Association of C-reactive Protein with Diabetic Retinopathy in Young People with Type-I Diabetes Mellitus
Article Information
Rawnok Jahan Rahman1*, Bedowra Zabeen2, Mohammad Zafar Khaled3, Shah-Noor Hassan3, A. K. M Shahidur Rahman4
1Vision Eye Hospital, Dhaka, Bangladesh
2Department of Paediatric Endocrinology, Changing Diabetes in Children (CDIC), Bangladesh Institute of Research and Rehabilitation in Diabetes, Endocrine and Metabolic Disorder (BIRDEM), Dhaka, Bangladesh
3Department of Ophthalmology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
4Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
*Corresponding author: Rawnok Jahan Rahman, Vision Eye Hospital, Dhaka, Bangladesh
Received: 16 January 2024; Accepted: 19 January 2024; Published: 03 February 2024
Citation: Rahman RJ, Zabeen B, Khaled MZ, Hassan SN, Rahman AKMS. Association of C-reactive Protein with Diabetic Retinopathy in Young People with Type-I Diabetes Mellitus. Journal of Ophthalmology and Research. 7 (2024): 16-24.
Share at FacebookAbstract
Background: Diabetic retinopathy (DR) is a chronic, progressive sight threatening disease of the retinal microvasculature and is a significant complication of diabetes mellitus (DM). Type l diabetes mellitus (T1DM) is one of the most common metabolic conditions in childhood and adolescence. C-reactive protein (CRP) plays a major role in the human innate immune response and serves as a stable plasma biomarker indicating the presence of low-grade systemic inflammation.
Objective: This study aimed to assess the association of C-reactive protein (CRP) with diabetic retinopathy in young people with T1DM.
Methods: A total of 60 T1DM patients were included; of them 30 T1DM patients with retinopathy (cases- Group I) and 30 patients of T1DM without retinopathy (controls- Group II), aged between 10 to 24 years. Complete clinical evaluation including history, physical examination, relevant ocular examinations like visual acuity test, slit lamp examination, fundus examination both direct and indirect ophthalmoscopy and color fundus photography were done accordingly. Serum high sensitivity CRP (hs-CRP) level was measured following standard procedure.
Results: It was observed that, mean hs-CRP was significantly increased in retinopathy group compare to without retinopathy group (5.08±1.29 mg/L versus 3.43±0.69 mg/L, p<0.001). Mean hs-CRP level had significant difference among National Screening Committee (NSC) grading of retinopathy (p<0.001). Mean hs-CRP level was gradually increased with NSC grades. An age-adjusted and sex-adjusted model revealed that patients with highest hs-CRP (>4 mg/L) were 13.1 times more likely to have DR. Serum hs-CRP had a significant strong positive correlation with HbA1c in Group I (r=+.831, p<0.001) and Group II (r=.666, p<0.001). Patients with retinopathy group had significantly higher duration of DM and HbA1c compare to without retinopathy group (p<0.05), but this relation was lost in multivariate logistic regression model.
Conclusion: C-reactive protein (CRP) is strongly associated with diabetic retinopathy. Serum CRP also significantly related to different grades of retinopathy.
Keywords
C-Reactive Protein (CRP), Diabetic Retinopathy (DR), Glycated Hemoglobin (HbA1c), Type 1 Diabetes Mellitus (T1DM), Young People
C-Reactive Protein (CRP) articles, Diabetic Retinopathy (DR) articles, Glycated Hemoglobin (HbA1c) articles, Type 1 Diabetes Mellitus (T1DM) articles, Young People articles
Article Details
1. Introuduction
Diabetes Mellitus (DM) is a complex metabolic disorder characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action, or both [1]. Around the world 463 million people have diabetes and in the South East Asia (SEA) region 997.4 million people have diabetes which will rise to 1.3 billion by 2045 [2]. Type l diabetes is one of the most common metabolic conditions in children and adolescents [3]. Type 1 diabetes mellitus (T1DM) once known as juvenile diabetes or insulin-dependent diabetes is a chronic condition in which there is partial or absolute deficiency of insulin [3]. T1DM can lead to numerous complications, with the most common long-term complications in children and adolescents include retinopathy, nephropathy and dyslipidemia [3]. The number of children developing this form of diabetes every year is increasing rapidly, especially among the youngest children [3]. In South East Asia; it was reported that estimated 184,100 children and adolescents under the age of 20 living with T1DM [2]. Diabetic retinopathy (DR) is one of the micro vascular complications of diabetes mellitus (DM), a major cause of avoidable blindness around the world [4]. Prevalence of retinopathy in type 1 diabetes mellitus (T1DM) among children and adolescent is ranges from 5.6% to 17% in South East Asian region [6, 7]. DR can be divided into non proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR) [5]. In the natural course, approximately 50% of patients with very severe NPDR progress to PDR within 1 year [5]. The most common early clinically visible manifestations of DR include micro aneurysm formation and intra-retinal hemorrhage [4]. Microvascular damage leads to retinal capillary non perfusion, cotton wool spots, increased number of hemorrhages, venous abnormalities [intra-retinal microvascular abnormalities (IRMA)] [5]. During this stage, increased permeability of the retinal vessels can result in retinal thickening (oedema) and/or exudates that may lead to a loss in central visual acuity [8]. Prolong duration of diabetes, poor glycemic control, hypertension, hyperlipidemia and genetic predisposition are the risk factors for the development of diabetic retinopathy [9]. Inflammation is now recognized as a critical contributor in the pathogenesis of diabetic retinopathy [10, 11]. It has been suggested that, hyperglycemia leads to the activation of pro-inflammatory cytokines (e.g., TNF-α, IL-6 and IL-12) that are crucial for macro and microangiopathy developments [12]. These cytokines may mediate the synthesis of acute phase proteins which are able to initiate and support inflammatory process in the vascular wall [12, 13]. In addition, various inflammatory markers have been implicated in the disease process and progression of diabetic retinopathy [14]. C-reactive protein (CRP) is a well-known inflammatory marker and a sign of inflammation in the walls of arteries [14]. C-reactive protein, an acute-phase reactant produced by liver, is an extremely sensitive marker of systemic inflammation [14]. The recent development of high-sensitivity assays for CRP (hs-CRP) has permitted detection of mild elevation of CRP, even within the normal range [14, 15]. Recent study showed that retinal microvascular caliber is associated with higher levels of highly sensitive CRP (hs-CRP) in Asians [15]. This supported the concept that retinal venular caliber may be a marker for low-grade systemic inflammation [16]. Many clinical studies conducted to investigate the association between CRP level and DR [14-16]. Some studies suggest that CRP level is associated with DR and with the severity of the disease among diabetic patients in children and adults [17-21]. In order to prevent blindness from diabetic retinopathy, prevention and early detection are mandatory. Therefore, this study was aimed to investigate the association of CRP with diabetic retinopathy in young people with TIDM and also to find out whether CRP could predict the presence of retinopathy.
2. Materials and Methods
This case control study was conducted at the Department of Ophthalmology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh from March 2019 to march 2021.This study was approved by the Ethical Review Committee, BSMMU, Dhaka, Bangladesh. A total of sixty (60) young people (age between 10-24 years) of both gender, diagnosed as type 1 diabetes mellitus (T1DM) with retinopathy (30 patients) and without retinopathy (30 patients) were included by purposive sampling technique as study population. Patients with any systemic disease other than diabetes mellitus, patients with any systemic infection, patients with any ocular disease other than diabetic retinopathy and patients having previous photocoagulation therapy and anti- vascular endothelial growth factor (VEGF) injection were excluded from the study. Then study population was divided into two following groups-
Group –I: Case (Type-1 diabetic young people with retinopathy)
Group –II: Control (Type-1 diabetic young people without retinopathy)
2.1 Study procedure
After selection, all patients and their guardians were informed about the purpose and procedure of the study. Informed written consent was taken from the patients or guardians prior to enrollment. Determination of the type 1 diabetes mellitus (T1DM) was made by the International Society for Pediatric and Adolescent Diabetes (ISPAD) criteria according to available clinical features and history [7, 22]. A complete clinical evaluation including history and physical examination was done to exclude any systemic disease or any infective condition other than diabetes mellitus. Relevant ocular examinations, visual acuity test and slit lamp examination were done to exclude any other ocular disease other than diabetic retinopathy in case group and any ocular disease in control group. Both direct and indirect ophthalmoscopic examination was done to diagnose diabetic retinopathy and exclude other ocular disease [23]. Examination findings were recorded accordingly. Diabetic retinopathy was evaluated after dilatation of pupil with direct and indirect ophthalmoscopy with color fundus photography. Microaneurysm, dot and blot hemorrhage, venous changes, hard exudates, new vessels on the disc or new vessels elsewhere were considered as diabetic retinopathy on the basis of National Screening Committee (NSC) classification [24].
2.2 Blood sample collection and analysis
With all aseptic precautions, 4 ml venous blood was collected by puncturing the antecubital vein of the patients. Then 2 ml of blood was collected in blood collection tube containing potassium ethylenediamine tetra-acetic acid (EDTA) for estimation of glycated hemoglobin (HbA1c) level and rest 2 ml of blood was collected in blood collection tube containing clot activator for estimation of serum high sensitivity C-reactive protein (hs-CRP). The blood collection tube was labeled with patient’s ID and sent for analysis. HbA1c level was measured by Capillary Electrophoresis Method in Minicap Flex Piercing, Sebia, France machine. Before estimation of serum hs-CRP, serum portion of blood sample was separated by standard laboratory techniques. Serum level of hs-CRP was measured by using Cardio Phase hs-CRP reagent by means of particle enhanced immune nephelometry using the BN ProSpec System, SIEMENS, USA machine.
2.3 Color fundus photograph (CFP)
Color Fundus Photograph (CFP) of both eyes was obtained by Canon CR-2 Digital Retinal Camera, Canon, USA. Fundus photographs were stored, viewed and processed using Canon Retinal Imaging Control Software (RICS).
2.4 Statistical analysis of data
After collection; all data were cross checked, compiled and verified accordingly. Statistical analysis of the results was done by using computer-based software Statistical Package for the Social Sciences (SPSS), version 26. The mean with standard deviation (±SD) values were calculated for continuous variables. The quantitative observations were indicated by frequencies and percentages. Comparisons between groups (continuous parameters) were done by unpaired ‘t’ test. Categorical parameters were compared by Chi-Square test. Independent ‘t’ test was applied to compare the mean value of serum hs-CRP level in diabetic patients without retinopathy and mean value of serum hs-CRP level in diabetic patients with retinopathy and ANOVA test was applied to compare among groups. Pearson’s correlation test and multivariate logistic regression analysis were done to predict association. A probability ‘p’ value of 0.05 or less was considered as significant.
3. Results and Observations
In this case control study total 60 young participants, age between 10-24 years were selected purposively according to the selection criteria. Among them 30 participants had type 1 diabetes mellitus (T1DM) with diabetic retinopathy who were considered as cases (Group I) and 30 participants had T1DM without diabetic retinopathy who were considered as controls (Group II). Among total 60 patients; 33(55%) patients were female and 27(45%) patients were male (Figure- 1). In the case group among 30 patients, 16(53.3%) patients were female and 14(46.7%) patients were male; in control group among 30 patients, 17(56.7%) patients were female and 13(43.3%) patients were male; there was no significant difference in gender distribution in both study groups (p= 0.795) (Table- 1). In this study, mean(±SD) age in group I was 21.30±2.49 years and that was 19.97±2.81 years in group II. Majority of the patients (70%) in case group was belonged to the age group 20-24 years and 50.0% patients in control group was in age group 20-24 years; no significant age difference was observed between the groups (p= 0.195) (Table- 1).
Table 1: Demographic profile of the study patients in two groups (N= 60)
|
Variables |
Group I (Cases) (n= 30) |
Group II (Controls) (n= 30) |
p value |
|
Gender |
|||
|
Female |
16(53.3%) |
17(56.7%) |
0.795ns |
|
Male |
14(46.7%) |
13(43.3%) |
|
|
Age (years) |
|||
|
Mean±SD |
21.30±2.49 years |
19.37±2.71 years |
|
|
Age groups (years) |
|||
|
10-15 |
1(3.3%) |
4(13.3%) |
0.195ns |
|
15-20 |
8(26.7%) |
11(36.7%) |
|
|
20-24 |
21(70.0) |
15(50.0%) |
|
Data were expressed as frequency with percentage and mean±SD, Chi-square test was done, ns= not significant
Table- 2 shows that age of onset of T1DM was significantly lower in case group compares to control group (11.0±2.10 versus 12.67±1.45, p<0.001). It was observed that, TIDM patients with retinopathy had significantly higher duration of diabetes mellitus (more than 10 years) (p<0.001). The difference of glycemic control (indicated by HbA1c level) between two groups was also statistically significant (11.17±2.25% versus 9.83±2.36%, p= 0.029) (Table- 2).
Table 2: Comparison of age of onset of diabetes mellitus (DM), duration of DM and HbA1c between TIDM patients with or without nephropathy (N= 60)
|
Variables |
Group I (Cases) (n= 30) |
Group II (Controls) (n= 30) |
p value |
|
Age onset of DM (years) |
|||
|
5-10 |
12(40.0%) |
1(3.3%) |
|
|
11-15 |
18(60.0%) |
27(90.0%) |
|
|
16-20 |
0(0.0%) |
2(6.7%) |
|
|
Mean±SD |
11.0±2.10 years |
12.67±1.45 years |
<0.001s |
|
Range (minimum – maximum) |
7 – 15 years |
10 – 16 years |
|
|
Duration of diabetes mellitus (years) |
|||
|
≥10 |
19(63.3%) |
7(23.3%) |
|
|
<10 |
11(36.7%) |
23(76.7%) |
|
|
Mean±SD |
10.3±3.14 years |
6.87±3.14 years |
<0.001s |
|
Range (minimum – maximum) |
5 – 15 years |
1 – 12 years |
|
|
HbA1c (%) |
|||
|
<7 |
1(3.3%) |
4(13.3%) |
|
|
7-9 |
5(16.7%) |
11(36.7%) |
|
|
>9 |
24(80.0%) |
15(50.0%) |
|
|
Mean±SD |
11.17±2.25% |
9.83±2.36% |
0.029s |
|
Range (minimum – maximum) |
7.6 %– 14.7 % |
6.4% – 14.0% |
|
Data were expressed as frequency with percentage and mean±SD, Unpaired t-test was done to analyze data, s= significant
In this study, the mean(±SD) high sensitivity C-Reactive Protein (hs-CRP) level of the T1DM patients having diabetic retinopathy was 5.08±1.29 mg/L and that was 3.43±0.69 mg/L in T1DM patients without having diabetic retinopathy. Therefore, a significantly increased hsCRP level was found in retinopathy group compare to without retinopathy group (p<0.001) (Table- 3).
Table 3: Comparison of serum high sensitivity C-Reactive Protein (hs-CRP) between Type I DM patients with or without nephropathy (N= 60)
|
High sensitivity C-Reactive Protein (hs-CRP) |
Group I (Cases) (n= 30) |
Group II (Controls) (n= 30) |
p value |
|
Mean±SD |
5.08±1.29 mg/L |
3.43±0.69 mg/L |
<0.001s |
|
Range (minimum – maximum) |
3.10 mg/L - 7.83 mg/L |
1.56 mg/L - 4.89 mg/L |
Data were expressed as mean±SD, Unpaired t-test was done to analyze data, s= significant
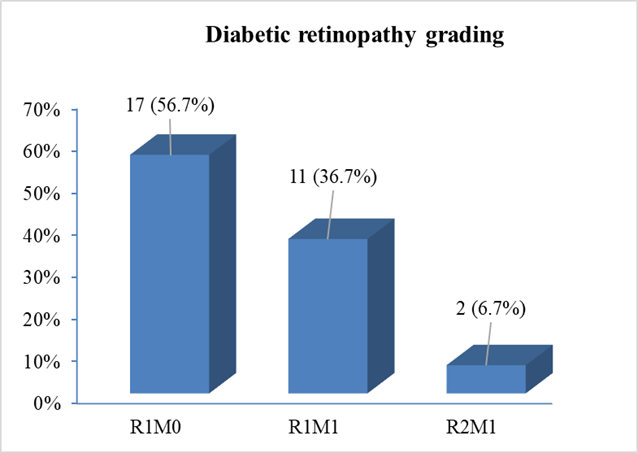
National Screening Committee (NSC) grading of diabetic retinopathy among the study cases revealed that, more than half [17(56.7%)] of the patients had R1M0 grading (mild to moderate non-proliferative diabetic retinopathy but no maculopathy). 11(36.7%) patients had NSC grading of RIMI (mild to moderate non-proliferative diabetic retinopathy with maculopathy) and only 2(6.7%) patients had NSC grading of R2M1 (severe non-proliferative diabetic retinopathy with maculopathy) (Figure- 2).
In this study, comparison of high sensitivity C-Reactive Protein (hs-CRP) level with NSC grading among the study patients having diabetic retinopathy was done. The mean hs-CRP level was 3.43±0.69mg/L in control group (Group- II). The mean hs-CRP level in R1MO (mild to moderate non proliferative diabetic retinopathy but no maculopathy) was 4.06±0.44 mg/L, in R1M1 ((mild to moderate non-proliferative diabetic retinopathy with maculopathy) was 6.23±0.49 mg/L and in NSC grading of R2M1 (severe non-proliferative diabetic retinopathy with maculopathy) was 7.36±0.66 mg/L. Mean hs-CRP level gradually significantly increased with advancement of NSC grade of diabetic retinopathy (p<0.001) (Table- 4).
Table 4: Comparison of serum high sensitivity C-Reactive Protein (hs-CRP) level among National Screening Committee (NSC) grading of Type I diabetes mellitus (T1DM) patients with or without retinopathy (N= 60)
|
Variable |
Group II |
Group I |
p value |
||
|
Controls (n= 30) R0M0 |
Cases (n= 30) |
||||
|
R1M0 |
R1M1 |
R2M1 |
|||
|
(n= 17) |
(n= 11) |
(n= 2) |
|||
|
Mean±SD |
Mean±SD |
Mean±SD |
Mean±SD |
||
|
Serum hs-CRP (mg/L) |
3.43±0.69 mg/L |
4.06±0.44 mg/L |
6.23±0.49 mg/L |
7.36±0.66 mg/L |
<0.001s |
Data were expressed as mean±SD, ANOVA test was done, s= significant
Group –I: Case (Type-1 diabetic young people with retinopathy)
Group –II: Control (Type-1 diabetic young people without retinopathy)
It was observed that serum high sensitivity C-Reactive Protein (hs-CRP) had a significant strong positive correlation with glycated hemoglobin (HbA1c) both in Group I (r=+0.831, p<0.001) and Group II (r=+0.666, p<0.001) (Table- 5).
Table 5: Correlation of high sensitivity C-Reactive Protein (hs-CRP) with glycated hemoglobin (HbA1c) in Type I diabetes mellitus (T1DM) patients (N= 60)
|
Variable |
Group I (Cases) (n=30) |
Group II (Controls) (n=30) |
||
|
r- value |
p value |
r- value |
p value |
|
|
HbA1c (%) |
0.831 |
<0.001s |
0.666 |
<0.001s |
Pearson’s correlation test was done, s= significant
Group –I: Case (Type-1 diabetic young people with retinopathy)
Group –II: Control (Type-1 diabetic young people without retinopathy)
Univariate and multivariate logistic regression analysis was performed with diabetic retinopathy as the dependent variable. Crude odds ratio (OR) shows; high sensitivity C-Reactive Protein (hs-CRP), poor glycemic control (HbA1c >9%) and duration of diabetes (more than 10 years) were individually strongly associated with diabetic retinopathy. In multivariate logistic regression after taking into consideration the effect of poor glycemic control and duration of T1DM, only elevated hs-CRP (>4 mg/L) significantly associated with diabetic retinopathy with an OR of 7.438 (p= 0.004) (Table- 6).
Table 6: Univariate and multivariate logistic regression analysis for prediction of diabetic retinopathy in Type I diabetes mellitus (T1DM) patients
|
Variables |
Univariate |
Multivariate |
||||
|
Crude OR |
95% CI |
p value |
Adjusted OR |
95% CI |
p value |
|
|
Duration T1DM (>10 years) |
2.26 |
1.32-3.87 |
0.002s |
3.377 |
0.94-12.09 |
0.062 |
|
HbA1c (>9%) |
2 |
0.98-4.09 |
0.028s |
1.509 |
0.37-6.11 |
6.11 |
|
hs-CRP (>4 mg/L) |
13.1 |
3.42-50.0 |
<0.001s |
7.438 |
1.91-29.04 |
0.004s |
OR= odds ratio, CI= Confidence Interval, s= significant
4. Discussion
Diabetic retinopathy (DR) is a major microvascular complication of diabetes mellitus (DM) and is a major cause of avoidable blindness around the world [5, 23]. The onset of diabetic retinopathy varies and depends on a number of predisposing risk factors, the main ones being glycemic control and duration of diabetes [9, 23]. However, the risk factors cannot always predict or explain the development of DR [9]. As the onset and progress of diabetic retinopathic changes are not predictable and cannot be determined without eye checkup in diabetic patients [24, 25, 26]. There is no screening tool which can easily perform with routine blood checkup of diabetic patient that can predict presence of diabetic retinopathy. A valid tool could help in early diagnosis of DR and early intervention will prevent progression and complication of DR. Hence, efforts should be directed to screening for early signs of diabetic retinopathy and modifiable risk factors. Diabetes mellitus is associated with low-grade inflammation and inflammatory markers play a major role in the development of diabetes and its microvascular complications, such as retinopathy [27]. Inflammation and endothelial dysfunction are the important components responsible for the progression of diabetic retinopathy [28]. C-Reactive Protein (CRP) is a well-accepted biomarker of acute inflammation and is also associated with chronic low-grade inflammation and neuro-degenerative diseases [29]. Previous studies have demonstrated a positive correlation between elevated CRP levels and type 1 and type 2 diabetes, as well as increased risks for diabetic complications, such as DR [30]. In this background, current case control study was aimed to investigate the association between serum high sensitivity C-Reactive Protein (hs-CRP) level and diabetic retinopathy in type-I diabetic young people and also to find out whether serum CRP can act as a predictor of early development of DR. A total of 60 young patients with type I diabetes mellitus (T1DM) aged between 10 to 24 years were included in this study and divided into two groups, 30 in Group I: Cases (Type-1 diabetic young people with retinopathy) and Group II: Controls (Type-1 diabetic young people without retinopathy). Diabetic retinopathy (DR) rarely develops before 10 years of age [31], ISPAD guidelines suggest starting screening for DR from age 11 years [26] and according to World Health Organization (WHO) this 10 to 20 years age range are defined as young people [32]; these were the reasons behind choosing this age range of 10-24 years.
In this study, the mean age was 21.30±2.49 years in case group and that was 19.97±2.81 years in control group; majority (70.0%) patients of case group were in 20-24 years age and 50.0% patients of control group were in age 20-24 years; age distribution in both study groups were almost similar, making the study more representative. Several studies have investigated the association between serum CRP and diabetic retinopathy but very few have investigated this association in type 1 diabetic among young people. Izuora et al., investigated this association in young people having almost similar age range to the current study; they mentioned in their study that the mean age of the participants was 26.7±7.2 years [19]. The mean age difference of the participants between two studies could be explained by the fact that; previous study included the age between 14 to 42 years, whether this study included 10 to 24 years age group [19]. In accordance; Rajab et al., investigate in 13 to 39 years age group of T1DM where mean age was 26.8±7.1 years [30]. In this current study we found that more than 50% of study participants were female in both group (53.3% with DR versus 56.7% without DR), the difference between male and female among the groups was not statistically significant (p= 0.795). It should be mentioned here that majority of young people with T1DM in Bangladesh are female as reported in a previous study [7]. Age of onset of TIDM had significantly lower in case group compares to control group (11.0±2.10 years versus 12.67±1.45 years, p<0.001). In this present study, TIDM patients with retinopathy group had a higher duration of DM with a mean of 10.3±3.14 years and that was 6.87±3.14 years in without DR patients. The difference between the mean duration of DM between the groups was statistically significant (p<0.001). Similar significant difference was observed in a couple of previous studies [19, 20, 30, 33]. One previous study found a strong association of longer duration of diabetes with diabetic retinopathy in a large cohort of 1227 young patients in Bangladesh [7]. Almost all studies that have investigated the association between CRP and DR found poor glycemic control to be highly associated with DR. In present study, the mean HbA1c in retinopathy group was 11.17±2.25% and in participants without retinopathy group was 9.89±2.36%; the difference was statistically significant (p= 0.029). In TIDM patients with retinopathy group significantly higher concentrations of HbA1c and duration of DM compare to without retinopathy group (p<0.05). The mean HbA1c level of this present study was much higher than most of the related previous studies [20, 21, 30, 33, 34]. The difference between these findings may be due to most of the participants of this current study were younger than previous studies. It was documented that younger age is associated with poor glycemic control [35]. In this study, 80% of the participants with DR had a HbA1c level above 9%, which is considered as poor glycemic control status [36].
In present study mean hs-CRP was significantly increased in retinopathy group compare to without retinopathy group in TIDM patients (p<0.001). The mean value of hs-CRP was 5.08±1.29 mg/L in TIDM patients having diabetic retinopathy and that was 3.43±0.69 mg/L in T1DM patients without diabetic retinopathy. In this current study; it was also seen that higher grades of retinopathy (according to NSC grading) showed relatively higher values of hs-CRP in case group and hs-CRP level gradually increased with NSC grade. The mean hs-CRP level of the participants having diabetic retinopathy group in R1MO (mild to moderate non proliferative diabetic retinopathy but no maculopathy) was 4.06±0.44 mg/L, in R1M1 ((mild to moderate non-proliferative diabetic retinopathy with maculopathy) was 6.23±0.49 mg/L and NSC grading of R2M1 (severe non-proliferative diabetic retinopathy with maculopathy) was 7.36±0.66 mg/L. Mean CRP was increased significantly among advanced NSC grading. Here we found a significant association between elevated levels of hs-CRP and more advanced degrees of diabetic retinopathy (p<0.001). These findings were consistent with similar previous studies [5, 19, 21, 28, 33, 37]. This finding indicated that, CRP as an inflammatory mediator that increase in diabetic retinopathy and has significance in diabetic patients without any systemic involvement. As CRP is a well-known inflammatory marker, it would be expected to be elevated in patients with poor glycemic status. In this present study, serum CRP had a significant strong positive correlation with HbA1c in Group I (r=+0.831, p<0.001) and Group II (r=+0.666, p<0.001).
In this study, after univariate logistic regression analysis; duration of DM was found to be strongly associated with DR. The unadjusted odds ratio was 2.26 and after adjusting for other confounder the significance of the relationship was lost (p= 0.062). In this context, Nowak et al., also found association between duration of DM and DR; however, the association was lost after multivariate regression analysis [21]. Univariate logistic regression analysis revealed that, there was a strong association between poor glycemic control and DR; the unadjusted odds ratio was 2.0 (p= 0.028), but in multivariate logistic regression analysis, the significance of this relationship was lost (p= 6.11). Logistic regression analysis in this study showed that in an age-adjusted and sex-adjusted univariate model patient with highest hs-CRP (>4 mg/L) were 13.1 times more likely to have DR (crude odds ratio of 13.10 with a p value <0.001). Crude odds ratio of univariate model also shows poor glycemic control (HbA1c >9%) and duration of diabetes (more than 10 years) were individually strongly associated with DR. In multivariate logistic regression after taking into consideration the effect of poor glycemic control and duration of DM, only elevated hs-CRP (>4 mg/L) significantly associated with DR with an OR of 7.438 (p= 0.004). This result was comparable with related previous studies [33, 34, 38].
This study suggested that serum hs-CRP level can act as a predictor for early development of DR and also as a biomarker to determine the severity of DR in young type1 diabetic people. As serum CRP can be done easily with routine blood examination of diabetic patient, CRP can be used as a screening tool for DR and progression of higher grades of diabetic retinopathy which help in early diagnosis and follow up of DR.
Conclusions
This study concluded that there is a strong association of C- Reactive Protein with diabetic retinopathy in young people with type 1 diabetes mellitus. With increasing severity of diabetic retinopathy, the mean serum CRP level also increased, the correlation is significant. Univariate logistic regression analysis found that serum CRP independently associated with diabetic retinopathy and this association becomes stronger after multivariate logistic regression analysis. Type I diabetic patients with retinopathy has significantly higher duration of diabetes and poor glycemic control. The predictive value of CRP in early detection of the diabetic subjects at high risk for developing DR, suggesting that inflammatory activity could be regarded as a useful and an inexpensive tool for screening the diabetic retinopathy, contributing to the prevention of progression to more severe stages by early diagnosis and treatment. CRP can also be used for follow up of progression of different grads of retinopathy. Hence C- Reactive Protein (CRP) can be used as a screening tool for diabetic retinopathy.
Limitations of the study
It was a single center study with a relatively small sample size.
Recommendations
A multicenter study with large population should be done to confirm the findings of this current study.
Conflicts of interest
All authors declared that they have no conflict of interest regarding this publication.
References
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes 2018. Diabetes care 41 (2018): S13-27.
- Aschner P, Karuranga S, James S, Simmons D, Basit A, Shaw JE, et al. The International Diabetes Federation’s guide for diabetes epidemiological studies. Diabetes research and clinical practice (2021): 172.
- Al-Agha AE, Alafif M, Abd-Elhameed IA. Glycemic control, complications, and associated autoimmune diseases in children and adolescents with type 1 diabetes in Jeddah, Saudi Arabia. Saudi medical journal 36 (2015): 26.
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes care 35 (2012): 556-64.
- Naik M, Kanjani I, Yadav S and Quadri SU. Association of High Sensitivity C-Reactive Protein (hs-CRP) with Diabetic Retinopathy. International Journal of Science and Research (IJSR) 6 (2017): 683-86.
- Amutha A, Mohan V. Childhood and adolescent onset type 1 diabetes in India. MGM Journal of Medical Sciences 1 (2013): 46-53.
- Zabeen B, Govender D, Hassan Z, Noble JA, Lane JA, Mack SJ, et al. Clinical features, biochemistry and HLA-DRB1 status in children and adolescents with diabetes in Dhaka, Bangladesh. Diabetes research and clinical practice 158 (2019): 107894.
- American Academy of Ophthalmology (AAO). Basic and Clinical Science Course: Section 12. San Francisco, CA: American Academy of Ophthalmology (2019-2020): 12.91-12.107.
- Lima VC, Cavalieri GC, Lima MC, Nazario NO, Lima GC. Risk factors for diabetic retinopathy: a case–control study. International journal of retina and vitreous 2 (2016): 1-7.
- Meleth AD, Agrón E, Chan CC, Reed GF, Arora K, Byrnes G, et al. Serum inflammatory markers in diabetic retinopathy. Investigative ophthalmology & visual science 46 (2005): 4295-301.
- Tang J, Kern TS. Inflammation in diabetic retinopathy. Progress in retinal and eye research 30 (2011): 343-58.
- McCarter RJ, Hempe JM, Gomez R, Chalew SA. Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes care 27 (2004): 1259-64.
- Yokoi M, Yamagishi SI, Takeuchi M, Ohgami K, Okamoto T, Saito W, et al. Elevations of AGE and vascular endothelial growth factor with decreased total antioxidant status in the vitreous fluid of diabetic patients with retinopathy. The British journal of ophthalmology 89 (2005): 673.
- May D, Francis EA, Norman M, Nelly N, Nameeth D, Shah AJ. Role of C- reactive protein in diabetic retinopathy. Int J Med Res Rev 5 (2017): 585-592.
- Morimoto Y, Conroy SM, Ollberding NJ, Kim Y, Lim U, Cooney RV, et al. Ethnic differences in serum adipokine and C-reactive protein levels: the multiethnic cohort. International journal of obesity 38 (2014): 1416-22.
- Kilpatrick ES, Keevil BG, Jagger C, Spooner RJ, Small M. Determinants of raised C-reactive protein concentration in type 1 diabetes. Qjm 93 (2000): 231-6.
- Wang XL, Dai Y, Chen XH. Changes of the concentration of serum adiponectin and high sensitivity C-reactive protein in type 2 diabetes mellitus patients with retinopathy. International Journal of Ophthalmology 10 (2010): 1699-701.
- Budak YU, Akdogan M, Huysal K. Relationship of PON1 activity and hsCRP concentration with disease status in patients with type 2 diabetes mellitus with and without retinopathy. International Journal of Diabetes in Developing Countries 33 (2013): 40-5.
- Izuora KE, Chase HP, Jackson WE, Coll JR, Osberg IM, Gottlieb PA, et al. Inflammatory markers and diabetic retinopathy in type 1 diabetes. Diabetes Care 28 (2005): 714-5.
- Mysliwiec M, Balcerska A, Zorena K, Mysliwska J, Lipowski P, Raczynska K. The role of vascular endothelial growth factor, tumor necrosis factor alpha and interleukin-6 in pathogenesis of diabetic retinopathy. Diabetes research and clinical practice 79 (2008): 141-6.
- Nowak M, Wielkoszynski T, Marek B, Kos-Kudla B, swietochowska E, Sieminska L, et al. Antioxidant potential, paraoxonase 1, ceruloplasmin activity and C-reactive protein concentration in diabetic retinopathy. Clinical and experimental medicine 10 (2010): 185-92.
- Mayer-Davis EJ, Kahkoska AR, Jefferies C, Dabelea D, Balde N, Gong CX, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatric diabetes 19 (2018): 7.
- Lueder GT, Silverstein J. Screening for retinopathy in the pediatric patient with type 1 diabetes mellitus. Pediatrics 116 (2005): 270-3.
- Harris M, Lacey L. Ensuring the effective delivery of diabetic eye screening for children and young people in England. Diabetes Care Child. Young People 3 (2014): 59-63.
- Zabeen B, Huda K, Nessa M, Ahmed F, Akhter S, Azad K. Retinopathy in Bangladeshi Youth cohort with diabetes-a multicenter study. Pediatr Diab 19 (2018): 104.
- Donaghue KC, Marcovecchio ML, Wadwa RP, Chew EY, Wong TY, Calliari LE, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Microvascular and macrovascular complications in children and adolescents. Pediatric diabetes 19 (2018): 262.
- Khaloo P, Qahremani R, Rabizadeh S, Omidi M, Rajab A, Heidari F, et al. Nitric oxide and TNF-α are correlates of diabetic retinopathy independent of hs-CRP and HbA1c. Endocrine 69 (2020): 536-41.
- Nalini M, Raghavulu BV, Annapurna A, Avinash P, Chandi V, Swathi N. Correlation of various serum biomarkers with the severity of diabetic retinopathy. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 11 (2017): S451-4.
- Luan YY, Yao YM. The clinical significance and potential role of C-reactive protein in chronic inflammatory and neurodegenerative diseases. Frontiers in Immunology 9 (2018): 1302.
- Rajab HA, Baker NL, Hunt KJ, Klein R, Cleary PA, Lachin J, et al. The predictive role of markers of Inflammation and endothelial dysfunction on the course of diabetic retinopathy in type 1 diabetes. Journal of Diabetes and its Complications 29 (2015): 108-14.
- Agroiya P, Alrawahi AH. Pediatric diabetic retinopathy: experience of a tertiary hospital in Oman. Middle East African Journal of Ophthalmology 26 (2019): 189.
- World Health Organization. Engaging young people for health and sustainable development: Strategic opportunities for the World Health Organization and partners (2018).
- Melo LG, Morales PH, Drummond KR, Santos DC, Pizarro MH, Barros BS, et al. Relationship between proliferative diabetic retinopathy and inflammatory markers in patients with type 1 diabetes in Brazil: a nested case control study. Ophthalmologica 243 (2020): 471-8.
- Laursen JV, Hoffmann SS, Green A, Nybo M, Sjølie AK, Grauslund J. Associations between diabetic retinopathy and plasma levels of high-sensitive C-reactive protein or von Willebrand factor in long-term type 1 diabetic patients. Current eye research 38 (2013): 174-9.
- Nanayakkara N, Ranasinha S, Gadowski AM, Davis WA, Flack JR, Wischer N, et al. Age-related differences in glycaemic control, cardiovascular disease risk factors and treatment in patients with type 2 diabetes: a cross-sectional study from the Australian National Diabetes Audit BMJ open 8 (2018): e020677.
- Rewers MJ, Pillay K, De Beaufort C, Craig ME, Hanas R, Acerini CL, et al. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatric diabetes 15 (2014): 102-14.
- Song J, Chen S, Liu X, Duan H, Kong J, Li Z. Relationship between C-reactive protein level and diabetic retinopathy: a systematic review and meta-analysis. PLoS One 10 (2015): e0144406.
- Sasongko MB, Wong TY, Jenkins AJ, Nguyen TT, Shaw JE, Wang JJ. Circulating markers of inflammation and endothelial function, and their relationship to diabetic retinopathy. Diabetic Medicine 32 (2015): 686-91.