in the ten years before 2008 to 1.74 (95%CI: 1.35-2.23) in the ten years after 2008, which may be due to the gradual popularization of pre-pregnancy and prenatal examinations, as well as to the improvement of people’s pregnancy care consciousness. However, there were no significant change in the OR values of exposure to chemical toxic substances during pregnancy, negative life events during pregnancy, colds during pregnancy, medication use during pregnancy as well as abnormal childbearing history between the ten years before 2008 and the ten years after 2008, indicating that these 5 risk factors may have an stable effect on the onset of CHD.
Of the 6 new factors emerged in the ten years after 2008, 2 factors were protective factors of CHD (periconceptional folic acid supplementation and periconceptional vitamin supplementation) and 4 factors were risk factors of CHD (living near major traffic roads (<50m) during pregnancy, living or working near factories during pregnancy, living in newly renovated rooms during pregnancy, and passive smoking during pregnancy). All the 6 new factors focus on the the living environments, lifestyles, and health care during pregnancy, which shows that people’s concerns on these aspects have been greatly increased. More importantly, it may also indicate that environmental pollution and ecological deterioration has become an important issue endangering maternal and fetal health in the ten years after 2008.
Although the clear biological mechanisms underlying the relationship between these risk factors and the onset of CHD remain to be determined, some relevant hypotheses have been proposed. Living in newly renovated rooms, living or working near factories, living near major traffic roads, exposure to chemical toxic substances, as well as passive smoking during pregnancy, all of them will expose pregnant women to environmental teratogens such as formaldehyde, benzene, organic solvents, heavy metals, carbon monoxide, ozone, pesticides, herbicides, and cigarette smoke etc., which may induce congenital malformations through the induction of oxidative stress [36, 51]. As for infections and colds during pregnancy, many pathogens of infections and colds could be transmitted vertically to fetus through placenta and increase the risk of congenital malformations. In addition, fever caused by infections and colds may lead to abnormal cell apoptosis during embryonic heart development and hence cause cardiac dysplasia [52-55]. The possible mechanism between medication use during pregnancy and CHD risk may be that, many medications have the ability to cross placental and blood-brain barriers, which may directly affect embryonic development and increase the risk of congenital malformations [56, 57]. Maternal stress caused by negative life events during pregnancy would lead to increased secretion of plasma adrenocorticotrophin, β-endorphin, glucocorticoids, and catecholamines, which may lead to changes in the fetal neurotransmitter systems and transcriptional mechanisms [40, 58]. Abnormal childbearing history, such as previous abortions, may result in some residual effects that may affect the development of subsequent embryo [59, 60]. In addition, the causes of previous abnormal pregnancies, such as genetic factors, uterine factors, maternal chronic diseases, etc., if not removed, will still affect subsequent embryonic development [60]. Moreover, having an abnormal childbearing history may lead to an increased use of medications, which may increase the possibility of congenital malformations induced by medications [36]. Periconceptional folic acid supplementation was found as an protective factor for CHD. It has been hypothesized that deficiency of folic acid during pregnancy would impair folate and/or homocysteine metabolism, result in hyperhomocysteinemia and impair methionine formation in the embryo, which may increase the risk of congenital malformations [2, 3, 60-62]. Periconceptional vitamin supplementation was another protective factor for CHD. Many vitamins play important roles during embryonic development. For example, vitamin B12 works as a coenzyme with folates in methionine, purine, and thymine synthesis, and one-carbon metabolism, vitamin C may protect embryos from oxygen free radical damages due to their antioxidant properties [62, 63].
There are some limitations in this meta-analysis. Firstly, all the 44 studies included in the final meta-analysis were case-control studies, which were susceptible to selection bias and information bias. Therefore, we evaluated the quality of each eligible study rigorously and eliminated studies with poor quality to increase the credibility of our results. Secondly, although significant heterogeneity were found in negative life events during pregnancy (the ten years before 2008), passive smoking during pregnancy, and living in newly renovated rooms during pregnancy, we did not carry out further analysis to clarify the source of heterogeneity due to the lack of enough studies. Thirdly, there were limited evidence regarding these newly emerged factors such as living near major traffic roads (<50m) during pregnancy, living in newly renovated rooms during pregnancy, etc., thus only several studies were included in our meta-analysis to explore the association between these new factors and the risk of CHD. Therefore, further studies should focus on these new risk factors, rather than those for which considerable evidence already exists.
Despite the limitations mentioned above, there are also some strengths in this meta-analysis. Firstly, to our best knowledge, this is the first meta-analysis comparing the major maternal risk factors of CHD in Chinese population between the ten years before 2008 and the ten years after 2008. And 6 new risk factors, focusing on the living environments, lifestyles, and health care during pregnancy, were further identified in this meta-analysis. Secondly, this meta-analysis covered a total of 12 risk factors of the onset of CHD, and each of them was analyzed carefully in our paper.
Conclusions
The major maternal risk factors of CHD in Chinese population has changed greatly between the ten years before 2008 and the ten years after 2008. And a total of 12 factors were identified as the current risk factors of CHD in Chinese population. Among these two periods, there are 6 same risk factors and 6 newly emerged risk factors of CHD. Of the 6 same risk factors, the OR value of infection during pregnancy has significantly changed, which may be due to the gradual popularization of pre-pregnancy and prenatal examinations and the improvement of people’s pregnancy care consciousness in recent years. And all the 6 new risk factors that emerged in the ten years after 2008 focus on the living environments, lifestyles, and health care during pregnancy. This shows that people’s concerns on these aspects have been greatly increased in recent years. More importantly, this may also indicate that environmental pollution and ecological deterioration has become an important issue endangering maternal and fetal health in the ten years after 2008.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant 81400072); the Natural Science Foundation of Shandong Province (grant ZR2013HQ047); the Teaching Reform Project of Cheeloo College of Medicine (grant qlyxjy-201867); the Humanities and Social Sciences Youth Team Project of Shandong University (grant IFYT1811, IFYT18036); and the Key Research and Development project of Shandong Province (grant 2018GSF118106).
Conflicts of interest
The authors report no conflicts of interest.
References
- Yu D, Zhuang Z, Wen Z, Zang X, Mo X. MTHFR. A1298C polymorphisms reduce the risk of congenital heart defects: a meta-analysis from 16 case-control studies. Ital J Pediatr 43 (2017): 108.
- Feng Y, Wang S, Chen R, Tong X, Wu Z, Mo X. Maternal Folic Acid Supplementation and the Risk of Congenital Heart Defects in Offspring: A Meta-Analysis of Epidemiological Observational Studies. Sci Rep 5 (2015): 8506.
- Xu A, Cao X, Lu Y, Li H, Zhu Q, Chen X,, et al. A Meta-Analysis of the Relationship Between Maternal Folic Acid Supplementation and the Risk of Congenital Heart Defects. Int Heart J 57 (2016): 725-728.
- Giannakoulas G, Vasiliadis K, Frogoudaki A, Ntellos C, Tzifa A, Brili S,, et al. Adult congenital heart disease in Greece: Preliminary data from the CHALLENGE registry. Int J Cardiol 245 (2017): 109-113.
- Greutmann M, Tobler D. Changing epidemiology and mortality in adult congenital heart disease: looking into the future. Future cardiology 8 (2012): 171-177.
- Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute [Internet]. [cited 2018]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Lai J, Han L, Xu S, Fang Y. Study on Risk Factors of Congenital Heart Disease in Newborns. J Prev Med Inf 17 (2001): 370-371.
- Qiu X, Qin Y, Xie X, Liu R, Pan N, Peng R, et al. Investigation on the Risk Factors for 1286 Cases of Congenital Heart Diseases. Journal of Tongji Medical University 28 (1999): 399-401.
- Zhang Z, Li Z, Ji C, Zhao P, Hong S, Li W, et al. The Effect of Mental-stress During Early Pregnancy on Congenital Heart Disease (CHD) Occurrence in the Offspring. Chinese Journal of Public Health 4 (1999): 35-36.
- Liu F, Tao F. Research on the Correlation between Maternal Negative Major Life Events and Congenital Heart Disease during Early Pregnancy. Journal of Applied Clinical Pediatrics 13 (2008): 988-990.
- Wu J, Chen H, Chen K, Liao F. Nested case-control study of risk factors of congenital heart disease in Zhoushan archipelagoe. Disease Surveillance 8 (2008): 510-514.
- Zhong Q, Qiu X, Zeng X, Lin N. Analysis of risk factors of congenital heart diseases and ventricular septal defects. Maternal and Child Health Care of China 18 (2017): 2497-2500.
- Wang H, Jing X, Li D. The study of effect between environmental risk factors and congenital heart disease. Journal of Taishan Medical College 5 (2007): 338-340.
- Hou J, Gui Y, Xi L, Zhang J, Chu C, Gao S. Case-control study of environmental risk factors on congenital heart disease. Fudan University Journal of Medical Sciences 34 (2007): 652-655.
- Yuan X, Wang H, Yan S, Wang X, Zhu X, Lu X. Results of surveillance on CHD in 10665 children andanalysis of its environmental risk factors. Maternal and Child Health Care of China 6 (2006): 781-783.
- Tan M, Huang M, Li D, Yi Z, Huang R. A Case-Control Study on the Relationship Between Evironmental Risk Factors Exposed in Early Pregnancy and Congenital Heart Disease. Journal of Environment and Health 23 (2006): 427-430.
- Gao L, Zhao Z, Li D, Jiang B, Hao F, Feng Q. Case-control study on environmental risk factors of congenital heart disease. Chinese Journal of Public Health 21 (2005): 37-38.
- Liu S, Chen J, Ji J. 1:2 Matched Case-control Study On the Risk Factors of Congenital Heart Disease. Chinese Journal of Natural Medicine 4 (2004): 219-223.
- Liu Y, Xu X, Liu Q, Zhan S, Chen Y, Wang J, et al. Analysis on growth and development of 70 children with congenital heart disease and the pathogenetic factors. Maternal and Child Health Care of China 27 (2013): 4477-4479.
- Zhu S, Wu Z, Wu Q. The Influencing Factors of Congenital Heart Disease in Infants in Haining. Zhejiang Journal of Preventive Medicine 25 (2013): 78-80.
- Zhang X, Zhao Z, You A, Zhao W, Chai C, Guo J, et al. Case-control study of maternal risk factors for perinatal congenital heart defects. Chinese Journal of Birth Health and Heredity 21(2013): 86-88.
- Yang L, Yang W, Wang X, Liu L, Zhang C. Relationship between exposure in progestational and pregnant period and risks of congenital heart disease among offsprings. Chinese Journal of Woman and Child Health Research 23 (2012): 148-150.
- Chen J, Huang G, Zhao J, Xie J, Chen H, Bao D. A Case-Control Study of Environmental Risk Factors of 76 Newborns with Congenital Heart Disease. Fujian Medical Journal 33 (2011): 39-41.
- Ouyang N, Du Q, Liu Z. Case-Control Study on Environmental Factors in Congenital Heart Disease. Journal of Central South University (Medical Sciences) 36 (2011): 159-164.
- Guo Y, Zhang G, Su H, Li J, Xu D, Yang X. The preliminary case-control study of risk factors of congenital heart disease. Chinese Journal of Clinicians 5 (2011): 4406-4413.
- Chen S, Long X, Hu D, Ma B, Chen J, Fang H, et al. Epidmiologcal Survey on Congenital Heart Disease the Infant in Tongling. Modern Preventive Medicine 4 (2010): 658-661.
- Mei J, Wang H. Prevalence of perinatal congenital heart disease and its influencing factors: a case-control study. Chinese Journal of Public Health 26 (2010): 1181-1182.
- Mi J, Meng C, Jia X, Zhang Y. 1:1 matched case-control study on risk factors of congenital heart diseases. Journal of ShanghaiJiaotong University (Medical Science) 30 (2010): 894-896.
- Li Y, Chen J, Chen Y, Niu T, Ma S. Analysis on the risk factors of congenital heart disease in children in Ningxia southern area. Ningxia Medical Journal 10 (2009): 882-884.
- Gong T, Li F, Shi J, Feng J, Tang X, Li X, et al. Relationship between risk factors during pregnancy, maternal MTHFR gene 677C→T, plasma Hey levels and congenital heart disease in children. Maternal and Child Health Care of China 24 (2009): 3117-3121.
- Li X, Li S, Mu D, Liu Z, Li Y, Lin Y, et al. The association between periconceptional folic acid supplementation and congenital heart defects: A case–control study in China. Prev Med 56 (2013): 385-389.
- Chen J, Lu S, Li W, Zhao J. A Case-control Study on Non-genetic Risk Factors of Congenital Heart Disease in Shengzhou from 2003 to 2012. Clinical Focus 29 (2014): 1285-1287.
- Lin Y, Chen X, Lin X, Liu M, Xu L, He D, et al. The case-control study of the antinomy and congenital heart disease. China Engineering Science 16 (2014): 73-78.
- Dong Z, Zhang Q, Yan S, Zhao B. Research on child health care screening for congenital heart disease based on information network platform mode. Maternal and Child Health Care of China 29 (2014): 2478-2480.
- Hu H, Liu Z, Li X, Li N, Deng Y, Deng K, et al. A case-control study on the relationship between non-genetic factors in early pregnancy and congenital heart disease. Maternal and Child Health Care of China 29 (2014): 3420-3424.
- Ou Y, Mai J, Zhuang J, Liu X, Wu Y, Gao X, et al. Risk factors of different congenital heart defects in Guangdong, China. Pediatr Res 79 (2015): 549-558.
- Lv G, Chen Z, Xu Z. High risk factors of fetal congenital heart diseases: a case-control analysis. Maternal and Child Health Care of China 18 (2016): 3818-3820.
- Bing P. The relationship between fluoride content and its felated factors and neonatal congenital heart diseases. Journal of Bengbu Medical College 41 (2016): 643-645.
- Yang M. Correlation between urinary fluride levels in pregnant women and neonates with congenital heart disease. Chinese Journal of Control of Endemic Diseases 31 (2016): 124-126.
- Zhang J, Zhao Y. Relationship between Non-genetic Factors in prepregnancy and early pregnancy and Fetal Congenital Heart Disease. Maternal and Child Health Care of China 11 (2016): 2351-2353.
- Jiang H. Comparative case research of influence by pregnancy non-genetic factor effect on congenital heart disease. Chinese Journal of Modern Drug Application 10 (2016): 19-21.
- Li L, Chen L, Lin Y, Gao S. Relationship between arsenic contents of pregnant women hair and fetal congenital heart disease. Strait Journal of Preventive Medicine 23 (2017): 9-12.
- Xiang O. Case control study of congenital heart disease related factors in perinatal infants in Wenzhou. Disease Surveillance 32 (2017): 504-508.
- Xu X, Qi G, Lu L, Wang Q, Chen Q. Influencing Factors of Congenital Heart Disease in Plateau Region: A Case-Control Study. Chinese Preventive Medicine 19 (2018): 1-4.
- Li F, Zhang Z, Guo L, Huo X, Du J, Miao M, et al. Associations of CYP450 gene polymorphism and exposed to environmental risk factors with congenital heart disease: a case-control study. Chinese Journal of Public Health 33 (2017): 1354-1359.
- Chen J, Wang C. Analysis on Risk factors of congenital heart diseases in Deyang City. China Modern Doctor 55 (2017): 108-110.
- Li L, Chen X, Lin Y, Wang L, Gao S. The relationship between fetal congenital heart disease and the content of chemical elements of hair in pregnant women. Strait Journal of Preventive Medicine 23 (2017): 11-13.
- Zhang R, Ma H, Yan H, Wang Y, Mi Y, Chen F, et al. Generalized linear model analysis of the relationship of four kinds of prenatal lifestyle factors and congenital heart disease. Journal of Xian Jiaotong University (Medical Sciences) 38 (2017): 332-336.
- Li L, Lin F, Lin Y, Huang X, Wang L, Gao S. Relationship between environmental factors during pregnancy and fetal congenital heart disease. Fujian Medical Journal 39 (2017): 31-34.
- Zhang J, Wang P, Jiang L, Chai J, Ma Y, Sun P. Case-control study on risk factors of congenital heart disease. Journal of Zhengzhou University (Medical Sciences) 52 (2017): 617-620.
- Hansen JM. Oxidative stress as a mechanism of teratogenesis. Birth Defect Res C 78 (2006): 293-307.
- Li M, Liu Z, Li X. Study on the relationship between maternal influenza during the first trimester of pregnancy and congenital heart diseases of their offspring. Maternal and Child Health Care of China 18 (2014): 2851-2854.
- Wang J. TORCH and pregnancy infection. Journal of Clinical Rational Drug Use 4 (2010): 126-127.
- Liu S, Liu J, Tang J, Ji J, Chen J, Liu C. Environmental Risk Factors for Congenital Heart Disease in the Shandong Peninsula, China: A Hospital-based Case-Control Study. J Epidemiol 19 (2009): 122-130.
- Zhang J, Huang G. Research progress in the etiology and prevention of congenital heart disease. Chinese Journal of Evidence Based Pediatrics 3 (2012): 231-238.
- Gao S, Wu Q, Zhang T, Shen Z, Liu C, Xu X, et al. Fluoxetine and congenital malformations: a systematic review and meta-analysis of cohort studies. Br J Clin Pharmacol 83 (2017): 2134-2147.
- Sadler TW. Selective serotonin reuptake inhibitors (SSRIs) and heart defects: Potential mechanisms for the observed associations. Reprod Toxicol 32 (2011): 484-489.
- Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. The Journal of Physiology 572 (2006): 31-44.
- Campaña H, Rittler M, Gili JA, Poletta FA, Pawluk MS, Gimenez LG, et al. Association between a Maternal History of Miscarriages and Birth Defects. Birth Defects Research 109 (2017): 254-261.
- Feng Y, Wang S, Zhao L, Yu D, Hu L, Mo X. Maternal Reproductive History and the Risk of Congenital Heart Defects in Offspring: A Systematic Review and Meta-analysis. Pediatr Cardiol 36 (2015): 253-263.
- Lee CN, Su YN, Cheng WF, Lin MT, Wang JK, Wu MH, et al. Association of the C677T methylenetetrahydrofolate reductase mutation with congenital heart diseases. Acta Obstet Gyn Scan 84 (2005): 1134-1140.
- Sarmah S, Muralidharan P, Marrs JA. Common congenital anomalies: Environmental causes and prevention with folic acid containing multivitamins. Birth Defect Res C 108 (2016): 274-286.
- Memon S, Pratten MK. Developmental toxicity of ethanol in chick heart in ovo and in micromass culture can be prevented by addition of vitamin C and folic acid. Reprod Toxicol 28 (2009): 262-269.