Assessment of Right Ventricular Systolic Dysfunction Using 2D STE to Predict Short Term Outcome in Patients with Heart Failure with Reduced Ejection Fraction
Article Information
Md. Rakibul Hasan1*, Tuhin Haque2, Nurul Islam3, Md. Asifudduza4, Md. Owashak Faysal5, Rahatul Quadir6, Sharmin Ali7, Hosne Ara Sonia8
1Junior Consultant of Cardiology. Labaid Cardiac Hospital, Dhaka, Bangladesh
2Professor of Cardiology, National Heart
Foundation Hospital & Research Institute, Dhaka, Bangladesh
3Senior Medical Officer of Cardiology, National Heart Foundation Hospital & Research Institute, Dhaka, Bangladesh
4Junior Consultant of Cardiology, Labaid Cardiac Hospital, Dhaka, Bangladesh
5Medical Officer, National Institute of
Cardiovascular disease (NICVD), Dhaka, Bangladesh
6Assistant Registrar, National Institute of Cardiovascular Diseases(NICVD), Dhaka, Bangladesh
7Medical Officer Cardiology, National Institute of Cardiovascular Diseases & Hospital (NICVD), Dhaka, Bangladesh
8Registrar, Department of Gynecology and Obstetrics, Labaid Specialized Hospital, Dhaka, Bangladesh
Corresponding author: Dr. Md. Rakibul Hasan, Junior Consultant of Cardiology. Labaid Cardiac Hospital, Dhaka, Bangladesh..
Received: May 06, 2023; Accepted: May 15, 2023; Published: July 07, 2023
Citation: Md. Rakibul Hasan, Tuhin Haque, Nurul Islam, Md. Asifudduza, Md. Owashak Faysal, Rahatul Quadir, Sharmin Ali, Hosne Ara Sonia. Assessment of Right Ventricular Systolic Dysfunction Using 2D STE to Predict Short Term Outcome in Patients with Heart Failure with Reduced Ejection Fraction. Card5logy and Cardiovascular Medicine. 7 (2023): 251-259.
Share at FacebookAbstract
Backgraound and Objectives: Right ventricular functional assessment is challenging using conventional methods due to its complex geometry. Speckle tracking imaging using 2D is a novel method for assessing right ventricular systolic function in heart failure patients. The aim of this study was to predict short term outcome by RVFWS using 2D speckle tracking echocardiography in patients with heart failure with reduced ejection fraction.
Methods: This Prospective Cohort Study was conducted in the Department of Cardiology, National Heart Foundation Hospital and Research Institute from April, 2019 to May, 2020. A total of 115 patients with heart failure with reduced ejection fraction were included after considering inclusion and exclusion criteria who underwent right ventricle function assessment using 2D STE .The patients were divided into two group according to RVFWS, In group 1,Normal Right ventricular systolic function ( RVFWS ≤ -19), In group 2, Abnormal Right ventricular systolic function (RVFWS >-19).Outcome were recorded after 6 months over telephone. Baseline characteristics, LVEF, TAPSE, PASP, RVGLS and outcome were compared between the two groups.
Results: RVFWS was obtained successfully in 115 patients. Adverse short term outcome was more common in abnormal RVFWS group. RVFWS was found to be a best independent predictor of worst prognosis, compared with other echo parameters. ROC curve and Area Under ROC curve demonstrated that RVFWS could effectively predict short term outcome in patients with heart failure with reduced ejection fraction (Area under ROC curve was 0.7360, 95% CI = 0.631-0.840, P value <0.001). According to ROC curve analysis, RVFWS - 17.00 % appeared to be a good cutoff value for predicting short term outcome in patients with heart failure with reduced ejection fraction (Sensitivity 79.3%, Specificity 65.1% & Accuracy 68.7%).
Conclusion: Abnormal Right ventricular free wall strain using 2D speckle tracing echocardiography was found to be a predictor of adverse short term outcome in patients with heart failure with reduced ejection fraction with good degree of sensitivity and specificity.
Keywords
2D Speckle tracing echocardiography; Right ventricular free wall strain (RVFWS); LVFE; TAPSE; PASP; RVGLS
Article Details
Introduction
Heart failure is a global pandemic, affecting at least 26 million people worldwide and increasing in prevalence [1]. The lifetime risk of developing heart failure for women and men at age 55 years is 29% and 33%, respectively [2]. Approximately 1-2% of the adult population in developed countries has heart failure, with the prevalence rising to ≥10% among persons 70 years of age. Heart failure incidence increases with age, rising from approximately 20 per 1,000 individuals 65 to 69 years of age to >80 per 1,000 individuals among those >85 years of age [3]. In Bangladesh, data regarding the prevalence and incidence of heart failure is absent [4]. In one cross sectional study carried out in a single tertiary cardiac hospital, among 14,009 total admitted patients throughout the study period ,1970 patients were found to have a diagnosis of heart failure, 14.1% of totally admitted patients had heart failure [5]. It is crucial to accurately estimate and predict prognosis to identify patients who would benefit from advanced therapies, such as cardiac transplantation and mechanical circulatory support, or those who should be referred to palliative or hospice care [6]. HF is a progressive disease that produces high rates of morbidity and mortality [7-9]. The etiology of systolic heart failure dramatically affects prognosis and treatment. CAD accounts for the vast majority of cases of systolic heart failure in the United States followed by hypertensive and dilated cardiomyopathies [10]. Survival rates for chronic heart failure range from 81% to 91% at 1 year and 52% to 63% at 5 years [11, 12]. Identifying the predictors of prehospitalization and mortality among HF patients is vital in helping physicians for the risk stratification of their HF patients and chart the best possible post-discharge plan [13]. Multiple variables have associations with worse outcome in heart failure: male gender, advancing age, low ejection fraction, high NYHA functional class, low hematocrit, and sodium level, high brain natriuretic peptide, low peak exercise oxygen uptake, wide QRS, renal failure, low blood pressure, elevated heart rate, and volume overload refractory to medical treatment [14, 15]. Echocardiography is recommended in HFrEF patients to assess LVEF in order to guide evidence-based pharmacological treatment and device therapy ICD and CRT and to identify etiology of HF [16]. LVEF remains the most widely used echocardiographic parameter for quantification of systolic function and is an established predictor of mortality in HFrEF [17, 18]. The left side of the heart is not the sole contributor to risk stratification, the RV also holds significant prognostic value in HFrEF patients [19]. RV systolic dysfunction and LV systolic dysfunction are closely related through shared fibres of interventricular septum [20]. The relation between RV dysfunction and poor exercise capacity and between preserved RV functions and good exercise capacity as well as better hemodynamics, even in severely reduced LVEF are well established [21]. RV systolic dysfunction was relatively common in patients with left sided heart failure [22]. Poor RV function is an important driver of adverse prognosis regardless of LV function [23]. RV evaluation involves quantification of afterload and pre-load. RV volumes may be measured using 3 dimensional echocardiography [24]. TAPSE has been proposed as a simple and reproducible parameter for quantitative assessment of RV systolic function, TAPSE is a widely recognized, clinically useful and feasible marker of right ventricular systolic dysfunction and has been proven to be a valuable prognostic marker in various cardiac diseases, including heart failure [25]. The base-to-apex shortening of the RV during systole was represented by TAPSE [26]. It is not able to highlight regional abnormalities [27]. During recent years, deformation imaging called myocardial mechanics, have been emerged as valuable tools for more comprehensive and reliable assessment of myocardial function and this creates a new window to assess the myocardial deformation [28]. Myocardial deformation study can be done by tissue Doppler, 2D or 3D based echocardiographic speckle tracking, Sonomicrometry & Tagged MRI [29]. Speckle tracking echocardiography is a relatively new, angle and user-independent technique used for the evaluation of myocardial function. Speckle tracking appears to be highly reproducible and minimally affected by intra-observer and inter-observer variability [30]. Speckle tracking has recently been extended for the analysis of RV function. This technique provides an objective quantification of global and segmental RV longitudinal strain [31, 32]. RV strain had a sensitivity of 60.5% and a specificity of 87.5% to determine RV dysfunction, global RV function using mean RV strain was more effective to diagnose RV dysfunction than segmental parameters [33].RV strain is an excellent predictor of outcome and accurate, highly-feasible, fast and reproducible echocardiographic technique that provides important information about RV mechanics [34] and has significantly better correlation with MRI based RVEF measurements compared to other indices of RV function [35].
Objectives
General objective
To predict short term outcome in patients with heart failure with reduced ejection fraction using echocardiographic parameters of right ventricular systolic dysfunction.
Specific objectives
- To assess LVEF by Simpson’s biplane
- To measure TAPSE using M mode echocardiography, PASP using TR Jet & Right ventricular strain by 2D STE in patients with heart failure with reduced ejection fraction.
- To record short term outcome in patients with heart failure with reduced ejection fraction.
- To detect the sensitivities & specificities of TAPSE, PASP & RV strain to predict short term outcome in patients with heart failure with reduced ejection fraction.
Methodology
This was a prospective cohort study. The study was carried out at the Department of Cardiology and at the Echocardiography lab of the National Heart Foundation Hospital and Research Institute, Mirpur, Dhaka, Bangladesh. The patients were selected by consecutive sampling. This study was conducted from April, 2019 to May, 2020. A total number of 115 patients of both sexes were included in the study..
Inclusion criteria
Patient in heart failure with reduced ejection fraction (EF<40%).
Exclusion criteria
- Age<18 years
- Acute coronary syndrome
- Primary valvular heart disease
- Congenital heart disease
- Heart failure post valvular and congenital heart surgery
- Cardiac tamponade
- Aortic dissection
- Acute pulmonary embolism
- Severe pulmonary disease
- Right ventricular infraction
Study procedure
Enrolled patients were categorized into two groups according to right ventricular function assessed by RVFWS using 2D STE. Meticulous history was taken and detailed clinical examination was performed in each patient. Demographic data such as age, sex, height, weight was noted. Risk factors were recorded for all patients. Patients baseline 12 lead ECG was performed. Blood sample was taken for Blood sugar, Hb%, WBC, Serum creatinine, S. electrolytes, SGPT and NT- Pro. BNP. Echocardiography assessment using conventional 2D, LVEF measured by Simpson’s biplane method, TAPSE, PASP & 2D STE was done for all patients.
Statistical analysis
After processing of all available data, statistical analysis of their significance was done. Obtained data were expressed in frequency, percentage, mean and standard deviation as applicable. Comparison between groups was done by Student’s -test & Mann Whitney u test for continuous variables. Categorical data will be analyzed by chi-square test & Fisher's exact test. ROC curves were constructed for RVFWS, RVGLS, LVEF, TAPSE & PASP for predicting adverse outcome were identified. The whole analysis was done with the help of computer based SPSS version 23.
P-value of ≤ 0.05 was considered as statistically significant.
Results
Table 1: Distribution of the demographic variables among the patients with normal and abnormal RVFWS group. (N=115)
|
Demographic variables |
RVFWS |
P value |
|
|
Normal strain ≤-19 (n=54) |
Abnormal strain >-19 (n=61) |
||
|
Age (years) |
|||
|
≤40 Yrs. |
2(3.7) |
6(9.8) |
|
|
41-50 Yrs. |
14(25.9) |
15(24.6) |
|
|
51-60 Yrs. |
27(50.0) |
20(32.8) |
|
|
61-70 Yrs. |
10(18.5) |
18(29.5) |
|
|
>70 Yrs. |
1(1.9) |
2(3.3) |
|
|
Mean ± SD |
55.63 ± 8.27 |
56.46 ± 11.42 |
0.660 |
|
Male |
48(88.9) |
49(80.3) |
0.207 |
|
Female |
6(11.1) |
12(19.7) |
|
|
BMI (kg/m2) |
|||
|
Under weight |
1(1.9) |
1(1.6) |
|
|
Normal |
39(72.2) |
41(67.3) |
|
|
Over weight |
12(22.2) |
18(29.5) |
|
|
Obese |
2(3.7) |
1(1.6) |
|
|
Mean ± SD |
24.10 ± 2.70 |
23.93 ± 2.45 |
0.717 |
Table 1 showed distribution of the demographic variables according to patients with normal and abnormal RVWFS group. Maximum patients age was between 51 to 60 years in both normal and abnormal RVWFS group. There was no statistically significant difference observed of age, sex and BMI between normal and abnormal RVFWS group.
Table 2: Distribution of the clinical profiles among the patients with
normal and abnormal RVFWS group (N=115)
|
Clinical profile |
RVFWS |
P value |
|
|
Normal strain ≤-19 (n=54) |
Abnormal strain >-19 (n=61) |
||
|
Shortness of Breath |
|||
|
Yes |
53(98.1) |
61(100.0) |
0.470 |
|
No |
1(1.9) |
0(0.0) |
|
|
NYHA grading |
|||
|
II |
40(75.5) |
23(37.7) |
<0.001 |
|
III |
11(20.8) |
27(44.3) |
|
|
IV |
2(3.8) |
11(18.0) |
|
|
Edema |
|||
|
Positive |
2(3.7) |
5(8.2) |
0.445 |
|
Negative |
52(96.3) |
56(91.8) |
|
|
JVP Raised |
1(1.9) |
5(8.2) |
0.212 |
|
JVP Not raised |
53(98.1) |
56(91.8) |
|
|
Mean Heart rate |
81.22 ± 9.97 |
84.74 ± 10.31 |
0.066 |
|
Mean SBP |
114.44 ± 14.36 |
107.21 ± 15.07 |
0.010 |
|
Mean DBP |
75.56 ± 9.04 |
70.16 ± 9.91 |
0.003 |
Table 2 showed distribution of the clinical profile according to patients with normal and abnormal RVFWS group. 98.1% patients had shortness of breath in normal RVFWS and on the other hand, all (100.0%) patients had shortness of breath in abnormal RVFWS group. In shortness of breath patients with normal RVFWS group, 75.5% patients were NYHA grading II, 20.8% patients were III and only 2.8% patients were IV NYHA grading and on the others hand, patients with abnormal RVFWS group, 37.7% patients were NYHA grading II, 44.3% patients were III and 18.0% patients were IV NYHA grading. There was statistically significant difference observed between NYHA grading and RVFWS group. There was no statistically significant difference observed of edema, JVP & heart rate between normal and abnormal RVFWS group. The mean SBP of the patients with normal RVFWS was 114.44±14.36 mm of Hg and the patients with abnormal RVFWS was 107.21±15.07 mm of Hg. The mean DBP of the patients with normal RVFWS was 75.56±9.04 mm of Hg and the patients with abnormal RVFWS was 70.16±9.91 mm of Hg. There was statistically significant difference observed of blood pressure between normal and abnormal RVFWS groups.
Table 3: Distribution of the etiology, co-morbidities and risk profile among the patients with normal and abnormal RVFWS group. (N=115)
|
Etiology, Co-morbidities/ Risk profile |
RVFWS |
P value |
|
|
Normal Strain ≤-19 (n=54) |
Abnormal Strain >-19 (n=61) |
||
|
IHD |
48(88.9) |
49(80.3) |
0.207 |
|
Cardiomyopathy |
|||
|
ICM |
6(11.1) |
16(26.2) |
0.040 |
|
DCM |
2(3.7) |
12(19.7) |
0.009 |
|
DM |
24(44.4) |
30(49.2) |
0.612 |
|
Hypertension |
23(42.6) |
25(41.0) |
0.861 |
|
CKD |
6(11.1) |
13(21.3) |
0.142 |
|
Anemia |
1(1.9) |
7(11.5) |
0.065 |
|
Dyslipidemia |
29(53.7) |
24(39.3) |
0.123 |
|
Smoking |
32(59.3) |
36(59.0) |
0.979 |
Table 3 showed distribution of the presence of etiology, co- morbidities& risk profile according to the patients with normal and abnormal RVFWS group. 88.9% patients were diagnosed as IHD in normal RVFWS group and 80.3% patients were diagnosed as IHD in abnormal RVFWS group. 11.1% patients were diagnosed as ICM in normal RVFWS and 26.2% patients were diagnosed as ICM in abnormal RVFWS group. 3.7% patients were diagnosed as DCM in normal RVFWS and 19.7% patients were diagnosed as DCM in abnormal RVFWS group. ICM & DCM were more prevalent among patients with abnormal RVFWS group. There was no statistically significance difference observed between etiology, co-morbidities and risk factors and RVFWS except cardiomyopathy.
Table 4: Mean distribution of the biochemical parameters among the patients with normal and abnormal RVFWS group. (N=115)
|
Biochemical parameters |
RVFWS |
P value |
|
|
Normal strain ≤-19 (n=54) |
Abnormal strain >-19 (n=61) |
||
|
Hb (gm/dl) |
13.09 ± 1.87 |
12.63 ± 1.91 |
0.202 |
|
WBC (/cu mm) |
9438.89 ± 2805.28 |
9772.13 ± 3768.25 |
|
|
Mean Rank |
56.60 |
59.24 |
0.672 |
|
RBS (mmol/L) |
8.28 ± 2.96 |
8.36 ± 3.13 |
|
|
Mean Rank |
58.15 |
57.87 |
0.964 |
|
S. Creatinine (mg/dl) |
1.31 ± 0.48 |
1.41 ± 0.49 |
0.260 |
|
S. Electrolytes |
|||
|
Na |
137.56 ± 2.72 |
136.85 ± 3.49 |
0.230 |
|
K |
4.09 ± 0.52 |
4.10 ± 0.55 |
0.889 |
|
Cl |
99.02 ± 3.73 |
97.37 ± 3.83 |
0.021 |
|
SGPT (U/L) |
41.02 ± 17.76 |
68.10 ± 106.54 |
|
|
Mean Rank |
53.98 |
61.56 |
0.221 |
|
NT-ProBNP (pg/ml) |
2033.69 ± 1833.95 |
2228.08 ± 1623.60 |
|
|
Mean Rank |
52.66 |
62.73 |
0.106 |
Table 4 showed mean distribution of the biochemical parameters according to patients with normal and abnormal RVFWS group. The mean of Cl level was 99.02±3.73 mmol/L and 97.37±3.83 mmol/L in normal RVFWS and abnormal RVFWS group respectively. There was no statistically significant (p>0.05) difference observed of any of biochemical parameters between normal RVFWS and abnormal RVFWS group except Chloride level.
Table 5: Distribution of echocardiographic parameters among the patient with normal and abnormal RVFWS group. (N=115)
|
Echocardiographic parameters |
RVFWS |
P value |
|
|
Normal Strain ≤-19 (n=54) |
Abnormal strain >-19 (n=61) |
||
|
Conventional |
|||
|
LVEF (%) |
33.24 ± 4.46 |
30.33 ± 5.76 |
0.003 |
|
TAPSE (mm) |
16.09 ± 3.19 |
13.03 ± 3.53 |
<0.001 |
|
PASP |
(n=26) 40.12 ± 14.64 |
(n=42) 42.62 ± 11.82 |
|
|
Mean Rank |
30.50 |
36.98 |
0.189 |
|
Strain |
(n=54) |
(n=61) |
|
|
RVGLS (%) |
-17.08 ± 3.56 |
-8.99 ± 5.79 |
|
|
Mean Rank |
32.55 |
80.53 |
<0.001 |
|
RVFWS (%) |
-23.50 ± 3.41 |
-10.61 ± 8.10 |
|
|
Mean Rank |
27.50 |
85.00 |
<0.001 |
Table 5 showed mean distribution of the echocardiographic parameters according to patients with normal and abnormal RVFWS group. The mean of LVEF level were 33.24±4.46 and 30.33±5.76 in normal RVFWS and abnormal RVFWS group respectively. The mean of TAPSE level were 16.09±3.19 mm and 13.03 (±3.53) mm in normal RVFWS and abnormal RVFWS respectively. The mean of PASP level were 40.12±14.64 and 42.62 (±11.82) in normal RVFWS and abnormal RVFWS group respectively. The mean rank of PASP were 30.50 and 36.98 in normal and abnormal RVFWS group. The mean of RVGLS level were -17.08±3.56 and -8.99±5.79 in normal RVFWS and abnormal RVFWS group respectively. The mean rank of RVGLS were 32.55 and 80.53 in normal and abnormal RVFWS group respectively. The mean of RVFWS level were -23.50
±3.41 and -10.61±8.10 in normal RVFWS and abnormal RVFWS group respectively. The mean rank of RVFWS were 27.50 and 85.00 in normal and abnormal RVFWS group respectively. There was statistically significant difference observed of echocardiographic parameters between normal and abnormal RVFWS group except PASP.
Table 6: Distribution of the medication at discharge among the patients with normal and abnormal RVFWS group (N=115)
|
RVFWS |
|||
|
Discharge medication |
Normal Strain ≤-19 |
Abnormal Strain >-19 |
P value |
|
(n=54) |
(n=61) |
||
|
Beta blocker |
49(90.7) |
51(83.6) |
0.257 |
|
ACEI/ARB |
43(79.6) |
43(70.5) |
0.260 |
|
ARNI |
7(13.2) |
12(19.7) |
0.356 |
|
Ivabradine |
13(24.1) |
17(27.9) |
0.644 |
|
MRA |
54(100.0) |
59(96.7) |
0.497 |
|
Diuretics |
53(98.1) |
61(100.0) |
0.470 |
Table 6 showed distribution of the medication at discharge according to patients with normal and abnormal RVFWS group. There was no statistically significance difference observed between medication at discharge among the patients with normal and abnormal RVFWS group.
Table 7: Distribution of the Revascularization by PTCA and influenza vaccination among the patient with normal and abnormal RVFWS group (N=115)
|
PTCA & influenza vaccination |
RVFWS |
p value |
|
|
Normal Strain ≤-19 (n=54) |
Abnormal Strain >-19 (n=61) |
||
|
PTCA |
9(16.7) |
10(16.4) |
0.969 |
|
Influenza Vaccination |
3(5.6) |
7(11.5) |
0.331 |
Table 7 showed the prevalence of revascularization done by PTCA and influenza vaccination given among the groups. There was no statistically significant difference observed between prevalence of revascularisation and influenza vaccination among the groups.
Table 8: Distribution of the outcomes at follow up among the patients with normal and abnormal RVFWS group (N=115)
|
Outcome variables |
RVFWS |
p value |
|
|
Normal strain ≤-19 (n=54) |
Abnormal Strain >-19 (n=61) |
||
|
Re-hospitalization due to Heart Failure |
5(9.3) |
21(34.4) |
0.001 |
|
Death |
4(7.4) |
13(21.3) |
0.036 |
|
Either hospitalization or death |
5(9.3) |
24(39.3) |
<0.001 |
Table 8 showed distribution of the patients according to outcome variables by groups. In normal RVFWS group, 9.3% patients were re-hospitalized and in abnormal RVFWS group, 34.4% patients were re hospitalized due to heart failure. In normal RVFWS group, 7.4% patients were death and 21.3% patients were death in abnormal RVFWS group. In normal RVFWS group, 9.3% patients were recorded either re-hospitalization or death and in abnormal RVFWS group, 39.3% patients were recorded either re-hospitalization or death. There was statistically significant difference observed between outcome variables and groups. i.e. re-hospitalization due to heart failure and death were higher in abnormal RVFWS than normal RVFWS group.
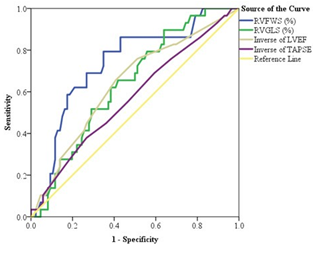
Table 9: Area Under the ROC (AUROC) curve of RVFWS, RVGLS, LVEF and TAPSE for the prediction of outcome in patients of heart failure (N=115)
|
Test Result Variables |
Area |
95% CI |
p value |
|
RVFWS (%) |
0.736 |
0.631-0.840 |
<0.001 |
|
RVGLS (%) |
0.640 |
0.532-0.747 |
0.025 |
|
Inverse of LVEF |
0.637 |
0.520-0.753 |
0.028 |
|
Inverse of TAPSE |
0.571 |
0.449-0.692 |
0.257 |
Table 9 & figure 1 showed the ROC and Area Under ROC curve of RVFWS, RVGLS, LVEF and TAPSE for the prediction of outcome variables in patients of heart failure. Area Under the ROC curve of RVFWS was 0.736, RVGLS was 0.640, Inverse of LVEF was 0.637 and Inverse of TAPSE was 0.571. Considering these variables, Area Under the ROC curve of RVFWS was higher than other variables.
i.e. RVFWS was the best predictor of outcome variables in patients of heart failure. There was statistically significant difference observed of Area Under the ROC curve of RVFWS, RVGLS and inverse of LVEF with true area.
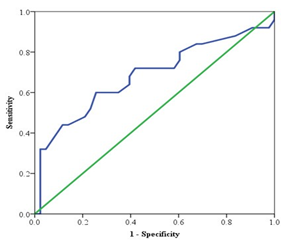
Table 10: Area Under the ROC (AUROC) curve of PASP for the prediction of outcome in patients of heart failure (n=68)
|
Test Result Variable |
Area |
95% CI |
p value |
|
PASP |
0.682 |
0.540-0.824 |
0.013 |
Table 10 and figure 2 showed the ROC curve and Area Under ROC curve of PASP for the prediction of outcome variables in patients of heart failure. Area Under the ROC curve of PASP was 0.682, There was statistically significant (p<0.05) difference observed of Area Under the ROC curve of PASP with true area.
Table 11: Validity test of different cut of values of RVFWS.
(N=115)
|
Parameters |
Cut of values |
||
|
-17.00 |
-19.00 |
-19.25 |
|
|
True positive |
23 |
24 |
25 |
|
False positive |
30 |
37 |
37 |
|
False negative |
6 |
5 |
4 |
|
True negative |
56 |
49 |
49 |
|
p value |
<0.001 |
<0.001 |
<0.001 |
|
Sensitivity |
79.3% |
82.8% |
86.2% |
|
Specificity |
65.1% |
57.0% |
57.0% |
|
Accuracy |
68.7% |
63.5% |
64.3% |
Table 11 showed the validity test of different cut of values of RVFWS. In cut of value of -17.00, sensitivity, specificity & accuracy were 79.3%, 65.1% & 68.7% respectively. In cut of value of -19.00, sensitivity, specificity & accuracy were 82.8%, 57.0% & 63.5% respectively. In cut of value of -19.25, sensitivity, specificity & accuracy 86.2%, 57.0% & 64.3% respectively. Though positive result of RVFWS was influence the bad outcome in patients of heart failure, increasing the cut of value of RVFWS, sensitivity was decreasing and specificity was increasing. At cut of value of -19.25, sensitivity was higher and specificity was lower and cut of value of -17.00, sensitivity was lower and specificity was higher. But accuracy was higher at cut of value of -17.00. So, we could refer the cut of value 17.00 from our data.
Table 12: Validity test of different Echocardiographic parameters. (N=115)
|
Parameters |
Echocardiographic parameters |
||
|
TAPSE (N=115) |
PASP (n=68) |
RVGLS (N=115) |
|
|
True positive |
22 |
18 |
26 |
|
False positive |
58 |
25 |
59 |
|
False negative |
7 |
7 |
3 |
|
True negative |
28 |
18 |
27 |
|
p value |
0.394 |
0.253 |
0.026 |
|
Sensitivity |
75.9% |
72.0% |
89.7% |
|
Specificity |
32.6% |
41.9% |
31.4% |
|
Accuracy |
43.5% |
52.9% |
46.1% |
Table 12 showed the validity test of different Echocardiographic parameters of RV function. In TAPSE, sensitivity, specificity & accuracy were 75.9%, 32.6% & 43.5% respectively. In PASP, sensitivity, specificity & accuracy were 72.0%, 41.9% & 52.9% respectively. In RVGLS, sensitivity, specificity & accuracy were 89.7%, 31.4% & 46.1% respectively. The test results of validity test of TAPSE, PASP and RVGLS were lower than RVFWS. So, RVFWS was the best predictor of adverse outcome in patients with heart failure with reduce ejection fraction.
Discussion
The main findings derived from the data analysis of the present study was that the abnormal RVFWS using 2D STE in patients with systolic heart failure patients was the best echo predictor of short term outcome. In normal RVFWS group, 9.3% patients and in abnormal RVFWS group, 39.3% patients had either re-hospitalization or death. There was statistically significant difference observed between outcome variables and groups. Adverse outcome due to heart failure re-hospitalization and death was higher in patients with abnormal RVFWS than patients with normal RVFWS group. Abnormal RVFWS group had lower LVEF, TAPSE, RVGLS, worse NYHA functional class, Lower SBP & DBP, abnormal RVFWS was more common in ICM and DCM population than normal RVFWS. Considering the independent variables, area under the ROC curve of RVFWS was higher than other variables. Area under the ROC curve of RVFWS was 0.736. RVFWS was the best echo predictor of short term outcome in patients of heart failure with reduced ejection fraction. In present study the mean age of the patients with normal and abnormal RVFWS groups were 55.63±8.27 and 56.46±11.42 respectively. There was no statistically significant difference observed of age between normal RVFWS and abnormal RVFWS group. Maximum patient’s age was between 51 to 60 years in both normal and abnormal RVWFS groups. In normal RVFWS group, 88.9% patients were male and 11.1% patients were female and in abnormal RVFWS group, 80.3% patients were male and 19.7% patients were female. In Bangladesh and developed countries various studies showed that, the female patients formed a small percentage. Rahman, et al. (2014) [36] found 25%. The mean BMI of the patients with normal RVFWS group was 24.10±2.70 kg/m2 and the patients with abnormal RVFWS group was 23.93±2.45 kg/ m2. 98.1% patients had shortness of breath in normal RVFWS group and on the other hand, all (100.0%) patients had shortness of breath in abnormal RVFWS group. In shortness of breath patients with normal RVFWS group, 75.5% patients were NYHA grading II, 20.8% patients were III and only 2.8% patients were IV NYHA grading and on the others hand, patients with abnormal RVFWS group, 37.7% patients were NYHA grading II, 44.3% patients were III and 18.0% patients were IV NYHA grading. Patients with more severe NYHA grading were in group with abnormal RVFWS compared with normal RVFWS group. From normal RVFWS group to abnormal RVFWS group worse NYHA functional class due to RV dysfunction patient’s functional capacity is more reduced than normal RV function due to oxygenation is more reduced in abnormal RVFWS group. Rahman, et al. (2014) [36] showed 98% patients with heart failure presented with shortness of breath in Bangladesh. In normal RVFWS group, only 3.7% patients had positive edema and in abnormal RVFWS group, 8.2% patients had positive edema. There was no statistically significant difference observed between edema and RVFWS. Only1.9% and 8.2% patients were raised JVP in normal RVFWS and abnormal RVFWS respectively. Rahman, et al. (2014) [36] showed that 40% of this patients presented with ankle edema, 25% presented with raised JVP which is discordant to my result may be due to selection of patient during discharge, during discharge edema and JVP may be subsided due to ongoing treatments. The mean heart rate of the patients in normal RVFWS group was 81.22±9.97 b/minute and the patients in abnormal RVFWS group was 84.74±10.31 b/minute. There was no statistically significant difference observed on heart rate between normal and abnormal RVFWS group. Hamada-Harimura, et al. (2018) [37] reported SBP & DBP, 117±20 & 65±13 mm Hg respectively. In developed counties, Damy, et al. (2012) [23] showed that, ischemic heart disease was present in 65% in heart failure patients. 11.1% patients had ICM in normal RVFWS and 26.2% patients had ICM in abnormal RVFWS group. 3.7% patients had DCM in normal RVFWS and 19.7% patients had DCM in abnormal RVFWS group. ICM and DCM were more in abnormal RVFWS than normal RVFWS because in cardiomyopathy, same pathophysiological process involves in both ventricles simultaneously. Rahman, et al. (2014) [36] showed that, 38.03% was diagnosed as Ischemic Cardiomyopathy in Bangladesh. Diabetics mellitus patients were present in 44.4% of normal RVFWS and 49.2% were in abnormal RVFWS group. Prevalence of hypertension was 42.6% in normal RVFWS and 41.0% in abnormal RVFWS group. Hamada-Harimura, et al. (2018) [37] observed that 57.3 % of their patients were hypertensive and 44.3% were diabetics. The mean of Hb level were 13.09 ±1.87 mg/dl and 12.63±1.91 mg/dl, the mean of serum creatinine level was 1.31±0.48 mg/dl and 1.41±0.49 mg/dl, the mean of NTpro. BNP level was 2033.69±1833.95 pg/ml and 2228.08±1623.60 pg/ml in normal RVFWS and abnormal RVFWS group respectively. The mean of LVEF level were 33.24±4.46 and 30.33±5.76, TAPSE level were 16.09±3.19 mm and 13.03±3.53 mm and RVGLS level were -17.08±3.56 and-8.99±5.79 in normal RVFWS and abnormal RVFWS group respectively. LVEF, TAPSE, and RVGLS were significantly influenced by the normal or abnormal RVFWS group. This study detected that more abnormal values of LVEF, TAPSE, RVGLS present in abnormal RVFWS group. No similar type study done previously to compare LVEF, TAPSE, PASP, RVGLS in between normal and abnormal RVFWS group. In RV dysfunction patients PASP is difficult to calculate using TR jet because TR jet is reduced in RV dysfunction patients.
Cameli, et al. (2013) [38] showed that, the prediction of cardiovascular events was greatest for RV free-wall longitudinal strain. Kossaify, (2015) [39] conclude that use of STE to directly monitor RV myocardial function may allow early sensitive detection of subclinical myocardial dysfunction with better risk stratification and timely institu¬tion of therapy. Mean values of RV-GLS were significantly lower than those of RVFWLS & both RV-GLS and RVFWLS were similarly able to significantly predict mortality. The predictive value of RV free-wall longitudinal strain was higher than that of RV GLS, TAPSE and tricuspid s’ Cameli, 2012 [40] showed that not RV-GLS but RVFWLS was associated with cardiac events. Their study showed that RV-GLS was more closely associated with LV systolic function than RVFWLS, these results collectively indicate that RV-fwLS is a more accurate marker of intrinsic RV systolic function and less reflective LV systolic function than RV-GLS. However, degree of RV myocardial fibrosis was poorly correlated with TAPSE. In the present study RVFWS with a cut of value of -19.00, predicted short term outcome in patients with HF with reduced ejection fraction with a sensitivity, specificity & accuracy of 82.8%, 57.0% & 63.5% respectively with the Cut of value of -17.00, sensitivity, specificity & accuracy of 79.3%, 65.1% & 68.7% respectively. Sensitivity was similar but specificity was higher & accuracy also higher at cut of value of -17.00. Therefore, we could refer the cut of value -17.00 from our data. Adverse events were best predicted using ROC curve. Hamada-Harimura, et al. (2018) [37] showed that patients with impaired RVFWLS, (≥-13.1%) reached the primary composite end point when compared with preserved RVFWLS. In the present study area under the ROC curve of RVFWS was 0.736, PASP was 0.682, RVGLS was 0.640, Inverse of LVEF was 0.637 and Inverse of TAPSE was 0.571, there was statistically significant difference observed of area under the ROC curve of RVFWS, RVGLS and inverse of LVEF & PASP with true area. Cameli, et al. (2013) [38] showed that the prediction of cardiovascular events was greatest for RV free-wall longitudinal strain, significantly higher than for other variables which is concordant with our results. A value of RVFWS ≥-15.3% in patients with preserved TAPSE was associated with an adjusted 2-fold increased risk of events. TAPSE measures displacement and is therefore subject to translational error of cardiac motion, which can be influenced by several variables including heart rate, respiratory rate, chamber size, and body size. As a measure of deformation, strain analysis strives to provide a method of contractile function assessment that corrects for translational error and is less dependent on imaging plane angle. Two dimensional RV systolic longitudinal strains calculated using speckle-tracking echocardiography has emerged as a feasible and reproducible measure of RV systolic function [41]. So, RVFWS was the best echo predictor of worse outcome in patients with heart failure with reduced ejection fraction.
Conclusion
The present study showed that abnormal RVFWS measurement using 2D STE was a best predictor of adverse short term outcome in patients with heart failure with reduced ejection fraction. RVFWS can actually assess RV function and better predictor of outcome than conventional RV functional assessment parameters like, TAPSE & PASP. RVFWS assessment help to early and accurately predict outcome which will reclassify HF risk score and help to intensify its management.
Study Limitations
Although the result of the study supports the hypothesis, there were some limiting factors which might have an effect on the results.
- Data was collected from single tertiary hospital and may not reflect general population.
- Only patients with good image quality were included in this
- Inter-vendor variability and frequent up gradation of the speckle tracking software results in changes to reference
Recommendations
RVFWS measurement using 2D speckle tracking echocardiography might play a role in the risk stratifications in HFrEF patients in addition to conventional RV assessment in routine clinical practice. Large scale, randomized and multicenter studies are needed to validate the findings of present study. If the utility of RVFWS is supported by future studies, this may be added to the existing modalities for evaluation of Right ventricular systolic dysfunction in patients with Heart failure with reduced ejection fraction.
Ethical Issue
The ethical clearance of the study was taken from the Institutional Ethical Review Board of National Heart Foundation Hospital and Research Institute.
References
- Ponikowski P, Anker SD, Alhabib KF, Cowie MR, Force, et al.Heart failure: preventing disease and death ESC Heart Failure 1 (2014): 4-15.
- Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure: The Rotterdam Study. European Heart Journal 25 (2004): 1614-1619.
- Mosterd A, Hoes AW. Clinical epidemiology of heart Heart 93 (2007): 1137-1146.
- Majumder Management of heart failure an update. Bangladesh Medical Journal 43 (2014): 36-45.
- Kabiruzzaman M, Malik FN, Ahmed N, Badiuzzaman M, Choudhury SR, et al. Burden of heart failure patients in a tertiary level cardiac hospital. Journal of Bangladesh College of Physicians and Surgeons 28 (2010): 24-29.
- Murninkas D, Alba AC, Delgado D, McDonald M, Billia F, et Right ventricular function and prognosis in stable heart failure patients. Journal of Cardiac Failure 20 (2014): 343-349.
- Wright SP, Verouhis D, Gamble G, Swedberg K, Sharpe N, et Factors influencing the length of hospital stay of patients with heart failure. European Journal of Heart Failure 5 (2003): 201-209.
- Allen LA, Smoyer Tomic KE, Wilson KL, Smith DM, Agodoa The inpatient experience and predictors of length of stay for patients hospitalized with systolic heart failure: comparison by commercial, Medicaid, and Medicare payer type. Journal of Medical Economics 16 (2013): 43-54.
- Whellan DJ, Zhao X, Hernandez AF, Liang L, Peterson ED, et al. Predictors of hospital length of stay in heart failure: findings from Get with the Guidelines. Journal of Cardiac Failure 17 (2011): 649-656.
- He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, et Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Archives of Internal Medicine 161 (2001): 996- 1002.
- Tsutsui H, Tsuchihashi-Makaya M, Kinugawa S, Goto D, Takeshita A, et Characteristics and outcomes of patients with heart failure in general practices and hospitals. Circulation Journal 71 (2007): 449-454.
- Taylor CJ, Ryan R, Nichols L, Gale N, Hobbs FR, et al. Survival following a diagnosis of heart failure in primary Family Practice 34 (2017): 161-168.
- Zaya M, Phan A, Schwarz Predictors of re- hospitalization in patients with chronic heart failure. World Journal of Cardiology 4 (2012): 23-30.
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, et al. 2005 WRITING COMMITTEE MEMBERS; Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, et FOCUSED UPDATE WRITING GROUP MEMBERS. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation 119 (2009): e391-e479.
- Ho KK, Anderson KM, Kannel WB, Grossman W, Levy
- Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 88 (1993): 107-115.
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: European Heart Journal 37 (2016): 2129-2200.
- Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, et The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. Journal of the American College of Cardiology 42 (2003): 736-742.
- Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, et al. Predictors of mortality and morbidity in patients with chronic heart failure. European Heart Journal 27 (2006): 65-75.
- Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, et Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. Journal of the American College of Cardiology 37 (2001): 183-188.
- Pennell Cardiovascular magnetic resonance. Circulation 121 (2010): 692-705.
- Aktas MK, Kim DD, Mcnitt S, Huang DT, Rosero SZ, et al. Right Ventricular Dysfunction and the Incidence of Implantable Cardioverter-Defibrillator Therapies. Pacing and Clinical Electrophysiology 32 (2009): 1501-1508.
- William V, El Kilany W. Assessment of right ventricular function by echocardiography in patients with chronic heart failure. The Egyptian Heart Journal 70 (2018): 173-179.
- Damy T, Kallvikbacka-Bennett A, Goode K, Khaleva O, Lewinter C, et Prevalence of, associations with, and prognostic value of tricuspid annular plane systolic excursion (TAPSE) among out-patients referred for the evaluation of heart failure. Journal of Cardiac Failure 18 (2012): 216-225.
- Ling LF, Marwick TH. Echocardiographic assessment of right ventricular function: how to account for tricuspid regurgitation and pulmonary JACC: Cardiovascular Imaging 5 (2012): 747-753.
- Kjaergaard J, Iversen KK, Akkan D, Møller JE, Køber LV, et Predictors of right ventricular function as measured by tricuspid annular plane systolic excursion in heart failure. Cardiovascular Ultrasound 7 (2009): 51.
- Nagy VK, Széplaki G, Apor A, Kutyifa V, Kovács A, et Role of right ventricular global longitudinal strain in predicting early and long-term mortality in cardiac resynchronization therapy patients. PloS one 10 (2015): e0143907.
- Focardi M, Cameli M, Carbone SF, Massoni A, De Vito R, et al. Traditional and innovative echocardiographic parameters for the analysis of right ventricular performance in comparison with cardiac magnetic European Heart Journal-Cardiovascular Imaging 16 (2015): 47-52.