Assessment of Residents Level of Knowledge in Pharmacovigilance
Article Information
Bouraoui OUNI1, Khadija MANSOUR2, Fatma HARRATH1, Raoudha SLIM1, Imen AKKARI3, Neila FATHALLAH1, Nesrine BENSAYED4
1Department of Pharmacology. Faculty of Medicine, University of Sousse, Tunisia
2Department of Pharmacology, EPS Fattouma Bourguiba, Faculty of Medicine, University of Monastir, Tunisia
3Department of Gastroenterology, Farhat Hached hospital, Sousse, Tunisia
4Department of Hematology, Farhat Hached hospital, Sousse, Tunisia
*Corresponding Author Bouraoui OUNI, Department of Pharmacology. Faculty of Medicine, University of Sousse, Tunisia
Received: October 18, 2023; Accepted: November 27, 2023 Published: January 12, 2024
Citation: Bouraoui OUNI, Khadija MANSOUR, Fatma HARRATH, Raoudha SLIM, Imen AKKARI, Neila FATHALLAH, Nesrine BENSAYED. Assessment of Residents Level of Knowledge in Pharmacovigilance. Archives of Clinical and Medical Case Reports 8 (2024): 05-09.
Share at FacebookAbstract
Introduction: Pharmacovigilance is a discipline aimed at monitoring, evaluating, managing, and pre-venting the adverse effects resulting from the use of medications. It is crucial in everyday medical practice and contributes to ensuring patient safety by identifying and managing the risks associated with drugs.
Objective: To assess the level of knowledge among residents in Tunisia regarding pharmacovigilance, with the aim of proposing a strategy to raise awareness in this field.
Materials and Methods: A cross-sectional observational study was conducted to evaluate the knowledge of residents (across all specialties) in pharmacovigilance. Data were collected using an anonymous questionnaire that included personal and professional information, knowledge about pharmacovigilance, reasons for underreporting adverse effects, and suggested corrective actions. The variables studied encompassed personal and professional factors, pharmacovigilance training, information sources, knowledge, and attitudes towards the reporting system. Statistical analysis was performed using the SPSS software, and a literature search was conducted to support the study.
Results: The survey conducted among 511 residents revealed that 65% were females and 35% were males. The most common medical specialty was general medicine with 36.6% of participants, followed by family medicine (34.6%) and surgical specialties (28.8%). The majority of residents were in their first year (35%), followed by second year (24.9%) and third year (23.7%). Regarding the residents’ knowledge of pharmacovigilance, 87.1% had heard about it during their medical studies. The majority (83%) accurately defined pharmacovigilance as the detection, evaluation, understanding, and prevention of adverse effects. However, almost all residents were not aware of the Tunisian pharmacovigilance system (53.2%) or the Sousse Regional Pharmacovigilance Center (30.1%). Regarding the residents’ knowledge of pharmacovigilance, 61.4% knew that reporting adverse effects was mandatory, but only 29.2% were aware of the healthcare professionals involved in this reporting. The majority of residents were unfamiliar with causality assessment methods in pharmacovigilance (97.3%) and the timeframe for reporting a serious adverse effect (96.5%). Concerning the reporting of adverse effects, most residents knew they should report all types of adverse effects but believed they didn’t report enough (83.6%). The majority of residents reported adverse effects to the national/regional pharmacovigilance center (91.2%).
Keywords
Pharmacovigilance; Resident of médecine; Adverse drug reaction
Article Details
Introduction
With the increasingly widespread use of pharmacological treatments, easy access to healthcare, and a rise in self- medication rates, pharmacovigilance has become an essential discipline in public health. It allows for the collection, analysis, and prevention of ad-verse effects of medications, which can lead to severe health issues for patients and result in significant economic costs [1]. Pharmacovigilance also helps optimize the use of medications by identifying high- risk populations, enhancing the quality of available drug information, and promoting their rational use. Ultimately, pharmacovigilance fosters patient trust in the healthcare system by ensuring the safety and efficacy of medications.
Key stages of pharmacovigilance include detection, recording and reporting, assessment, communication, and risk management measures. Detecting adverse effects can be achieved through various sources, such as spontaneous reports from healthcare professionals, clinical studies, post-market trials, pharmacodependence data, and electronic reporting systems. Once detected, adverse effects are recorded and reported to relevant authorities. The assessment of adverse effects involves a detailed analysis of available data, aiming to determine their severity, frequency, and relationship with the use of the medication. This might require further studies, such as observational studies or pharmacopidemiological analyses, to assess potential risks in specific populations. Un siècle d’événements tragiques a joué un rôle essentiel dans le façonnement des structures et des processus actuels de développement des médicaments, notamment en ce qui concerne la pharmacovigilance (PV) [2].Citons la tragédie de la thalidomide en 1961 qui a déclenché une chaîne d’activités faisant partie d’un effort mondial visant à éviter une récidive de telles situations [3].
En effet, dans les années 1950 et 1960, la thalidomide était un médicament largement prescrit pour soulager les nausées et les insomnies chez les femmes enceintes. Cependant, ce médicament est considéré comme responsable de la naissance de bébés présentant des malformations congénitales connues sous le nom de phocomélie (notamment l’absence ou l’atrophie des membres supérieurs et parfois inférieurs). Cette tragédie a conduit à une prise de conscience mondiale sur la nécessité de garantir la sécurité des médicaments. Les conséquences désastreuses de la thalidomide ont incité les autorités réglementaires à renforcer leurs exigences en matière d’essais cliniques et de surveillance des médicaments [4,5]. Studying the knowledge of residents regarding pharmacovigilance holds paramount importance in ensuring patient safety, enhancing clinical practice, and contributing to medical education. This approach can have a significant impact on public health by bolstering physicians’ understanding of pharmacovigilance, thereby promoting more informed utilization of medications and improved management of associated risks.
Methods
In the context of this study, a cross-sectional analytical observational study was conducted among students in the third cycle of medical studies in the regions of Sousse, Kairouan, Sidi-Bouzid, and Kasserine. The studied population included individuals currently enrolled in the third cycle of medical studies. Students encountered within hospitals during the data collection period who agreed to participate were included in the study. Those absent during the data collection period or who refused to participate were excluded from the study. The study spanned a duration of five months, starting from April 1st and concluding on August 30th, 2022. It covered the regions of Sousse, Kairouan, Sidi-Bouzid, and Kasserine.
We utilized an anonymous questionnaire to gather data from medical students in the third cycle across the four medical faculties in Tunisia, who met the specified inclusion criteria. The questionnaire underwent pretesting and validation. It comprised sections on participants’ personal and professional details, knowledge regarding pharmacovigilance, reasons behind underreporting, and corrective actions.
Statistical analysis
The literature review was conducted using the databases Pub Med, Science Direct, and EM Consulte, as well as the repositories of theses from both Tunisia and abroad. The organization and management of references were handled using the software Zotero.
Results
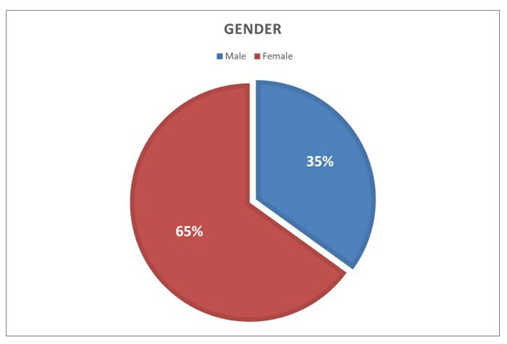
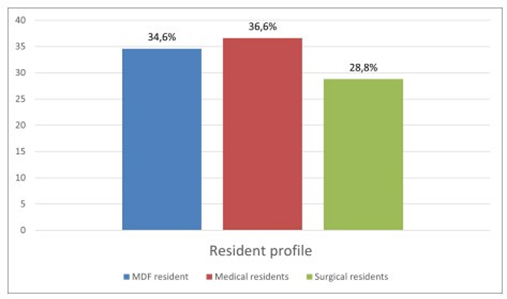
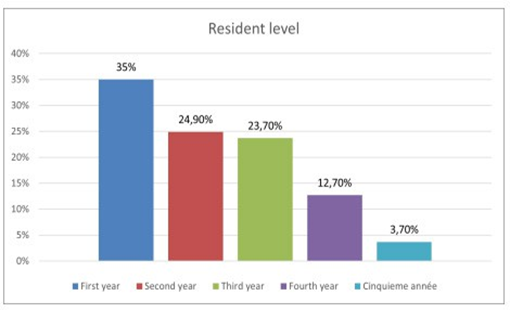
Among 750 residents were working in the Sousse region. Only 511 participated in our study, with a participation rate of 68%. Out of the 511 residents who responded to the questionnaire, 332 were women (accounting for 65%) and 179 were men (35%), resulting in a gender ratio of 0.53 (Figure 1). Figure 2 displays the distribution of residents according to their specialties, indicating a predominance of the medical specialty in our population, with a frequency of 187 participants (equivalent to 36.6%). This was followed by Family Medicine with 177 participants (34.6%), and finally, the surgical specialty, which encompassed 147 participants (28.8%), for our study, we observed a predominance of the first year of specialty level, with 197 participants, accounting for 35% (Figure 3). For residents’ knowledge of pharmacovigilance, we observed that Five hundred and eight residents surveyed had already heard of pharmacovigilance. Four hundred and forty-five during their medical studies (87.1%).52 (10.2%) during internships. Five residents had heard about pharmacovigilance from the press (1%) and 6 (1.2%) from one or more colleagues. (Table 1).
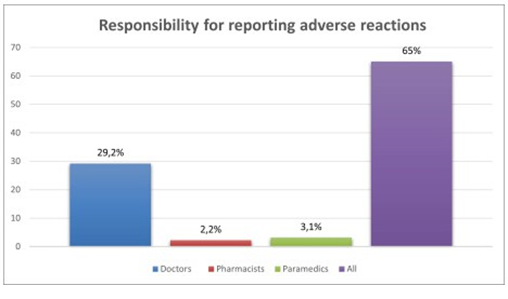
The analysis of residents’ knowledge of which healthcare professionals should report adverse events showed that 149 participants (29.2%) thought it was only the role of doctors 16 (3.1%) thought it was the role of paramedics only 11 thought it was the role of pharmacists and the remaining 332 (65%) participants reported that it was the role of all the healthcare professionals mentioned (Doctors, Pharmacists, Paramedics) (Figure 4) Four hundred thirty residents (84.1%) were able to recognize the severity criteria of an adverse effect, including death, life-threatening conditions, hospitalization or prolonged hospital stay, incapacity or disability, and birth defects in a baby born to a woman exposed to a medication during pregnancy.
Thirty-nine residents (7.6%) felt that death was a criterion for the severity of an adverse event, and 25 (4.9%) felt that life-threatening illness was also a criterion. Hospitalization or prolongation of hospitalization and incapacity or disability were considered to be criteria for the severity of an adverse event by 0.2% and 0.6% of residents respectively. Finally, the presence of malformations in the baby of a woman exposed to a drug during pregnancy was a criterion of adverse reaction severity for 0.8% of residents. The current state of pharmacovigilance and the causes of under-reporting of adverse reactions (Figure 5).
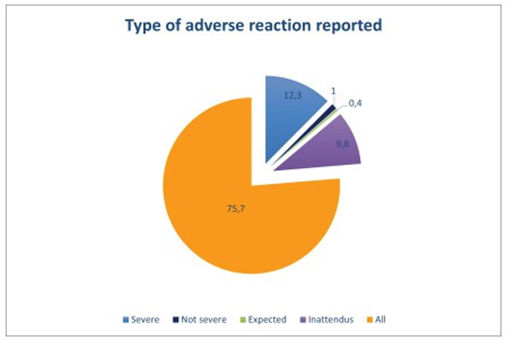
According to our study Three hundred and eighty- seven (75.7%) residents knew that they should report all adverse events, whether serious, non-serious, expected or unexpected, Sixtythree (12.3%) of residents were convinced that they should report serious adverse events and 50 (9. 8%) thought they should report unexpected adverse events, Only 5 (1%) thought they should report non-serious adverse events and 2 (0.4%) expected adverse events, Four hundred and twenty-seven residents (83.6%) thought they did not report enough adverse events. Table 2 represents the main reasons for residents’ non-reporting of adverse effects. we have suggested a few ways of improving the reporting of undesirable effects:
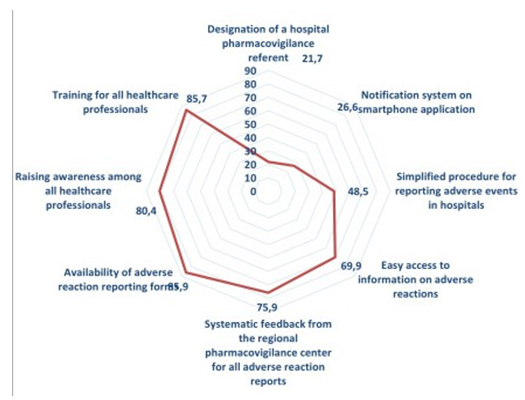
The strategy most suggested concerned the availability of adverse event reporting forms (85.9%), followed by training for all healthcare professionals (85.7%), then awareness- raising for all healthcare professionals (80.4%) and systematic feedback from the CRPV for all adverse event reports (also 75.9%), then easy access to information on adverse events (69.9%). Secondly, residents also suggested simplifying the procedure for reporting adverse reactions to the hospital (48.5%), a reporting system on a Smartphone application (26.6%) and finally the appointment of a pharmacovigilance referent at the hospital (21.7%) (Figure 6).
|
Because adverse reactions are known |
115 |
22.5 |
|
Because residents don’t know what to do |
92 |
18 |
|
Because it takes too long |
63 |
12.3 |
|
Because residents feel that reporting ARs is not their role |
9 |
1.8 |
|
Because residents have not encountered an AR |
23 |
4.5 |
|
Because imputability is difficult to prove |
33 |
6.5 |
Table 1: Distribution of residents according to the first time they
heard about PV.
|
The first time residents heard about pharmacovigilance was: |
Headcount |
Percentage (%) |
|
During medical studies |
445 |
87.1 |
|
During internships |
52 |
10.2 |
|
From the press |
5 |
1 |
|
From one or more colleagues |
6 |
1.2 |
|
Never |
3 |
0.6 |
|
Total |
511 |
100 |
Table 2: Causes of under-reporting of adverse events by residents.
|
Causes of under-reporting of adverse reactions by physicians |
Headcount |
Percentage (%) |
|
Because adverse reactions are frequent |
36 |
7 |
|
Because adverse reactions are benign |
126 |
24.7 |
Discussion
The cross-sectional analytical observational study conducted among postgraduate medical students in the regions of Sousse, Kairouan, Sidi-Bouzid, and Kasserine to assess their knowledge of pharmacovigilance has yielded interesting results. The collected data indicated that the students’ knowledge of pharmacovigilance was moderate, with significant variations across the different studied regions.
Our study primarily relied on fixed variables that enabled us to analyze the data based on gender, level, and residents’ profiles. The study results might indicate that women have a better understanding of pharmacovigilance than men. This trend could be attributed to the fact that women are more health-conscious and likely to seek information and advice about medications before using them. The pharmacovigilance knowledge among residents is moderate, with gaps in certain key areas such as reporting adverse effects and managing risks associated with medication use. This information could be used to guide pharmacovigilance training policies and contribute to improving patient safety.
The analysis of data regarding residents’ pharmacovigilance practices during their clinical training suggests that improvements are necessary to ensure that residents apply pharmacovigilance principles in their clinical practice. Providing ongoing pharmacovigilance training to residents and implementing protocols to ensure proper pharmacovigilance practices in healthcare facilities would be important steps to consider [6]. Our results could also include an analysis of the main causes for non-reporting of adverse effects by residents. These causes could encompass a lack of knowledge and skills in pharmacovigilance, absence of prior training in the field, as well as administrative and logistical barriers. The findings could be used to formulate recommendations for training and raising awareness among residents about pharmacovigilance, as well as identifying obstacles to adverse effects reporting and proposing solutions to overcome them.
Regarding the legal age status of residents, there was no significant correlation with their knowledge of pharmacovigilance or their practice of adverse effects reporting. This suggests that all residents require proper pharmacovigilance training, regardless of their experience or status.
Numerous studies are available on PubMed and Google Scholar that explore pharmacovigilance knowledge and practices among physicians and residents. Some of these studies present results similar to our conducted study, while others reveal differing outcomes based on the studied populations, methodologies employed, and countries of origin.
A study conducted in France in 2016 [7] revealed that the majority of medical residents had heard of pharmacovigilance but possessed limited knowledge about adverse effects reporting procedures. The authors recommended ongoing training to enhance the pharmacovigilance knowledge of interns.
A study carried out in Italy in 2010 [8] demonstrated that medical residents’ knowledge of pharmacovigilance was weak, particularly in terms of adverse effects severity criteria and reporting procedures. The authors suggested targeted training to improve residents’ pharmacovigilance skills.
A study conducted in Nepal in 2014 [9] indicated that most medical residents were aware of the importance of pharmacovigilance but had limited knowledge about reporting procedures. The authors recommended continuous training to enhance residents’ pharmacovigilance skills.
Comparing our study with other similar studies has confirmed the lack of pharmacovigilance knowledge among medical residents, although certain outcomes vary. These results underscore the necessity to enhance pharmacovigilance training and awareness among healthcare professionals in training.
Conclusion
The results of our cross-sectional study in the regions of Sousse, Kairouan, Sidi Bouzid, and Kasserine reveal an average level of knowledge in pharmacovigilance but underscore notable gaps, particularly in adverse event reporting and the assessment of benefits/risks of medications. This need for improvement also applies to the Sousse Regional Pharmacovigilance Center, where underreporting of adverse effects remains a major challenge. Our recommendations align with strengthening pharmacovigilance training within medical programs, integrating this discipline into curricula, raising awareness through regional events, and fostering collaboration among residents, health authorities, and pharmaceutical companies. Together, we can enhance patient safety, demanding cooperation among all stakeholders to shape a safer and higher quality medical future.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Funding Sources
No funds, grants, or other support was received to assist with the preparation of this manuscript.
Consent
“Written informed consent was obtained from the patient to publish this report in accordance with the journal’s patient consent policy”
Acknowledgements: None
References
- Felix T, Jordan J, Akers C, et Improvements are needed. Expert Opinion on Drug Safety. Current state of biologic pharmacovigilance in the European Union (2019).
- Beninger P. Pharmacovigilance: An Overview. Clinical Therapeutics (2018): 40.
- Organisation mondiale de la Santé. Indicateurs de l’OMS pour la pharmacovigilance: un manuel pratique pour l’évaluation des systèmes de Genève: Organisation mondiale de la Santé. (2019).
- Histoire de la pharmacovigilance Jacques Caron, Michaël Rochoy, Louise Gaboriau, Sophie Gautier Centre régional de pharmacovigilance, CHRU de Lille,1, place de Verdun, 59045 Lille cedex, Publié par Elsevier Masson SAS pour les Société française de pharmacologie et de thérapeutique (2016).
- Susanne C. Un médicament « monstrueux » : débats publics et couverture médiatique de la tragédie de la thalidomide au Canada„” Denyse Baillargeon Related information Département d’histoire, Université de Montréal (1961-1963).
- Brown P, Bahri P. Engagement’ of patients and healthcare professionals in regulatory pharmacovigilance: establishing a conceptual and methodological Eur J Clin Pharmacol (2019).
- Arimone Y, Bidault I, Dutertre J, et al. Updating the French Method for the Causality Assessment of Adverse Drug Reactions. Therapies (2013).
- Marques J, Ribeiro-Vaz I, Pereira A, et al. A survey of spontaneous reporting of adverse drug reactions in 10 years of activity in a pharmacovigilance centre in International Journal of Pharmacy Practice. (2014).
- Jha N. Need for involving consumers in Nepal’s pharmacovigilance system. (2014).