Assessment of Patients Affected by Treatment-Resistant Depression: Findings from a Real-World Study in Italy
Article Information
Perrone Valentina1,?, Sangiorgi Diego1, Andretta Margherita2, Ducci Giuseppe3, Forti Bruno4, Francesa Morel Pier Cesare5, Gambera Marco6, Maina Giuseppe7, Mencacci Claudio8, Mennini Francesco9, Zanalda Enrico10, Degli Esposti Luca1
1CliCon S.r.l., Health, Economics and Outcomes Research Ravenna, Italy
2HTA Unit, Azienda Zero, Padova, Italy
3Mental Health Department, ASL Roma 1, Rome, Italy
4Mental Health Department - Azienda ULSS (AULSS) n 1 "Dolomiti" - Veneto Region, Italy
5Janssen-Cilag SpA, Milano, Italy
6“OSPEDALE P. PEDERZOLI” Casa di Cura Privata S.p.A., Peschiera del Garda, Verona, Italy
7Department of Neuroscience "Rita Levi Montalcini", University of Turin, University Hospital San Luigi Gonzaga Italy
8Department of Neuroscience, ASST Fatebenefratelli Sacco, Milan, Italy
9EEHTA - CEIS (Centre for Economic and International Studies) , Faculty of Economics, University of Rome "Tor Vergata", Rome, Italy and Institute for Leadership and Management in Health Care, Kingston University, London; 10Department of Mental Health ASL TO3 and AOU San Luigi Gonzaga, Collegno, TO, Italy.
*Corresponding Author: Dr. Valentina Perrone, Clicon Srl, Health, Economics and Outcomes Research Via Salara, 36, 48100 Ravenna, Italy
Received: 22 May 2020; Accepted: 01 June 2020; Published: 24 June 2020
Citation:
Perrone Valentina, Sangiorgi Diego, Andretta Margherita, Ducci Giuseppe, Forti Bruno, Francesa Morel Pier Cesare, Gambera Marco, Maina Giuseppe, Mencacci Claudio, Mennini Francesco, Zanalda Enrico, Degli Esposti Luca. Assessment of Patients Affected by Treatment-Resistant Depression: Findings from a Real-World Study in Italy. Journal of Psychiatry and Psychiatric Disorders 4 (2020): 104-117.
Share at FacebookAbstract
The study aims to estimate the number of patients affected by treatment-resistant depression (TRD) in Italy, and to analyse the therapeutic pathways and drug utilization in real-world settings. This retrospective study is based on administrative databases of Italian Entities. Data were re-proportioned on the Italian population. Adult patients were included from July 2011 to December 2017 if they underwent ≥2 antidepressant (AD) cycles matching the following criteria: i) duration of ≥4 weeks for each cycle; ii) presence of ≥1 prescription for each AD at the maximum dosage reported in the datasheet; iii) grace period of ≤ 30 days to switch between AD; iv) if no changing occurs, treatment maintained ≥9 months. Patients prescribed antipsychotics or mood stabilizers in the 6-months prior inclusion were excluded. Sensitivity analyses were performed taking into account ≥6 and ≥8 weeks with the same AD or considering the mean maximum dosage (84.2% of maximum dosage). Considering the Italian population, 101,455 patients were estimated to have TRD according to the inclusion/exclusion criteria applied and represented the 4.2% of patients prescribed AD in Italy. For thresholds of ≥6 and ≥8 weeks for cycle duration, TRD patients were estimated to be 97,223 and 96,213, respectively. When the mean maximum dosage criterion was selected, 130,049 TRD patients were identified: of them, 45,198 had ?a psychiatric visit/hospitalization, 32,341 of which added another AD/mood-stabilizer/antipsychotic to the current therapy. In conclusion, we gave an estimation at the National level of TRD patients in Italy, considering different AD dosages and AD cycle durations to provide realistic scenarios.
Keywords
Antidepressant; Depressive disorder; Therapeutic pathways; Treatment-resistant depression
Antidepressant articles, Depressive disorder articles, Therapeutic pathways articles, Treatment-resistant depression articles
Antidepressant articles Antidepressant Research articles Antidepressant review articles Antidepressant PubMed articles Antidepressant PubMed Central articles Antidepressant 2023 articles Antidepressant 2024 articles Antidepressant Scopus articles Antidepressant impact factor journals Antidepressant Scopus journals Antidepressant PubMed journals Antidepressant medical journals Antidepressant free journals Antidepressant best journals Antidepressant top journals Antidepressant free medical journals Antidepressant famous journals Antidepressant Google Scholar indexed journals Depressive disorder articles Depressive disorder Research articles Depressive disorder review articles Depressive disorder PubMed articles Depressive disorder PubMed Central articles Depressive disorder 2023 articles Depressive disorder 2024 articles Depressive disorder Scopus articles Depressive disorder impact factor journals Depressive disorder Scopus journals Depressive disorder PubMed journals Depressive disorder medical journals Depressive disorder free journals Depressive disorder best journals Depressive disorder top journals Depressive disorder free medical journals Depressive disorder famous journals Depressive disorder Google Scholar indexed journals Therapeutic pathways articles Therapeutic pathways Research articles Therapeutic pathways review articles Therapeutic pathways PubMed articles Therapeutic pathways PubMed Central articles Therapeutic pathways 2023 articles Therapeutic pathways 2024 articles Therapeutic pathways Scopus articles Therapeutic pathways impact factor journals Therapeutic pathways Scopus journals Therapeutic pathways PubMed journals Therapeutic pathways medical journals Therapeutic pathways free journals Therapeutic pathways best journals Therapeutic pathways top journals Therapeutic pathways free medical journals Therapeutic pathways famous journals Therapeutic pathways Google Scholar indexed journals Treatment-resistant depression articles Treatment-resistant depression Research articles Treatment-resistant depression review articles Treatment-resistant depression PubMed articles Treatment-resistant depression PubMed Central articles Treatment-resistant depression 2023 articles Treatment-resistant depression 2024 articles Treatment-resistant depression Scopus articles Treatment-resistant depression impact factor journals Treatment-resistant depression Scopus journals Treatment-resistant depression PubMed journals Treatment-resistant depression medical journals Treatment-resistant depression free journals Treatment-resistant depression best journals Treatment-resistant depression top journals Treatment-resistant depression free medical journals Treatment-resistant depression famous journals Treatment-resistant depression Google Scholar indexed journals Major Depressive Disorder articles Major Depressive Disorder Research articles Major Depressive Disorder review articles Major Depressive Disorder PubMed articles Major Depressive Disorder PubMed Central articles Major Depressive Disorder 2023 articles Major Depressive Disorder 2024 articles Major Depressive Disorder Scopus articles Major Depressive Disorder impact factor journals Major Depressive Disorder Scopus journals Major Depressive Disorder PubMed journals Major Depressive Disorder medical journals Major Depressive Disorder free journals Major Depressive Disorder best journals Major Depressive Disorder top journals Major Depressive Disorder free medical journals Major Depressive Disorder famous journals Major Depressive Disorder Google Scholar indexed journals years lived with disability articles years lived with disability Research articles years lived with disability review articles years lived with disability PubMed articles years lived with disability PubMed Central articles years lived with disability 2023 articles years lived with disability 2024 articles years lived with disability Scopus articles years lived with disability impact factor journals years lived with disability Scopus journals years lived with disability PubMed journals years lived with disability medical journals years lived with disability free journals years lived with disability best journals years lived with disability top journals years lived with disability free medical journals years lived with disability famous journals years lived with disability Google Scholar indexed journals treatment-resistant depression articles treatment-resistant depression Research articles treatment-resistant depression review articles treatment-resistant depression PubMed articles treatment-resistant depression PubMed Central articles treatment-resistant depression 2023 articles treatment-resistant depression 2024 articles treatment-resistant depression Scopus articles treatment-resistant depression impact factor journals treatment-resistant depression Scopus journals treatment-resistant depression PubMed journals treatment-resistant depression medical journals treatment-resistant depression free journals treatment-resistant depression best journals treatment-resistant depression top journals treatment-resistant depression free medical journals treatment-resistant depression famous journals treatment-resistant depression Google Scholar indexed journals European Medicines Agency articles European Medicines Agency Research articles European Medicines Agency review articles European Medicines Agency PubMed articles European Medicines Agency PubMed Central articles European Medicines Agency 2023 articles European Medicines Agency 2024 articles European Medicines Agency Scopus articles European Medicines Agency impact factor journals European Medicines Agency Scopus journals European Medicines Agency PubMed journals European Medicines Agency medical journals European Medicines Agency free journals European Medicines Agency best journals European Medicines Agency top journals European Medicines Agency free medical journals European Medicines Agency famous journals European Medicines Agency Google Scholar indexed journals Local Health Unit articles Local Health Unit Research articles Local Health Unit review articles Local Health Unit PubMed articles Local Health Unit PubMed Central articles Local Health Unit 2023 articles Local Health Unit 2024 articles Local Health Unit Scopus articles Local Health Unit impact factor journals Local Health Unit Scopus journals Local Health Unit PubMed journals Local Health Unit medical journals Local Health Unit free journals Local Health Unit best journals Local Health Unit top journals Local Health Unit free medical journals Local Health Unit famous journals Local Health Unit Google Scholar indexed journals Anatomical-Therapeutic Chemical articles Anatomical-Therapeutic Chemical Research articles Anatomical-Therapeutic Chemical review articles Anatomical-Therapeutic Chemical PubMed articles Anatomical-Therapeutic Chemical PubMed Central articles Anatomical-Therapeutic Chemical 2023 articles Anatomical-Therapeutic Chemical 2024 articles Anatomical-Therapeutic Chemical Scopus articles Anatomical-Therapeutic Chemical impact factor journals Anatomical-Therapeutic Chemical Scopus journals Anatomical-Therapeutic Chemical PubMed journals Anatomical-Therapeutic Chemical medical journals Anatomical-Therapeutic Chemical free journals Anatomical-Therapeutic Chemical best journals Anatomical-Therapeutic Chemical top journals Anatomical-Therapeutic Chemical free medical journals Anatomical-Therapeutic Chemical famous journals Anatomical-Therapeutic Chemical Google Scholar indexed journals
Article Details
1. Introduction
Major Depressive Disorder (MDD) is a debilitating and life-threatening mental illness leading to functional disability in affected individuals [1]. MDD is associated with significant morbidity and mortality [2], and represents a worldwide public health-concern. According to the Global Burden of Disease (GBD) 2010 study, MDD is in the top 30 most common disease [3] and the World Health Organization ranked depression as the single largest contributor to global disability, representing the 7.5% of all years lived with disability (YLD) in 2015 [4]. The most recent epidemiological estimates reported a predicted point prevalence for MDD of 4.7% (4.4%-5.0%) globally [5].
Several treatment options either pharmaceutical or non-pharmaceutical are currently available to manage MDD, with a consistent number of antidepressant drugs (AD) developed over the last 50 years [6]. However, MDD burden is mainly due to unsuccessful treatments. In a significant proportion of MDD patients (around 20-30%), therapeutic response is only partially or not achieved with the majority of first-line treatment strategies, and sequential interventions are often required [7, 8]. MDD is indeed associated with high rates of treatment failure and relapse that increase with the number of treatment steps [9]. MDD patients who do not satisfactorily respond to adequate treatments are considered to have treatment-resistant depression (TRD).
TRD imposes a significant health and social burden as it has considerable effects on patients quality of life and a major impact on their family as well. TRD is regarded as a complex phenomenon influenced by the high degree of heterogeneity in depression subtypes and psychiatric comorbidities [10]. Over the years, many clinical definitions of TRD were reported, however a standard definition does not currently exist. The European Union’s Committee for Human Proprietary Medicinal Products (CHMP) defined TRD as an inadequate response (i.e. a lack of clinically meaningful improvement) despite the consecutive treatment of at least two AD (belonging to the same or different class) at adequate doses, prescribed for adequate duration with adequate affirmation of treatment adherence [11].
A standard approach for the management of TRD is not yet established. The strategies suggested by the international guidelines for second-line treatment of MDD and that are usually applied in clinical routine care include: dose escalation of the AD dispensed, switching to another AD, combination of 2 or more AD and augmentation of a non-antidepressant agents as antipsychotics or mood stabilizers to the ongoing AD therapy [2, 12].
The lack of a universal definition for this condition results in the wide variation of TRD estimate rates observed in literature. Moreover, the subjectivity involved in the assessment of the proper criteria to delineate TRD (such as therapy duration, daily dose and number of treatments) challenges the use of administrative claims databases, which act as tools to study large number of patients in real-world health-care settings. Cepeda et al.[7] build a data-driven definition to discriminate between individuals with and without TRD considering the number of distinct antidepressants (≥3) or antipsychotics (≥1) prescribed over the last year.
As far as Italy is concerned, to the best of our knowledge, the only analysis from which an estimation of TRD patients can be derived is provided by the Italian Medicines Agency (AIFA) and it regards patients that in 1 year receive 3 different antidepressant prescriptions [13]. This administrative observation does not indicate the selection of extraction criteria to define a specific resistant population. Therefore, real-world data to select a potential and more realistic TRD population in Italy applying European Medicines Agency (EMA) definition are needed. In this context, the aims of the present study were to estimate the number of patients affected by TRD in Italy, and to provide a full characterization of their therapeutic pathways and drug utilization in an Italian real-world setting.
2. Methods
2.1 Data source
This observational retrospective analysis was based on administrative databases of one Italian Region and one Local Health Unit (LHU), and data were re-proportioned on the Italian population. To perform the analysis, data were retrieved from the following databases: beneficiaries’ database that contains all demographic data for patients analysed (deaths included); pharmaceutical database providing data on prescription as Anatomical-Therapeutic Chemical (ATC) code, number of packages, number of units per package, and prescription date; hospitalisation database that includes all hospitalization data; outpatient specialist services database, which contains date of prescription, type, description activity of diagnostic tests and visits for patients in analysis.
The patient code in each database allowed electronic linkage between all different databases. To guarantee patients’ privacy, an anonymous univocal numeric code was assigned to each subject included in the study, in full compliance with the European General Data Protection Regulation (GDPR) (2016/679). Based on this procedure, the extracted data did not permit - either directly or indirectly - the identification of patients involved in the analysis and no identifiers related to patients were provided to the authors. All the results of the analyses were produced as aggregated summaries. According to the Italian law [14], this study has been notified to the local Ethics Committee of the Region and LHU involved in the study and the Ethic Committees have approved the study. According to the pronouncement of the Data Privacy Guarantor Authority (General Authorization for personal data treatment for scientific research purposes – 01/03/2012 and following further measures) data treatment using encrypted retrospective information is authorized without patient Informed Content when the collection is impossible due to organizational reasons.
2.2 Study design
All patients ≥18 years old were included if they presented between July 1st, 2011 and December 31st, 2017 at least 2 AD cycles who met the four TRD criteria reported in Figure 1 and listed in the following: i) cycle duration of ≥ 4 weeks with the same treatment, as short period treatments could indicate safety issues or diagnosis other than MDD; ii) presence of at least one prescription for each AD therapy at the maximum dosage labelled in the summary of product characteristics (SMPC), to avoid patients treated with low doses of AD due to different diagnoses and to identify moderate or severe MDD patients for which dose optimization is suggested [15]; iii) a grace period of ≤ 30 days is observed to switch from one AD to another, in order to narrow down to MDD patients who actually require a switch of therapy and not to include, for instance, patients with well-being periods without treatment; iv) if no changing occurs, treatment should be maintained at least for 9 months, reflecting the typical course of treatment with AD for an MDD episode. AD cycles who met the 4 TRD criteria could also be not consecutive, i.e. the presence of one or more AD cycles not responding to such criteria between 2 TRD cycles was allowed. Combination of two AD was considered as a single cycle.
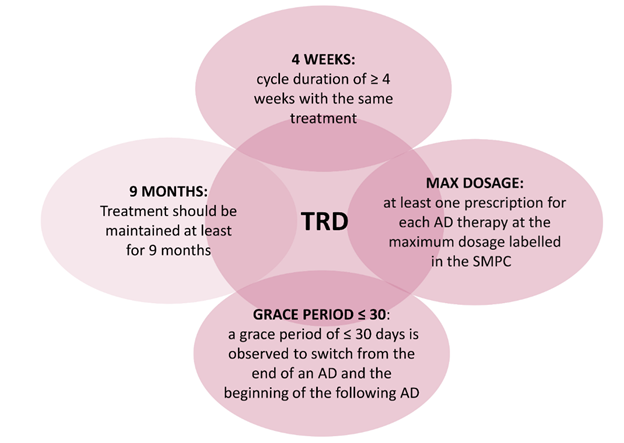
Figure 1: Criteria applied to the EMA definition of TRD for the identification of the study population.
Abbreviations: AD, antidepressant; TRD, treatment-resistant depression; SMPC, summary of product characteristics.
The index date (ID) corresponded to the date of first AD prescription during inclusion period. To refine the study population, patients in treatment with antipsychotics (ATC code: N05A) or mood stabilizers (ATC code: N03A) in the 6 months prior ID (baseline period) were excluded as they could be affected by other psychiatric conditions. Patients were followed-up starting from ID until end of continuous eligibility or death. The study design is shown in (Figure 2).
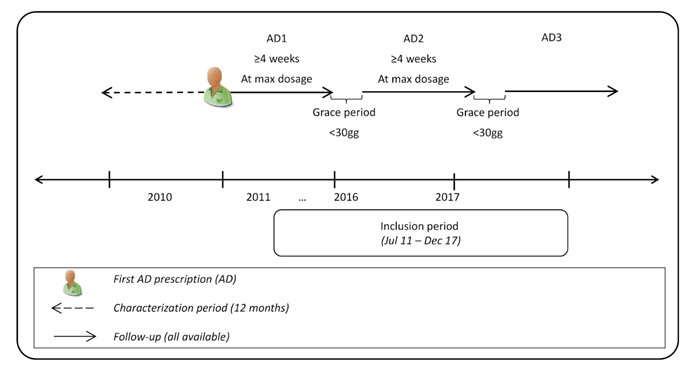
Figure 2: Study design.
Abbreviations: AD, antidepressant.
2.3 Study variables
Previous use of antipsychotics or mood stabilizers was assessed in the 6 months prior the index AD prescription. Concerning treatment patterns, the percentage of patients with each AD (ATC code N06A) was calculated as the sum of patients with a specific AD divided by the total number of patients, and percentage of patients according to number of AD calculated as the sum of patients according to number of AD divided by the total number of patients. During follow-up, the following resources consumption was analysed: psychiatric visit (codes 94.12.1, 94.19.1), neurologic visit (code 89.13), psychiatric hospitalizations [Major Diagnostic Categories 19 (mental disorders)]. Percentage of patients with each resource was calculated as the sum of patients with a specific resource divided by the total number of patients. Add-on was defined as the presence of AD/mood stabilizers/antipsychotics during follow-up.
2.4 Sensitivity analysis
In order to assess how different TRD criteria could have influenced the results of the study, we conducted 2 different sensitivity analyses considering:
- Only patients treated for ≥ 6 and ≥ 8 weeks with the same treatment instead of ≥ 4 weeks;
- AD therapy prescribed at a dosage corresponding to the ratio between mean dosage for the TRD patients and maximum dosage according to the SMPC (mean maximum dosage). In this analysis, an estimate of the number of TRD patients for each Italian Region and autonomous province was attempted. This estimate was calculated multiplying the TRD patients resulting from the analysis by the ratio between subjects treated with AD in each Region/autonomous province and subjects treated with AD in Italy. The number of individuals treated with AD was taken from the Italian report on mental health of Ministry of Health [16].
2.5 Statistical analysis
Continuous variables were reported as mean ± standard deviation (SD), categorical variables were expressed as frequencies and percentages. All statistical analyses were performed using STATA SE, version 12.0.
3. Results
Considering the Italian population, 133,835 patients were estimated to be affected by TRD according to the criteria applied. Among them, 101,455 patients without antipsychotics or mood stabilizers prescriptions in the 6 months prior ID were included in the study, representing the 4.2% of patients treated with AD in Italy (Figure 3).
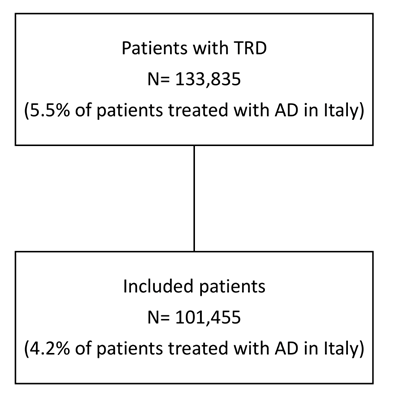
Figure 3: Flowchart of included patients.
Abbreviations: TRD, treatment-resistant depression; AD, antidepressant.
The more prescribed AD was venlafaxine (21.9%), followed by paroxetine (14.1%), duloxetine (12.6%), escitalopram (11.5%), sertraline (10.8%), citalopram (5.9%), clomipramine and bupropion (5.1% both), fluoxetine (4.4%), amitriptyline (3.8%) and other drugs (4.8%). During follow-up, 38.7% of patients were prescribed with 3 different AD, 27.1% with 4, 16.6% with 5, 8.9% with 6 and 8.7% received 7 or more AD. Mean of cycles duration was far above the threshold of 4 weeks and it progressively decreased as the number of cycles increased (Figure 4): mean duration was 21 weeks for the first cycle, 18 weeks for the second one, 17 weeks for third and fourth cycles and 16 weeks from the fifth cycle onwards. Given these results, a sensitivity analysis was undertaken considering higher thresholds of cycle duration with the same AD treatment: patients treated for ≥ 6 weeks with same AD were 97,223, while those treated for ≥ 8 weeks were 96,213. During follow-up, 35,260 patients had a psychiatric visit/hospitalization and 25,230 required an add-on (Figure 5A). Specifically, add-ons with other AD/mood stabilizers/antipsychotics were observed in the 8.7% of patients at the first cycle, 22.2% at second cycle and 57.3% from third cycle onwards. Starting from the third cycle, 34.7% of patients had one or more psychiatric visit/hospitalization and 24.9% had both psychiatric visit/hospitalization and add-on therapy.
One of the TRD criteria applied required at least one prescription for each AD at the maximum dosage labelled in SMPC. However, the maximum dosage could be limited by the onset of adverse events, and the strategy of dose increasing could not be effective for all classes of AD medications [17, 18].
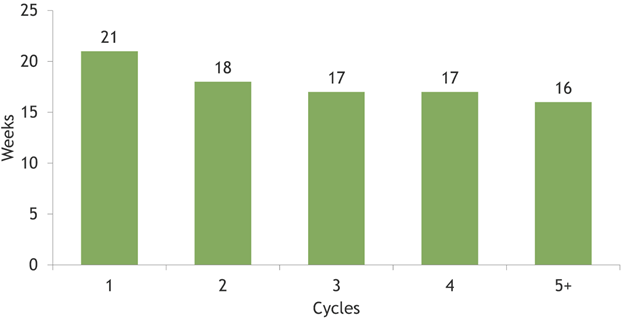
Figure 4: Mean time (weeks) of cycles duration.
A further sensitivity analysis was then performed, considering the 84.2% of maximum dosage instead, that corresponded to the mean maximum dosage. In this analysis, 130,049 patients were identified who met the 4 TRD criteria and accounted for the 5.4% of patients prescribed AD in Italy. This result provided a more likely estimate of TRD patients in Italy. Considering this criterion, an estimate of distribution of TRD patients across Italian Regions/autonomous province was ventured, as shown in Figure 6. Among the 130,049 patients, 45,198 were estimated to have a psychiatric visit/hospitalization, 32, 341 an add-on as well as a psychiatric visit/hospitalization (Figure 5B).
Notes: * Patients fulfilling the selected criteria that required a psychiatric visit or psychiatric hospitalization; **Patients that required also an add-on therapy, defined as presence of other antidepressants/mood stabilizers/antipsychotics.
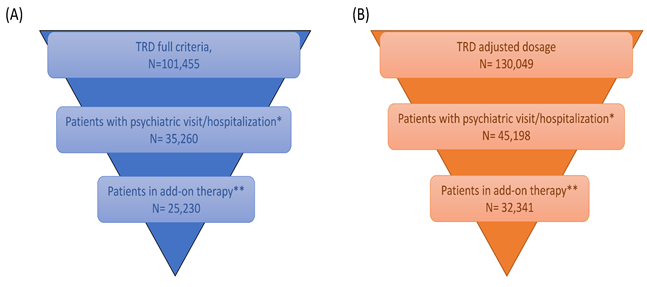
Figure 5: Patient distribution according to psychiatric visit/hospitalization considering (A) maximum dosage labelled in SMPC and (B) ratio between mean dosage prescribed/maximum dosage labelled in SMPC.
Notes: The estimate was obtained multiplying the TRD patients resulting from the analysis (N= 130,049) by the ratio between subjects treated with antidepressants in each Region/autonomous province and subjects treated with antidepressants in Italy.
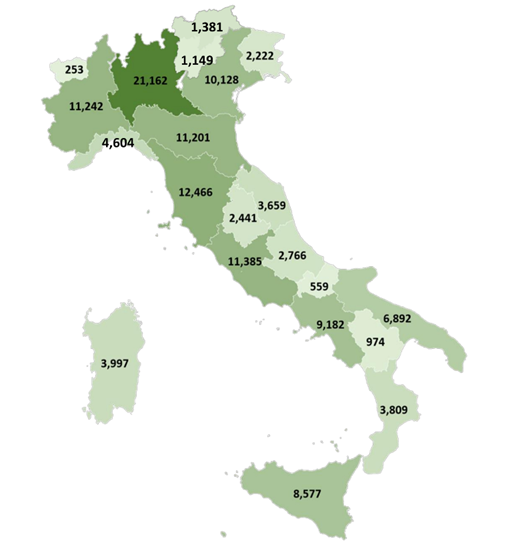
Figure 6: Estimate of the number of TRD patients with mean maximum dosage (N= 130,049) for each Italian Region and autonomous province.
4. Discussion
The substantial burden of MDD is likely attributable to the one-third of patients developing TRD. Hence, a thorough characterization of TRD patients in real-world settings is necessary to get a better understanding of this condition. Nevertheless, to date almost no evidences are available on TRD Italian patients. In the present study, we sought to address this issue by providing for the first time an estimation of TRD patients in the Italian population and describing their therapeutic pathway and drug utilization in Italian settings of clinical practice. To perform the analysis, we decided to stick to the definition of TRD stated by the EMA [11] exploiting 4 criteria to assess the adequate treatment duration, dosage and adherence to therapy. The results were then projected to the Italian population.
According to the majority of international guidelines [18] and the European Group for the Study of Resistant Depression (GSRD) [19], a sufficient treatment duration for an adequate AD trial administration should be of at least 4 weeks prior to analyse treatment response. In this study, we first applied a threshold of 4 weeks, that was far exceeded as mean cycles duration was found to range between 16 and 21 weeks. Olgiati et al. [20] highlighted that, given the complex nature of TRD, some patients may require longer treatment duration, and suggested that, regardless early signs of no response, an AD treatment should be continued for at least other 4 weeks. Moreover, in the STAR*D study was observed that the majority of patients achieved response or remission at or after 8 weeks of AD therapy [21]. We performed a sensitivity analysis considering a thresholds of 6 and 8 weeks, and our results showed that the overall sample changed in a negligible rate of patients, thus corroborating the validity of the criteria applied.
The TRD patients identified were estimated to account for the 5.4% of patients with AD in Italy. This percentage is lower if compared to what reported in literature for prevalence of TRD in MDD patients. However, it should be acknowledged that AD may be prescribed for conditions other than depression: in an Italian survey, Carta et al. [22] found that around half of the subjects assuming AD does not present a lifetime diagnosis of MDD, pointing out the broader use of AD.
A specific treatment for TRD has not yet been approved in Europe [23]. Adding an ulterior AD or other non-antidepressant agents to the current therapy regimen is considered a common strategy for patients resistant to 2 or more classes of AD [8]. Consistently, our findings showed that overall around one-fourth of patients required an add-on, which was observed in more than half of patients starting from the third cycle of therapy.
In our study, the “adequate dosage” considered in the TRD criteria was at first the maximum dosage labelled in SMPC. In deciding a proper dose, clinicians tend to prescribe low dose of AD to minimize risk of adverse events and, in case of partial response, a dose escalation is advised to improve the chances of positive outcomes [24]. Still, Fife et al.[25] reported that around one-third of the first AD therapy does not reach the minimum effective dose when it is stopped or switched. In line with the concept aforementioned, we selected the mean maximum dosage as criterion to reproduce a more realistic scenario of TRD population in Italy. Our findings estimated 130,049 patients with TRD in Italy.
The present study has some limitations. Our cohort of patients reflected real clinical practice, and the results must be interpreted taking into account the limitations related to the observational nature of the study, which was based on data collected from administrative databases. First, there is not a consensus definition for TRD: the present study tried to overcome this limitation using the most common accepted definition and performed sensitivity analysis to take into account different scenarios. Nevertheless, the selected criteria could have over- or under- estimated the number of TRD patients. Another limitation was the lack of clinical information related to comorbidities, the severity of the pathology in terms of depressive episodes, other clinical considerations as persistence of symptoms and potential confounders that could have influenced our results. Furthermore, data on the use of pharmacological treatments were retrieved from medical prescriptions and dispensing, and actual drug use data were not available as well as the reasons requiring additional therapies. Moreover, the distribution of TRD patients at a regional level was a temptative estimate that may not reflect the actual situation in each Region/autonomous province.
5. Conclusion
This analysis gave an estimate at the National level of TRD patients in Italy. Our results showed that when the mean maximum dosage was selected as criterium, TRD population increased from 101,455 to 130,049. Although each single criterion taken alone could not be reliable for TRD identification, the 4 criteria together along with the exclusion of patients already and stably treated with antipsychotics and/or mood stabilizers could represent an accurate way to target the TRD population through data collected from administrative databases.
Acknowledgements
This study was funded by Janssen Italy. The views expressed here are those of the authors and not necessarily those of the sponsor.
Conflict of Interest
VP, DS and LDE report no conflicts of interest in this work. Clicon S.r.l. is an independent company. The agreement signed by Clicon S.r.l. and Janssen Italy does not create any entityship, joint venture or any similar relationship between parties. Neither CliCon S.r.l. nor any of their representatives are employees of Janssen Italy for any purpose. PCFM is employee of Hemar Department Janssen Italy Janssen Italy. MA, GD, BF, MG, CM, FM report no conflict of interest. GM has been consultant/speaker for: Angelini, Boheringer, Fb Health, Innovapharma, Italfarmaco, Janssen, Otsuka, Lundbeck, Sanofi. EZ has been consultant for Johnson & Johnson, Otzuka, Lundbeck.
References
- Malhi GS, Mann JJ. Depression. The Lancet 392 (2018): 2299-2312.
- Caraci F, Calabrese F, Molteni R, et al. International Union of Basic and Clinical Pharmacology CIV: The Neurobiology of Treatment-resistant Depression: From Antidepressant Classifications to Novel Pharmacological Targets. Ohlstein EH, editor. Pharmacol Rev 70 (2018): 475-504.
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380 (2012): 2163-2196.
- World Health Organization. Depression and other common mental disorders: global health estimates. World Health Organization; 2017 p. Licence: CC BY-NC-SA 3.0 IGO.
- Ferrari AJ, Somerville AJ, Baxter AJ, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med 43 (2013): 471-481.
- Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: From monoamines to glutamate. Exp Clin Psychopharmacol 23 (2015): 1-21.
- Cepeda MS, Reps J, Fife D, et al. Finding treatment-resistant depression in real-world data: How a data-driven approach compares with expert-based heuristics. Depress Anxiety 35 (2018): 220-228.
- de Sousa RT, Zanetti MV, Brunoni AR, et al. Challenging Treatment-Resistant Major Depressive Disorder: A Roadmap for Improved Therapeutics. Curr Neuropharmacol 13 (2015): 616-635.
- McIntyre RS, Filteau M-J, Martin L, et al. Treatment-resistant depression: Definitions, review of the evidence, and algorithmic approach. Journal of Affective Disorders 156 (2014): 1-7.
- Gaynes BN, Asher G, Gartlehner G, et al. Definition of Treatment-Resistant Depression in the Medicare Population [Internet]. Rockville, MD: Agency for Healthcare Research and Quality (US); 2018 [cited 2019 Sep 18]. (AHRQ Technology Assessments).
- Clinical investigation of medicinal products in the treatment depression [Internet]. European Medicines Agency. 2018 [cited 2019 Sep 18].
- Ijaz S, Davies P, Williams CJ, et al. Psychological therapies for treatment-resistant depression in adults. Cochrane Common Mental Disorders Group, editor. Cochrane Database of Systematic Reviews [Internet]. 2018 May 15 [cited 2019 Sep 13].
- The Medicines Utilisation Monitoring Centre. National Report on Medicines use in Italy. Year 2015. Rome: Italian Medicines Agency (2016).
- Agenzia Italiana del Farmaco (AIFA). Guideline for the classification and conduction of the observational studies on medicines. [Internet] (2010).
- Ionescu DF, Rosenbaum JF, Alpert JE. Pharmacological approaches to the challenge of treatment-resistant depression. Dialogues Clin Neurosci 17 (2015): 111-126.
- Rapporto salute mentale. Analisi dei dati del Sistema informativo per la salute mentale (SISM) [Internet]. [cited 2019 Dec 6].
- Luchini F, Cosentino L, Pensabene L, et al. Depressione resistente al trattamento: stato dell’arte Parte II. Trattamento. Rivista di Psichiatria 49 (2014): 228-240.
- MacQueen G, Santaguida P, Keshavarz H, et al. Systematic Review of Clinical Practice Guidelines for Failed Antidepressant Treatment Response in Major Depressive Disorder, Dysthymia, and Subthreshold Depression in Adults. Can J Psychiatry 62 (2017): 11-23.
- Bartova L, Dold M, Kautzky A, et al. Results of the European Group for the Study of Resistant Depression (GSRD) — basis for further research and clinical practice. The World Journal of Biological Psychiatry 20 (2019): 427-448.
- Olgiati P, Serretti A, Souery D, et al. Early improvement and response to antidepressant medications in adults with major depressive disorder. Meta-analysis and study of a sample with treatment-resistant depression. J Affect Disord 227 (2018): 777-786.
- Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163 (2006): 28-40.
- Carta MG, Aguglia E, Bocchetta A, et al. The Use of Antidepressant Drugs and the Lifetime Prevalence of Major Depressive Disorders in Italy. Clin Pract Epidemiol Ment Health 6 (2010): 94-100.
- Jaffe DH, Rive B, Denee TR. The humanistic and economic burden of treatment-resistant depression in Europe: a cross-sectional study. BMC Psychiatry 19 (2019): 247.
- Pandarakalam JP. Challenges of Treatment-Resistant Depression. Psychiat Danub 30 (2018): 273-284.
- Fife D, Blacketer C, Reps JM, et al. Database Studies of Treatment-Resistant Depression Should Take Account of Adequate Dosing. Prim Care Companion CNS Disord (2018).
