Biochemical Markers of Mineral Bone Disorder in Chronic Kidney Disease Patients on Maintenance Hemodialysis
Article Information
Sumon Dhar1*, Md Masud Iqbal2, Dilip Kumar Debnath3, Md. Zakir Hussain4, Md. Al Mahmud4, Sujon Sarker5, Md. Reaz Uddin6, Mt. Anjumanara Khatun1, Marjoa Humaira Mekhola7, A. K. M Shahidur Rahman7
1Assistant Registrar, Department of Pediatric Nephrology, National Institute of Kidney Diseases and Urology (NIKDU), Dhaka, Bangladesh
2Professor (Former Head of the Department), Department of Nephrology, National Institute of Kidney Diseases and Urology (NIKDU), Dhaka, Bangladesh
3Associate Professor, Department of Nephrology, National Institute of Kidney Diseases and Urology (NIKDU), Dhaka, Bangladesh
4Assistant Professor, Department of Nephrology, National Institute of Kidney Diseases and Urology (NIKDU), Dhaka, Bangladesh
5Medical Officer (Hemodialysis Unit), Department of Nephrology, Shaheed Ziaur Rahman Medical College, Bogura ,Bangladesh
6Assistant Professor, Department of Pediatric Nephrology, National Institute of Kidney Diseases and Urology (NIKDU), Dhaka, Bangladesh
7Medical Officer, Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
*Corresponding Author: Dr. Sumon Dhar, Assistant Registrar, Department of Pediatric Nephrology, National Institute of Kidney Diseases and Urology (NIKDU), Dhaka, Bangladesh
Received: 12 September 2023; Accepted: 25 September2023; Published: 03 October 2023
Citation: Dhar S, Iqbal MM, Debnath DK, Hussain MZ, Mahmud MA, Sarker S, Uddin MR, Khatun MA, Mekhola MH, Rahman AKMS. Biochemical Markers of Mineral Bone Disorder in Chronic Kidney Disease Patients on Maintenance Hemodialysis.Archives of Nephrology and Urology. 6 (2023): 104-110.
Share at FacebookAbstract
Mineral and bone disorder (MBD) in chronic kidney disease (CKD) is associated with increased morbidity and mortality. The CKDMBD syndrome is comprised of biochemical abnormalities, vascular calcification, and bone fragility, and each is consistently associated with elevated risks for morbidity and mortality across the spectrum of CKD. This study was aimed to assess the biochemical markers of mineral bone disorder in chronic kidney disease patients on maintenance hemodialysis. This cross-sectional study was conducted at National Institute of Kidney Diseases and Urology (NIKDU), Dhaka, Bangladesh from March 2022 to September 2022. A total of 160 cases with end-stage renal disease (ESRD) on maintenance hemodialysis (MHD) were enrolled. Demographic profile, detailed case history with clinical examination findings of each study patient was recorded and relevant investigations were done accordingly. The mean age of the study patients was 45.9±12.4 years and their mean duration of dialysis was 2.0±0.8 years. Maintained intact parathyroid hormone (iPTH) was in 55% of study patients and maintained bone alkaline phosphatase (BALP) was in 83.7% of study patients. Total 20.6% of study patients had high turnover and 24.4% had low turnover bone disease according to iPTH. Most of the study participants had history of taking oral phosphate binder, calcium and activated form of vitamin D. Among the total participants, 8.1% had hypocalcemia, 46.9% had hypercalcemia, 56.9% had hyperphosphatemia, 15.6% had hypophosphatemia, and 77.5% had vitamin D deficiency. This study concluded that, abnormalities of biochemical markers of mineral bone disorder are common in maintenance hemodialysis patients despite of oral supplementation.
Keywords
Biochemical Markers, Chronic Kidney Disease (CKD), Hemodialysis, Mineral Bone Disorder (MBD)
Biochemical Markers articles; Chronic Kidney Disease (CKD) articles; Hemodialysis articles; Mineral Bone Disorder (MBD) articles
Biochemical Markers articles Biochemical Markers Research articles Biochemical Markers review articles Biochemical Markers PubMed articles Biochemical Markers PubMed Central articles Biochemical Markers 2023 articles Biochemical Markers 2024 articles Biochemical Markers Scopus articles Biochemical Markers impact factor journals Biochemical Markers Scopus journals Biochemical Markers PubMed journals Biochemical Markers medical journals Biochemical Markers free journals Biochemical Markers best journals Biochemical Markers top journals Biochemical Markers free medical journals Biochemical Markers famous journals Biochemical Markers Google Scholar indexed journals Chronic Kidney Disease articles Chronic Kidney Disease Research articles Chronic Kidney Disease review articles Chronic Kidney Disease PubMed articles Chronic Kidney Disease PubMed Central articles Chronic Kidney Disease 2023 articles Chronic Kidney Disease 2024 articles Chronic Kidney Disease Scopus articles Chronic Kidney Disease impact factor journals Chronic Kidney Disease Scopus journals Chronic Kidney Disease PubMed journals Chronic Kidney Disease medical journals Chronic Kidney Disease free journals Chronic Kidney Disease best journals Chronic Kidney Disease top journals Chronic Kidney Disease free medical journals Chronic Kidney Disease famous journals Chronic Kidney Disease Google Scholar indexed journals Hemodialysis articles Hemodialysis Research articles Hemodialysis review articles Hemodialysis PubMed articles Hemodialysis PubMed Central articles Hemodialysis 2023 articles Hemodialysis 2024 articles Hemodialysis Scopus articles Hemodialysis impact factor journals Hemodialysis Scopus journals Hemodialysis PubMed journals Hemodialysis medical journals Hemodialysis free journals Hemodialysis best journals Hemodialysis top journals Hemodialysis free medical journals Hemodialysis famous journals Hemodialysis Google Scholar indexed journals Mineral Bone Disorder articles Mineral Bone Disorder Research articles Mineral Bone Disorder review articles Mineral Bone Disorder PubMed articles Mineral Bone Disorder PubMed Central articles Mineral Bone Disorder 2023 articles Mineral Bone Disorder 2024 articles Mineral Bone Disorder Scopus articles Mineral Bone Disorder impact factor journals Mineral Bone Disorder Scopus journals Mineral Bone Disorder PubMed journals Mineral Bone Disorder medical journals Mineral Bone Disorder free journals Mineral Bone Disorder best journals Mineral Bone Disorder top journals Mineral Bone Disorder free medical journals Mineral Bone Disorder famous journals Mineral Bone Disorder Google Scholar indexed journals end-stage renal disease articles end-stage renal disease Research articles end-stage renal disease review articles end-stage renal disease PubMed articles end-stage renal disease PubMed Central articles end-stage renal disease 2023 articles end-stage renal disease 2024 articles end-stage renal disease Scopus articles end-stage renal disease impact factor journals end-stage renal disease Scopus journals end-stage renal disease PubMed journals end-stage renal disease medical journals end-stage renal disease free journals end-stage renal disease best journals end-stage renal disease top journals end-stage renal disease free medical journals end-stage renal disease famous journals end-stage renal disease Google Scholar indexed journals bone mineral density articles bone mineral density Research articles bone mineral density review articles bone mineral density PubMed articles bone mineral density PubMed Central articles bone mineral density 2023 articles bone mineral density 2024 articles bone mineral density Scopus articles bone mineral density impact factor journals bone mineral density Scopus journals bone mineral density PubMed journals bone mineral density medical journals bone mineral density free journals bone mineral density best journals bone mineral density top journals bone mineral density free medical journals bone mineral density famous journals bone mineral density Google Scholar indexed journals renal osteodystrophy articles renal osteodystrophy Research articles renal osteodystrophy review articles renal osteodystrophy PubMed articles renal osteodystrophy PubMed Central articles renal osteodystrophy 2023 articles renal osteodystrophy 2024 articles renal osteodystrophy Scopus articles renal osteodystrophy impact factor journals renal osteodystrophy Scopus journals renal osteodystrophy PubMed journals renal osteodystrophy medical journals renal osteodystrophy free journals renal osteodystrophy best journals renal osteodystrophy top journals renal osteodystrophy free medical journals renal osteodystrophy famous journals renal osteodystrophy Google Scholar indexed journals parathyroid hormone articles parathyroid hormone Research articles parathyroid hormone review articles parathyroid hormone PubMed articles parathyroid hormone PubMed Central articles parathyroid hormone 2023 articles parathyroid hormone 2024 articles parathyroid hormone Scopus articles parathyroid hormone impact factor journals parathyroid hormone Scopus journals parathyroid hormone PubMed journals parathyroid hormone medical journals parathyroid hormone free journals parathyroid hormone best journals parathyroid hormone top journals parathyroid hormone free medical journals parathyroid hormone famous journals parathyroid hormone Google Scholar indexed journals bone alkaline phosphatase articles bone alkaline phosphatase Research articles bone alkaline phosphatase review articles bone alkaline phosphatase PubMed articles bone alkaline phosphatase PubMed Central articles bone alkaline phosphatase 2023 articles bone alkaline phosphatase 2024 articles bone alkaline phosphatase Scopus articles bone alkaline phosphatase impact factor journals bone alkaline phosphatase Scopus journals bone alkaline phosphatase PubMed journals bone alkaline phosphatase medical journals bone alkaline phosphatase free journals bone alkaline phosphatase best journals bone alkaline phosphatase top journals bone alkaline phosphatase free medical journals bone alkaline phosphatase famous journals bone alkaline phosphatase Google Scholar indexed journals adynamic bone disease articles adynamic bone disease Research articles adynamic bone disease review articles adynamic bone disease PubMed articles adynamic bone disease PubMed Central articles adynamic bone disease 2023 articles adynamic bone disease 2024 articles adynamic bone disease Scopus articles adynamic bone disease impact factor journals adynamic bone disease Scopus journals adynamic bone disease PubMed journals adynamic bone disease medical journals adynamic bone disease free journals adynamic bone disease best journals adynamic bone disease top journals adynamic bone disease free medical journals adynamic bone disease famous journals adynamic bone disease Google Scholar indexed journals
Article Details
1. Introduction
Chronic kidney disease (CKD) constitutes a public health problem that is estimated to affect >10% of the global population and the prevalence of which has increased in recent years [1]. Mineral and bone disorder (MBD) is one of the important complications caused by chronic kidney disease (CKD), especially in hemodialysis patients and MBD can decrease the quality of life among CKD patients [2]. According to the KDIGO (Kidney Disease Improving Global Outcomes), CKD-MBD Update Work Group 2017; CKD-MBD is a systemic disorder of mineral as well as bone metabolism due to CKD manifesting with one or combination of abnormalities of calcium, parathyroid hormone (PTH), phosphate, or vitamin D metabolism, which ultimately cause abnormalities of bone turn over, volume linear growth or strength, mineralization and vascular or soft tissue calcification [3]. The bone mineral metabolism abnormalities start during the early stages of CKD as renal function decreases, long before the need for renal replacement therapy and can be positively or negatively influenced by the treatment strategy employed [3]. It is recommended that all relevant biochemical parameters should be monitored and control at the early stages of CKD, before the need for dialysis [4]. Secondary hyperparathyroidism with elevated plasma parathyroid hormone (PTH) is a central component in the pathogenesis of CKD-MBD [4]. Phosphate retention, low plasma calcium, and high fibrinogen growth factor- 23 (FGF- 23) with subsequently decreased plasma calcitriol concentration and all contribute to the evolvement of secondary hyperparathyroidism [5]. Among patients with a wide range of renal dysfunctions including end-stage renal disease (ESRD), vitamin D deficiency showed associations with different cardiovascular events, vascular calcification, vascular endothelial dysfunction and cardiovascular mortality [6]. Lack of sufficient vitamin D in its native form, as reflected by low circulating 25(OH) vitamin D concentrations, is frequent in CKD patients; 25(OH) vitamin D is now recognized as an important component in the maintenance of bone health in CKD [5, 6]. Guidelines recommended that, regular measurement of 25(OH) vitamin D concentration and supplementation if levels fall below30 ng/ml [5]. On the other hand, the relationship between serum phosphorus and cardiovascular morbidity and mortality is complex but may involve the direct effects of increased phosphorus on renal bone disease and extra-skeletal calcification [7]. It was reported that, the prevalence of renal bone disease ranges from 33.3% to 57% among dialytic patients in different geographical areas [8, 9, 10]. Slouma et al. determined the usefulness of measuring serum bone turnover and bone mineral density (BMD) in hemodialysis patients [11]. Study on the Western population reveals that, the use of BMD markers in combination with bone mineral density (BMD) estimation has the potential of improving the diagnosis and the treatment of renal osteodystrophy (ROD) [12]. In this background the objective of this current study was to assess the biochemical markers of mineral bone disorder in chronic kidney disease patients on maintenance hemodialysis.
2. Methodology
This cross-sectional study was conducted at the National Institute of Kidney Diseases and Urology (NIKDU), Dhaka, Bangladesh from March 2022 to September 2022. A total of 160 patients with end-stage renal disease (ESRD) on maintenance hemodialysis (MHD) were enrolled in this study by purposive sampling technique following selection criteria. The study was approved by the ethical review committee of NIKDU. Adult patients (age 18 years or above) of both sexes, on maintenance hemodialysis for at least 3 months were included. Patients with recent fractures, cases with known malignant tumors, and patients with known obstructive liver disease were excluded.
2.1 Study procedure
All the end-stage renal disease (ESRD) patients on maintenance hemodialysis (MHD) attended at NIKDU, Dhaka, Bangladesh were approached for this study. After describing the aim, purpose and procedure of the study, a total of 160 patients were finally enrolled who met the inclusion and exclusion criteria. Informed written consent was taken from each study participant prior to enrollment. A detailed case history was collected and relevant clinical examination was done. Demographic and clinical variables of the study patients were recorded accordingly. Then mineral and bone disorder parameters like- serum/plasma bone alkaline phosphatase (BALP), alkaline phosphatase (ALP), calcium, phosphate, vitamin D and intact parathyroid hormone (iPTH) levels along with dialysis accuracy variables such as- serum albumin, uric acid, hemoglobin (Hb) levels and Kt/V of each study patients were analyzed following standard procedure. All information regarding history of patients, clinical examination findings, and investigation repots were recorded in a data collection sheet.
2.2 Blood sample collection and analysis
With all aseptic precaution 5 ml of venous blood were drawn from peripheral vein. Each blood sample was put into three different tubes for hematological/biochemical investigations and allowed to clot for half an hour and then the samples were centrifuged at 3000 rpm for 15 minutes in room temperature (22°C-24°C) and then serum was stored in ultra-deep freezer (-20°C) until analysis. All analysis was done following standard laboratory procedure.
2.3 Data analysis
After collection of all data, then data editing, validation and cross-checking were done to remove the inconsistencies. Analysis was done by using the computer (Windows) based software Statistical Package for Social Sciences (SPSS) version- 24. Continuous variables were expressed as mean with standard deviation (±SD) and categorical variables were expressed as numbers (n) with percentages (%). Pearson correlation analysis was done to see the linear association between continuous variables. Statistical significance was set as a 95% confidence level at 5% acceptable error level. A p value <0.05 was considered as statistically significant.
3. Results
This study was intended to evaluate the biochemical markers of mineral bone disorder (MBD) in chronic kidney disease (CKD) patients on hemodialysis. A total of 160 CKD patients on maintenance hemodialysis (MHD) were enrolled in this study. It was observed that mean age of the study patients was 45.9±12 years; majority of the participants (54.4%) was less than 50 years and 45.6% was more than 50 years age group; of them 53% was male and rest 47% was female; the male to female ratio was 1.1:1 (Table- 1). Mean body mass index (BMI) of the study patients was 23.2±3.8 kg/m2. Majority (43.8%) of the participants had duration of dialysis 1-4 years followed by 28.9% participants had duration of dialysis less than 1 year and 27.5% participants had duration of dialysis more than 4 years. Mean duration of dialysis among the study patients was 2±0.8 years (Table- 1).
Table- 1: Basic characteristics of the study patients (N= 160)
|
Characteristics of the patients |
Findings [Mean±SD; n (%)] |
|
Age (years) |
45.9±12 |
|
Age group (years) |
|
|
<50 |
87 (54.4) |
|
≥50 (50 and above) |
73 (45.6) |
|
Gender |
|
|
Male |
85 (53.1) |
|
Female |
75 (46.9) |
|
Male to female ratio |
1.1:1 |
|
BMI (kg/m2) |
23.2±3.8 |
|
Duration of dialysis |
|
|
<1 year |
46 (28.7) |
|
1-4 years |
70 (43.8) |
|
>4 years |
44 (27.5) |
|
Mean duration of dialysis (years) |
2±0.8 |
Data expresses as mean with standard deviation (±SD) and frequency (n) with percentage (%)
Regarding the causes of chronic kidney disease (CKD); majority (28.8%) had glomerulonephritis (GN) followed by 21.3% had diabetic nephropathy (DN), 6.8% had hypertensive nephropathy, 5% had obstructive uropathy and 1.8% had developed CKD due to renal stone disease and 2.5% patients was diagnosed as polycystic kidney disease. Besides, 33.8% patients had ESRD of unknown causes (Figure- 1).
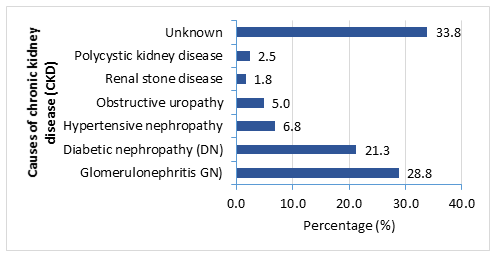
Figure- 1: Causes of chronic kidney disease (CKD) among the study patients (N= 160)
Laboratory investigations of the study patients revealed that; mean hemoglobin (Hb) level was 10.2±1.8 gm/dl, mean bone alkaline phosphatase (BALP) level was 43.8±25.9 ng/ml, corrected mean calcium level was 9.4±0.8 mg/dl, mean phosphate level was 5.9±2.3 mg/dl, mean albumin level was 3.8±0.5 mg/dl, mean uric acid level was 7.3±1.3 mg/dl, mean vitamin D level was 29.4±17.2 nmol/l, mean alkaline phosphatase (ALP) level was 172.6±63.4 U/L, mean intact parathyroid hormone (iPTH) level was 55.42±23.6 pmol/lit and mean Kt/V was 1.32±0.3 (Table- 2).
Table- 2: Laboratory investigations findings of the study patients (N= 160)
|
Investigation findings |
Mean±SD |
|
Hemoglobin (Hb) (gm/dl) |
10.2±1.8 |
|
Bone alkaline phosphatase (BALP) (ng/ml) |
43.8±25.9 |
|
Corrected calcium (mg/dl) |
9.4±0.8 |
|
Phosphate (mg/dl) |
5.9±2.3 |
|
Albumin (mg/dl) |
3.8±0.5 |
|
Uric acid (mg/dl) |
7.3±1.3 |
|
Vitamin D (nmol/l) |
29.4±17.2 |
|
Alkaline phosphatase (ALP) (U/L ) |
172.6±63.4 |
|
Intact parathyroid hormone (iPTH) (pmol/lit) |
55.42±23.6 |
|
Kt/V |
1.32±0.3 |
Among all study participants, 74.4% had a history of phosphate binder intake, 58.1% had a history of oral calcium intake and 86.3% had a history of activated form of vitamin D intake (Table- 3).
Table- 3: Frequency of oral phosphate binder, calcium and vitamin D supplements intake among the study patients (N= 160)
|
Supplement intake* |
Frequency (%) |
|
Phosphate binder intake |
119 (74.4) |
|
Calcium based |
93 (58.1) |
|
Sevelamer |
15 (9.4) |
|
Lanthanum |
3 (1.9) |
|
Iron based (Ferric citrate) |
8 (5) |
|
Oral calcium intake |
93 (58.1) |
|
500mg/day |
75 (46.9) |
|
1000mg/day |
18 (11.3) |
|
Calcitriol intake |
138 (86.3) |
|
0.25 mcg |
127 (79.4) |
|
0.5mcg |
11 (6.9) |
*Multiple responses
Analyzing the mineral and bone disorder (MBD) parameters among the participants; we observed that 46.9% of study cases had high, 8.1% had low and 45% had maintained calcium levels. Besides, 56.9% had high, 15.6% had low and 27.5% had maintained phosphate level. However, 20.6% had high, 24.4% had low and 55% had maintained intact parathyroid hormone (iPTH) level. But 77.5% had low and 22.5% had maintained vitamin D level. On the other hand 16.3% had high and 83.8% had maintained bone alkaline phosphatase (BALP) level, While 55% had high and 45% had maintained alkaline phosphatase (ALP) level (Table- 4).
Table- 4: Distribution of mineral and bone disorder (MBD) parameters among the study patients (N= 160)
|
MBD parameters |
High n (%) |
Low n (%) |
Maintained n (%) |
|
Calcium (mg/dl) |
75 (46.9) |
13 (8.1) |
72 (45) |
|
Phosphate (mg/dl) |
91 (56.9) |
25 (15.6) |
44 (27.5) |
|
iPTH (pmol/l) |
33 (20.6) |
39 (24.4) |
88 (55) |
|
Vitamin D (nmol/l) |
0 (0) |
124 (77.5) |
36 (22.5) |
|
BALP (ng/ml) |
26 (16.3) |
0 (0) |
134 (83.8) |
|
ALP (U/L) |
88 (55) |
0 (0) |
72 (45) |
Values within the parentheses indicate corresponding percentages.
According to the bone alkaline phosphatase (BALP) >44 ng/ml, 16.3% of the patients had high turnover bone disease, but 83.7% study patients had maintained BALP level (Figure- 2). On the other hand, according to the intact parathyroid hormone (iPTH) level; 20.6% of the patients had high turn-over bone disease (iPTH≥64 pmol/lit) and 24.4% had low turn-over bone disease (iPTH<16 pmol/lit); while 55% study patients had maintained iPTH level (Figure- 3).
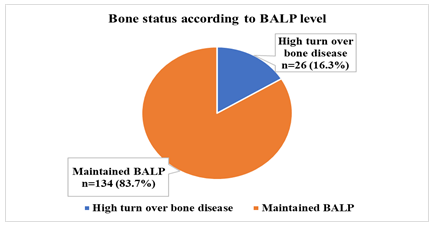
Figure- 2: Distribution of the study patients having bone disease according to BALP level (N= 160)
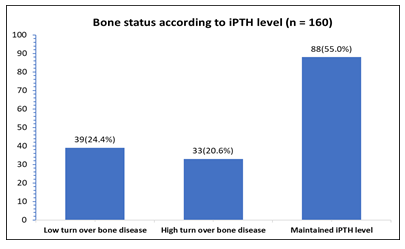
Figure- 3: Distribution of the patients by bone disease according to intact parathyroid hormone (iPTH) level (N= 160)
In this study, bone alkaline phosphatase (BALP) was negatively correlated with hemoglobin (Hb), uric acid, albumin and Kt/V levels; where correlation with uric acid and albumin showed statistically significant (p<0.05). Alkaline phosphatase (ALP) was positively correlated with Hb and uric acid levels, but negatively correlated with albumin and Kt/V levels; here correlation with Hb showed statistically significant (p<0.05). Serum calcium was positively correlated with Hb, uric acid, albumin and Kt/V levels; but correlation with albumin level was statistically significant (p<0.01). Similarly serum phosphorus was positively correlated with Hb, uric acid, and albumin levels but negatively correlated with Kt/V level, while correlation with uric acid level showed statistically significant (p<0.01). Intact parathyroid hormone (iPTH) level was positively correlated with Hb and albumin levels and negatively correlated with uric acid and Kt/V levels; where correlation with uric acid level was statistically significant (p<0.05). Serum vitamin D level was positively correlated with serum uric acid and albumin levels, but negatively correlated with Hb and Kt/V levels; however these correlation were not statistically significant (p>0.05) (Table- 5).
Table- 5: Correlation of dialysis adequacy parameters with MBD parameters
|
Dialysis adequacy parameters |
Coefficient correlation (r) and p value |
BALP |
ALP |
Calcium |
Phosphate |
iPTH |
Vitamin D |
|
Hb |
Coefficient correlation (r) |
-0.077 |
0.2 |
0.065 |
0.021 |
0.059 |
-0.15 |
|
p value |
0.175 |
0.007 |
0.214 |
0.397 |
0.236 |
0.083 |
|
|
Uric acid |
Coefficient correlation (r) |
-0.167 |
0.109 |
0.081 |
0.291 |
-0.031 |
0.095 |
|
p value |
0.017 |
0.085 |
0.153 |
<0.01 |
0.349 |
0.115 |
|
|
Albumin |
Coefficient correlation (r) |
-0.144 |
-0.054 |
0.356 |
0.12 |
0.112 |
0.095 |
|
p value |
0.035 |
0.247 |
<0.01 |
0.066 |
0.079 |
0.117 |
|
|
Kt/V |
Coefficient correlation (r) |
-0.03 |
-0.06 |
0.094 |
-0.012 |
-0.037 |
-0.054 |
|
p value |
0.354 |
0.225 |
0.118 |
0.44 |
0.32 |
0.24 |
p- value was determined by Pearson correlation test
4. Discussion
Disturbances in mineral metabolism and bone disease are common among chronic kidney disease (CKD) patients and causes considerable morbidity and mortality [2]. Serial measurement of calcium, phosphate, intact parathyroid hormone (iPTH), vitamin D and bone alkaline phosphatase (BALP) play an important role to assess the severity and monitor the treatment response of mineral bone disorder (MBD) [2, 3, 4]. Diagnosis of mineral bone disorder by bone biopsy is the gold standard for accurately classifying the type of renal osteodystrophy but it is an invasive procedure and facilities for histomorphometry are not universally available. Bone mineral density testing by dual-energy X-ray absorptiometry is not routinely available and affordable. Therefore, serial measurement of these markers may be helpful to reduce disease severity of these patients by early diagnosis and treatment. This study was aimed to assess the biochemical markers of mineral bone disorder among chronic kidney disease patients on maintenance hemodialysis. Total 160 CKD patients on maintenance hemodialysis (MHD) were enrolled; mean age of the study patients was 45.9±12 years; majority (54.4%) of the participants was less than 50 years and 53% of them were male. Mean body mass index (BMI) of the study patients was 23.2±3.8 kg/m2. Majority (43.8%) of the participants had duration of dialysis 1-4 years; mean duration of dialysis among the study patients was 2±0.8 years. Majority (28.8%) of the study patients had glomerulonephritis (GN) followed by diabetic nephropathy (DN), hypertensive nephropathy, obstructive uropathy, polycystic kidney disease and 33.8% patients had ESRD of unknown causes. Most of the participants had history of taking oral phosphate binder, calcium and activated form of vitamin D supplements. In this study according to the BALP level, 16.3% of the study patients had high turnover bone disease; but according to iPTH level, 20.6% of the patients had high turn-over bone disease and 24.4% had low turn-over bone disease; however 83.7% and 55% study patients had maintained BALP and iPTH levels respectively. In this context Seek et al. found, the prevalence of CKD-MBD was 66. 9%; among them 72.2% had high turnover bone disease and 27.8% patients had low turnover bone disease [13]. Ahmed et al. reported that, hyper parathyroid bone disease was prevalent among 55% of their study patients and low bone turnover in 30% of the study patients [14]. This current study found that 56.9% of the hemodialytic patients had hyperphosphatemia which was similar to the previous study by Bala et al.; they observed more than half of the study population had serum phosphate levels above the target level [15]. Ahmed et al. found 74% of their study patients had hyperphosphatemia among the CKD patients on hemodialysis [14]. Young et al. also found 51.6% of their study patients had increased phosphate levels [16]. It was reported that, despite the advances in hemodialysis, with the invention of more effective dialysis membranes and the use of ultrapure dialysate, the removal of phosphate is still inadequate [16]. Therefore, one of the reasons that could have accounted for this high prevalence of hyperphosphatemia is the ineffective removal of phosphate by conventional hemodialysis. Among the study patients; we found 58.1% had a history of oral calcium intake as a phosphate binder. Hypocalcemia was observed in 8.1% of the patients and 75.6% had hyperparathyroidism (maintained iPTH and high turnover group) along with 24.4% had hypoparathyroidism. In a related study, Babua et al. found 44.7% had hypocalcemia and 39.2% had hyperphosphatemia; but the prevalence of abnormal calcium and phosphate levels was found to increase among maintenance hemodialysis patients [17]. Because of the dynamic nature and complexity of bone homeostasis, it is difficult to rely on a single biochemical marker as a surrogate test of bone formation [18]. We found that 77. 5% of our study patients had vitamin D deficiency, although majority (86.6%) of them had history of vitamin D intake; this finding was supported by a couple of related previous study [19, 20]. In general; factors that could account for vitamin D deficiency in patients with CKD include reduced dietary intake and absorption, loss of vitamin D binding protein in urine, increased levels of FGF- 23 in CKD and inadequate sunlight exposure [20, 21]. In this study, bone-specific alkaline phosphatase (BALP) was negatively correlated with hemoglobin (Hb), uric acid, Kt/V and albumin levels. While alkaline phosphatase (ALP) was positively correlated with Hb and uric acid levels, but negatively correlated with albumin level and Kt/V. Serum calcium was positively correlated with Hb, uric acid, albumin and Kt/V levels. Serum phosphate level was positively correlated with Hb, uric acid, albumin and Kt/V levels. Intact parathyroid hormone (iPTH) level was positively correlated with Hb and albumin levels but negatively correlated with uric acid and Kt/V levels. Serum vitamin D was positively correlated with serum uric acid and albumin levels, but negatively correlated with Hb and Kt/V levels. In a similar study Fayed et al. documented that, vitamin D level was positively correlated with serum albumin level among CKD patients [22]. Serum vitamin D levels were directly associated with serum albumin, which was also revealed by another related study [20]. Bala et al. suggested that hyperparathyroidism and 25–OH vitamin D deficiency were common in hemodialysis patients and hypocalcemia with hyperphosphatemia were strong predictors for developing secondary hyperparathyroidism [15]. In this current study according to iPTH, hyperparathyroidism was observed among a large percentage of the study participants, which was consistent with a similar previous study [15]. While Carol Moore et al. documented that; among CKD patients- adynamic bone disease (ABD) was 16%, mild to moderate hyperparathyroidism was 72%, severe hyperparathyroidism was 12% on bone histology; while osteomalacia or mixed uremic osteodystrophy were not observed [23]. Therefore the findings of this current study were supported by a couple of previous studies [14- 24].
5. Conclusion
This study demonstrated that, abnormalities of biochemical markers of mineral bone disorder are common among chronic kidney disease patients on maintenance hemodialysis; although patients on oral supplementation of calcium, phosphate binder and vitamin D. Therefore, measuring the markers of mineral bone disorder and their association with dialysis adequacy parameters can help to predict the outcome and to prevent mortality.
Limitations of the study
It was a single center study with a relatively small sample size. In this study bone biopsy for bone histomorphometry was not done. Moreover, the study was conducted over a short period of time.
Recommendation
Multi-center studies with large sample sizes are recommended to confirm the findings of this study.
Conflict of interest
All authors declared that they have no conflict of interest regarding this publication.
References
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. Jama. 298 (2007):2038-2047.
- Heaf JG. Chronic kidney disease-mineral bone disorder in the elderly peritoneal dialysis patient. Peritoneal Dialysis International. 35 (2015):640-644.
- Ketteler M, Block GA, Evenepoel P, et al Executive summary of the 2017 KDIGO Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney international.;92 (2017):26-36.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney international. Supplement. 76 (2009):S1-130.
- Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney international. (2007):31-38.
- London GM, Guérin AP, Verbeke FH, et al. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. Journal of the American Society of Nephrology. 18(2007):613-620.
- Adeney KL, Siscovick DS, Ix JH, et al Association of serum phosphate with vascular and valvular calcification in moderate CKD. Journal of the American Society of Nephrology: JASN. 20(2009):381-387.
- Locatelli F, Mastrangelo F, Redaelli B, et al. Italian Cooperative Dialysis Study Group. Effects of different membranes and dialysis technologies on patient treatment tolerance and nutritional parameters. Kidney international.50 (1996):1293-1302.
- Jaroš A, Zadrazil J, Konecný K, et al. Single photon bone densitometry in hemodialysis patients. Biomedical papers. Acta Univ. Palacki. Olomuc., Fac. Med. 142 (1999): 135-138.
- Changsirikulchai S, Domrongkitchaiporn S, Sirikulchayanonta V, et al. Renal osteodystrophy in Ramathibodi Hospital: histomorphometry and clinical correlation. JOURNAL-MEDICAL ASSOCIATION OF THAILAND. 83 (2000):1223-1232.
- Slouma M, Sahli H, Bahlous A, et al. Mineral bone disorder and osteoporosis in hemodialysis patients. Advances in Rheumatology. 60(2020):1-7.
- Bednarek-Skublewska A, Chrapko B, Ksiazek A. Skeletal scintigraph and some bone turnover markers in the diagnosis of renal osteodystrophy in hemodialysis patients. PolskieArchiwumMedycynyWewnetrznej. 110(2003):943-950.
- Seck SM, Dahaba M, Ka EF, et al. Mineral and bone disease in black african hemodialysis patients: a report from senegal. Nephro-urology monthly. 4 (2012):613.
- Ahmed HA, Elzorkany KM, Yasein YS, et al. Prevalence of mineral bone disorders among hemodialysis patients in Menoufia Governorate, Egypt. Menoufia Medical Journal. 30 (2017):687.
- Bala W, Raquel D, Saraladevi N. Biochemical markers of mineral bone disorder in South African patients on maintenance haemodialysis. African Health Sciences. 17(2017):445-452.
- Young EW, Akiba T, Albert JM, et al, Mendelssohn DC, Jadoul M. Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). American Journal of Kidney Diseases.44 (2004):34-38.
- Babua C, Kalyesubula R, Okello E, Kakande B, Sebatta E, Mungoma M, Mondo CK. Cardiovascular risk factors among patients with chronic kidney disease attending a tertiary hospital in Uganda: cardiovascular topics. Cardiovascular journal of Africa.26(2015):177-180.
- Moorthi RN, Moe SM. Recent advances in the noninvasive diagnosis of renal osteodystrophy. Kidney international. 84 (2013):886-94.
- Porter A, Gilmartin C, Srisakul U, et al. Prevalence of 25-OH vitamin D deficiency in a population of hemodialysis patients and efficacy of an oral ergocalciferol supplementation regimen. American journal of nephrology. 37(6):568-574.
- Watanabe Y. Vitamin D supplementation for chronic kidney disease according to the guideline issued by DOQI. Clinical Calcium. 2004 May 1;14 (2004):778-785.
- Fernandez-Juarez G, Luno J, Barrio V, et al. vitamin D levels and renal disease progression in patients with type 2 diabetic nephropathy and blockade of the renin-angiotensin system. Clinical journal of the American Society of Nephrology: CJASN. (2013):1870.
- Fayed, A., El Nokeety, M.M., Heikal, A.A., et al. Urine albumin and serum uric acid are important determinants of serum 25 hydroxyvitamin D level in pre-dialysis chronic kidney disease patients. Renal Failure, 41 (2019): 540–546.
- Moore C, Yee J, Malluche H. D. Dialysis.Clin J Am SocNephrol. 4 (2009):1484-1493.
- Choudhary R, Yadav C, Jain P, et al. Prevalence of Mineral Bone Disease in Chronic Kidney Disease Patients using Biochemical Markers. Journal of Clinical & Diagnostic Research. 14 (2020).
