Assessment of the Safety of Enoxaparin a Low Molecular Weight Heparin for Prophylaxis against Deep Venous Thrombosis in Patients with Chronic Kidney Disease on Hemodialysis
Article Information
Sonali Vadi1, Sanjay P. Shah2, Kenneth Yim3, Ifeanyi Egbunike4
1Department of Critical Care Medicine, Kokilaben Dhirubhai Ambani Hospital and Medical Research Centre, Mumbai, India
2Department of Pulmonary Medicine, Northwest Hospital Center, Randallstown, Maryland, USA
3Director of Inpatient Hemodialysis- Davita Dialysis, University of Maryland Medical Center Midtown Campus, Baltimore, Maryland, USA
4Director of Pharmacy, Department of Pharmacy Services, University of Maryland Medical Center Midtown Campus, Baltimore, Maryland, USA
*Corresponding author: Sonali Vadi, Department of Critical Care Medicine, Kokilaben Dhirubhai Ambani Hospital and Medical Research Centre, Mumbai, India
Received: 19 January 2022; Accepted: 27 January 2021; Published: 01 March 2022
Citation: Sonali Vadi, Sanjay P. Shah, Kenneth Yim, Ifeanyi Egbunike. Assessment of the Safety of Enoxaparin a Low Molecular Weight Heparin for Prophylaxis against Deep Venous Thrombosis in Patients with Chronic Kidney Disease on Hemodialysis. Archives of Clinical and Biomedical Research 6 (2022): 269-276.
Share at FacebookAbstract
Study objective To analyze the incidence of complications following enoxaparin use in patients on hemodialysis.
Study design and patients This retrospective chart review study included 200 patients equally divided into two groups, those who received enoxaparin and those who were administered unfractionated heparin (UFH). Primary hypothesis was that enoxaparin is safe and equally effective as UFH for the prophylaxis of venous thromboembolism (VTE) in chronic kidney patients on hemodialysis in adjusted dosage. Safety parameter assessed was the incidence of bleeding. Secondary end point was that enoxaparin is safe as UFH in the elderly patients on hemodialysis in adjusted dosage.
Measurements and main results There were no significant differences in any of these parameters except that patients in the Enoxaparin group had a greater decline in platelets compared to the UFH group. Overall incidence of bleeding requiring packed red cell transfusion was 3 in LMWH group vs. 2 in UFH group, p = NS. None of the patients developed DVT during the therapy. There was no statistical difference between the groups in primary outcomes for patients > 65 years of age.
Conclusion The incidence of venous thromboembolism and bleeding complications were low suggesting that further evaluation of enoxaparin in this specific population is warranted.
Keywords
Enoxaparin; Deep vein thrombosis prophylaxis; Chronic kidney disease; Hemodialysis
Article Details
1. Introduction
The Food and Drug Administration has not approved the use of enoxaparin, a low molecular weight heparin (LMWH) for prophylaxis against deep vein thrombosis (DVT) in the End Stage Renal Disease (ESRD) population on maintenance hemodialysis (HD). Enoxaparin is excreted through the kidneys and its half-life is hence prolonged in patients with impaired renal function. Drug accumulation can potentially lead to increased bleeding risk [1] in these patients. Hence, UFH is preferred over LMWH in patients with chronic kidney disease. Measurement of anti-factor Xa (anti-Xa) concentrations is recommended for renal impaired patients [2], when LMWH is used for management of thromboembolic disorder. Given that the monitoring of anti-Xa levels or aPTT is not recommended when LMWH or UFH is used for prophylaxis against venous thromboembolism, the safety of LMWH can be assessed by monitoring thromboembolic event rates, bleeding rates, and incidence of heparin-induced thrombocytopenia. Hospital use of un-fractionated heparin (UFH) was recalled on January 2008 due to the discovery of contaminated batches. Enoxaparin was hence administered to patients for DVT prophylaxis in an adjusted dosage. This retrospective chart-review study aims to analyze the incidence of complications following enoxaparin use in patients on hemodialysis.
2. Methods
2.1 Study location
This study was conducted at the University of Maryland Medical Center Midtown Campus, Baltimore, MD with ethics approval from the Institutional Review Board. Need for consent was waived in view of the retrospective nature of the study.
2.2 Study objective
The objective of this study was to determine the comparative incidence of venous thromboembolism (VTE) and to investigate the rate of bleeding between UFH and enoxaparin. Secondary end point is that enoxaparin is safe and equally effective as UFH in patients aged > 65 years on hemodialysis, in adjusted dosage.
2.3 Study design
This was a retrospective case-controlled chart-review study. Patients were divided into two groups: Group 1, those administered LMWH, and Group 2, those administered UFH. Enoxaparin was administered in a dose of 30mgs subcutaneously, every 48 hours. These patients were historically compared to those administered UFH from September 2007 till February 2008 in a dose of 5000 units subcutaneously, every 8 hours. There were 100 patients in each group.
Data was abstracted from medical charts and included:
- Age of patients
- Weight
- Admission diagnosis
- Risk factors for DVT: immobilization, CCF, age > 70 years, COPD, pre-existing venous disease (chronic venous insufficiency, post-thrombotic syndrome, varicose veins), DM, preexisting malignancy, dehydration, history of DVT, previous AMI, severe infection
- Recent h/o intravenous drug use 3, 4
- World Health Organization (WHO) performance status5
- Calculated creatinine clearance
- Type of heparin used during hemodialysis (for priming the circuit)
- Indication for UFH and LMWH prophylaxis
- Other medications: Aspirin, Clopidogrel, Warfarin
- Other factors associated with a higher risk of bleeding: coagulopathy, post-resuscitation status, recent trauma / surgery, platelet levels
2.4 Patient population
All patients included in the study were hospitalized hemodialysis patients with ESRD who received DVT prophylaxis from March 2008 for a period of 6 months. As we did not monitor anti-Xa levels, and because the time to occurrence of bleeding can be variable, only those patients already confirmed to be on a regular outpatient hemodialysis schedule prior to admission were included, such that follow-up history could be obtained in evaluating the occurrence of any complication post discharge. Patients who received UFH for a minimum of 5 days, or a minimum of 3 doses of LMWH were included. None of these patients had received heparin in any form in the prior 48 hours. The reason for this washout period was to eliminate any carry-over effects. The exclusion criteria included: contraindication to UFH or LMWH, intracranial hemorrhage in previous 6 months, ocular or neurosurgery in past 4 weeks, acute signs of DVT or pulmonary thromboembolism, or gastrointestinal ulcer.
Definitions
- Major bleeding was defined by the following:
- Bleeding leading to death
- The need for transfusion of >/= 2 units packed red blood cells (PRBC)
- Therapeutic intervention (by surgical team or interventional radiology)
- Acute fall in hemoglobin by >/= 3gm/dl with hypotension or hypoxia not related to hemodialysis
- Intracranial, intraocular, or retroperitoneal bleeding
- Minor bleeding was defined as:
- Petechiae
- Ecchymoses
- Superficial bleeding of gums
- Microscopic hematuria
- Blood-tinged sputum
- Mild epistaxis
- Vaginal bleeding not requiring PRBC transfusion
- Duration of anticoagulation therapy was calculated in days
- Fall in platelet count defined by a 50% drop in baseline
- Occurrence of VTE- deep venous thrombosis and/or pulmonary thromboembolism upto 1 day after the end of prophylaxis
- WHO performance status
Grade 0 (able to carry out all normal activity without restriction)
Grade 1 (restricted in physically strenuous activity, but ambulatory and able to do light work)
Grade 2 (ambulatory and capable of all self-care, but unable to carry out any work)
Grade 3 (capable of only limited self-care, confined to bed or chair 50% of waking hours)
Grade 4 (completely disabled, cannot carry on any selfcare), and WHO grade 0 (able to carry out all normal activity without restriction)
2.5 Statistical analysis
Statistical analysis included calculations of means and standard deviations (SD) for continuous variables. Discrete values were compared between groups using chi-square while continuous variables were compared using a student’s t-test. A p value of < 0.05 signified statistical significance.
3. Results
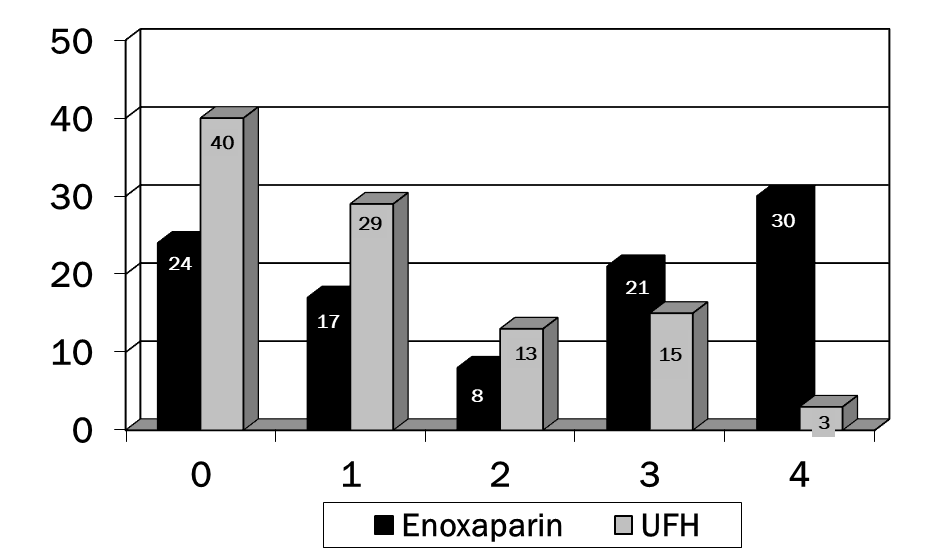
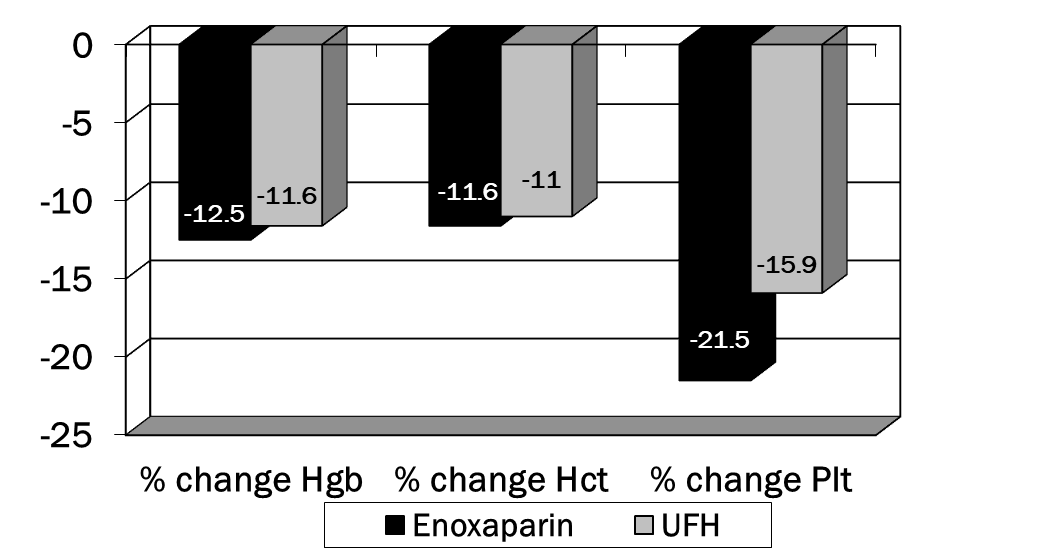
A total of 200 charts were reviewed to obtain data on 200 patients (100 patients in each group). Patient demographics can be found in table 1. There were no significant differences in any of these parameters. In table 2, the presence of several of the risk factors for VTE were different in the enoxaparin vs. UFH groups. Significantly more patients in the enoxaparin group were immobilized and had chronic obstructive airway disease. The UFH group contained more patients with pre-existing malignancy and history of DVT. An average of 2.8 and 2.5 risk factors were documented in the two groups, respectively. Enoxaparin was administered for an average of 11.8 days (WHO performance status of 4) while UFH for an average of 9.1 days (WHO performance status of 0). p<0.001 was for comparison of WHO Performance Status for enoxaparin vs. UFH (Figure 1). No patients developed venous thromboembolism in either groups. No major episode of bleeding was observed in both groups. There were no significant differences in any of these parameters except that patients in the enoxaparin group had a greater decline in platelets compared to the UFH group (Figure 2). Overall incidence of bleeding requiring packed red cell transfusion was 4 in the enoxaparin group vs. 2 in the UFH group, p<0.05 (Tables 3,4). None of the patients developed DVT during the therapy. Table 5 highlights the comparison of enoxaparin vs. UFH in patients aged > 65 years. There were no statistical differences between the two groups in primary outcomes for this age group.
|
Characteristic |
Enoxaparin (n=100) |
UFH (n=100) |
p-value |
|
Age, mean +/- SD (yrs) |
59.0 +/- 13.3 |
59.0 +/- 13.3 |
NS |
|
Sex, Male/Female, n |
61/39 |
53/47 |
NS |
|
Pre-Rx platelet levels mean +/- SD |
234.1 +/- 102.7 |
246.6 +/- 107.3 |
NS |
|
Hb prior to starting Rx, mean +/- SD |
10.4 +/- 2.0 |
10.6 +/- 1.9 |
NS |
|
HCT prior to starting Rx, mean +/- SD |
32.9 +/- 6.4 |
33.0 +/- 6.0 |
NS |
|
On additional anticoagulant/antiplatelet (warfarin, ASA, clopidogrel, or dipyridamole), n |
33 |
31 |
NS |
|
WHO performance status |
p<0.001 |
||
|
0 |
24 |
40 |
|
|
1 |
17 |
29 |
|
|
2 |
8 |
13 |
|
|
3 |
21 |
15 |
|
|
4 |
30 |
3 |
Table 1: Baseline demographic and clinical characteristics (NS= not significant)
|
Risk Factor |
Enoxaparin (n=100) |
UFH (n=100) |
p-value |
|
Immobilization, n |
56 |
30 |
p<0.001 |
|
Congestive Cardiac Failure, n |
18 |
15 |
NS |
|
Age > 70 yrs, n |
24 |
25 |
NS |
|
Chronic Obstructive Pulmonary Disease, n |
29 |
16 |
p=0.028 |
|
Pre-existing venous disease, n |
6 |
11 |
NS |
|
Diabetes Mellitus, n |
52 |
54 |
NS |
|
Pre-existing malignancy, n |
3 |
16 |
p=0.002 |
|
Dehydration, n |
1 |
0 |
NS |
|
History of Deep Venous Thrombosis, n |
4 |
12 |
p=0.037 |
|
Previous Acute Myocardial Infarction, n |
25 |
26 |
NS |
|
Severe infection, n |
15 |
11 |
NS |
|
Intravenous Drug Usage, n |
10 |
9 |
NS |
|
Coagulopathy, n |
1 |
0 |
NS |
|
Post-cardiopulmonary resuscitation status, n |
4 |
0 |
NS |
Table 2: Risk factors for venous thromboembolism (NS= not significant)
|
Outcome |
Enoxaparin (n=100) |
UFH (n=100) |
p-value |
|
Venous Thromboembolism (VTE), n |
0 |
0 |
NS |
|
Bleeding leading to death, n |
0 |
0 |
NS |
|
Acute fall in Hemoglobin (Hgb) >/=3, n |
4 |
2 |
NS |
|
Need for >/=2 packed red blood cell, n |
22 |
20 |
NS |
|
Transfusion due to therapy |
4 |
2 |
NS |
|
Fall in platelet count by 50%, n |
3 |
2 |
NS |
|
Composite outcome* |
7 |
4 |
NS |
Table 3: Safety outcomes [*Composite outcomes of venous thromboembolism + (bleeding leading to death, acute fall in Hgb or platelet count); NS= not significant]
|
% Change from baseline to lowest point on therapy: |
Enoxaparin (n=100) |
UFH (n=100) |
p-value |
|
Hemoglobin, mean +/- SD |
-12.5 +/- 10.8 |
-11.6 +/- 12.0 |
NS |
|
Hematocrit, mean +/- SD |
-11.6 +/- 10.5 |
-11.0 +/- 11.0 |
NS |
|
Platelets, mean +/- SD |
-38.2 |
-15.9 +/- 16.4 |
p=0.019 |
Table 4: Additional Safety parameters (NS= not significant)
|
Outcome |
Enoxaparin (n=39) |
UFH (n=44) |
p-value |
|
Venous thromboembolism (VTE), n |
0 |
0 |
NS |
|
Bleeding leading to death, n |
0 |
0 |
NS |
|
Acute fall in Hemoglobin (Hgb) >/=3, n (%) |
1 (2.6) |
1 (2.3) |
NS |
|
Need for >/=2 Packed red blood cell, n (%) |
9 (23.1) |
9 (20.5) |
NS |
|
Transfusion due to therapy |
1 (2.6) |
1 (2.3) |
NS |
|
Fall in platelet count by 50%, n (%) |
2 (5.1) |
1 (2.3) |
NS |
|
Composite outcomes* |
3 (7.6) |
2 (4.5) |
NS |
*p<0.001 for comparison of WHO Performance Status for Enoxaparin vs. UFH
Table 5: Safety outcomes in elderly patients (age > 65 years) [Composite outcomes= of effectiveness (VTE) + safety (bleeding leading to death, acute fall in Hgb or platelet count as above); (NS= not significant)]
4. Discussion
Advanced chronic kidney disease is a risk factor for bleeding. Several published studies do not support the use of enoxaparin in this population due to this increased incidence of bleeding [6]. Use of LMWH in patients with creatinine clearance < 30ml/min was shown to have a higher risk for major bleeding in a meta-analysis (odds ratio 2.25; 95% CI 1.19 to 4.27; p=0.013) [1]. Although there is insufficient data to support the use of enoxaparin, real-world circumstances influenced our decision as an institution to use this agent for prophylaxis against VTE in hospitalized hemodialysis patients. Traditional doses of enoxaparin used for DVT prophylaxis vary from 30 mg every 48 hours, to 30-40 mg daily. We used the lower dosing regimen in our institution due to concerns surrounding higher risk of bleeding risk in this patient population. Several observations can be made from our study. Although the study was underpowered to detect a significant difference in our effectiveness and safety outcome measures, the overall incidence of these parameters was extremely low when compared with published literature. Our study population consisted of inner-city patients with a higher prevalence of IVDU which may further influence the difference in the rates of VTE and bleeding complications. In addition, we compared patients based on time periods and found significant differences in VTE risk factors. We found no increased rate of bleeding with LMWH use in hospitalized ESRD patients on hemodialysis. Enoxaparin was also noted to be safe for DVT prophylaxis in this patient population. This is a retrospective study with convenience sampling. No screening was performed to evaluate for DVT pre-discharge.
4.1 Study highlights
- What is the current knowledge on the topic?
The Food and Drug Administration has not approved the use of enoxaparin, a low molecular weight heparin (LMWH) for prophylaxis against deep vein thrombosis in the End Stage Renal Disease (ESRD) population on maintenance hemodialysis (HD). Enoxaparin is excreted through the kidneys and its half-life is hence prolonged in patients with impaired renal function. Drug accumulation can potentially lead to increased bleeding risk in these patients. Hence, UFH is preferred over LMWH in ESRD patients on maintenance HD.
- What question did this study address?
The safety of LMWH can be assessed by monitoring thromboembolic event rates, bleeding rates, and incidence of heparin-induced thrombocytopenia.
- What does this study add to our knowledge?
Overall, the incidence of VTE and bleeding complications were low suggesting that further evaluation of enoxaparin in this specific population is warranted.
- How might this change clinical pharmacology or translational science?
A prospective trial of patients randomized to enoxaparin and UFH would best provide definitive answers to the relative efficacy and safety of these agents among ESRD patients on hemodialysis. Furthermore, if proven to be effective and safe, this finding would open up a new horizon for future research in the preventive management of clotting and thrombosis of vascular accesses in hemodialysis. This is a chronic and ongoing issue associated with significant morbidity and quality of life issues.
References
- Lim W, Dentali F, Eikelboom JW, et al. Meta-analysis: low-molecular-weight heparin and bleeding in patients with severe renal insufficiency. Ann Intern Med 144 (2006): 673
- Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest 119 (2001): 64S-94S.
- Syed FF, Beechings NJ. Lower-limb deep-vein thrombosis in a general hospital: risk factors, outcomes and the contribution of intravenous drug use. QJM 98 (2005): 139-145.
- Baldeweg SE. Injecting drug use is a major risk factor for deep vein thrombosis. BMJ 321 (2000): 10-18.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5 (1982): 649-655.
- Gerlach AT, Pickworth KK, Seth SK, et al. Enoxaparin and bleeding complications: A review in patients with and without renal insufficiency. Pharmacotherapy 20 (2017): 771-775.