Anti-inflammatory Effects of Pentraxin 3 in Human Visceral Adipocytes by Reducing Reactive Oxygen Species Production
Article Information
Taiki Tojo1, Minako Yamaoka-Tojo2*
1Sagamihara Kyodo Hospital, Sagamihara, Japan
2Kitasato University School of Allied Health Sciences, Sagamihara, Japan
*Corresponding author: Minako Yamaoka-Tojo, Kitasato University School of Allied Health Sciences, 1-15-1 Kitasato, Minami-ku, Sagamihara 252-0373, Japan
Received: 24 February 2021; Accepted: 03 March 2021; Published: 10 March 2021
Citation: Taiki Tojo, Minako Yamaoka-Tojo. Anti-inflammatory Effects of Pentraxin3 in Human Visceral Adipocytes by Reducing Reactive Oxygen Species Production. Cardiology and Cardiovascular Medicine 5 (2021): 213-217.
Share at FacebookAbstract
Background: Metabolic syndrome (MetS) is associated with an increased risk of developing atherosclerotic cardiovascular diseases. Pentraxin 3 (PTX3) is an acute phase protein strongly expressed by advanced atherosclerotic lesions.
Methods and Results: To determine the functional role of PTX3 in MetS patients, cultured human visceral adipocytes were treated with human recombinant PTX3 (hr- PTX3) in various conditions. Reactive oxygen species (ROS) production from differentiated adipocytes (detected by dichlorodihydrofluorescein diacetate) was markedly reduced by the treatment of hr-PTX3 (44% reduction, P < 0.01). The inhibition was dependent on the intracellular lipid contents identified by functional knock down of Niemann-Pick C1-like 1 (NPC1L1), a cholesterol transporter, using small interfering RNA.
Conclusions: PTX3-induced ROS reduction in human adipocytes suggested that MetS patients with reduced levels of PTX3 are high-risk patients for cardiovascular disease. In patients with MetS, circulating levels of PTX3 may be a novel biomarker to help predict the effectiveness of total risk management for cardiovascular prevention.
Keywords
Pentraxin 3
Article Details
1. Background
Metabolic syndrome (MetS) is associated with an increased risk of developing atherosclerotic cardiovascular diseases [1]. Increased secretion of proinflammatory cytokines by adipose tissue and dysfunctional vascular endothelium are important factors to progress atherosclerosis in patients with MetS [2]. Pentraxin 3 (PTX3) is an acute phase protein that belongs to the pentraxin super family, a group of proteins that also includes C-reactive protein (CRP) and serum amyloid P (SAP) component. PTX3 belongs to the long pentraxins, whereas CRP and SAP are recognized as classic short pentraxins. PTX3 is strongly expressed by advanced atherosclerotic lesions as primary inflammatory response in association with macrophage and neutrophils infiltration [3]. PTX3 is accordingly considered a potential specific biomarker for atherosclerotic lesions. We hypothesized that circulating PTX3 could be a useful biomarker for systemic inflammation in patients with MetS. To examine the functional role of PTX3 in MetS patients, we cultured human visceral adipocytes and treated with human recombinant PTX3 (hr-PTX3).
2. Material and Methods
2.1 Human visceral adipocytes culture
To clarify the functional role of PTX3 in MetS patients, we cultured human visceral adipocytes and stimulated with human recombinant PTX3 (hr-PTX3) in the basal media or the growth factor-riched differentiation media. Primary human visceral adipocytes were cultured in the basal media (PBM2) and differentiated for about 3 weeks in the differentiation growth media (PGM2). Human primary cells and their media were purchased from Lonza Walkersville, Inc. (Walkersville, MD). When cells were growth enough, adipocytes were treated with hr-PTX3 (Abnova, Taipei, Taiwan) in PBM2 or PGM2.
2.2 Measurement of cytokines
Concentrations of human PTX3 and adiponectin in cell culture supernatant were determined by enzyme-linked immune sorbent assay (ELISA) using the Human Pentraxin 3 /TSG-14 ELISA System (Perseus Protepmics Inc., Tokyo, Japan) and the CircuLexTM human adiponectin ELISA Kit (CycLex Co., Ltd., Nagano, Japan), respectively. Samples were processed according to the manufacturer’s instructions.
2.3 Functional analysis for PTX3-treated adipocytes
Adipocytes-derived reactive oxygen species (ROS) production was measured by CM- H2 DCFDA (Molecular Probes Inc., Eugene, OR) with or without PEG-catalase (100 U/mL) as a negative control. To reduce intracellular lipid contents in adipocytes, small interference RNA (siRNA) was used for functional knockdown of cholesterol transporter, Niemann-Pick C1-like 1 (NPC1L1), using siRNA (human NPC1L1 siRNA; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The intracellular lipid contents were detected by AdipoRed (Takara, Japan) and cell viability was confirmed by MTT assay (Roche Applied Science, Mannheim, Germany).
3. Results
3.1 Functional role of PTX3 in human adipocytes
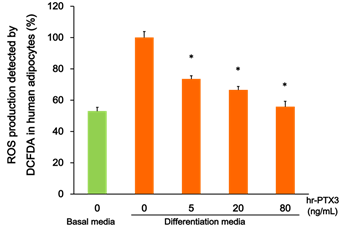
To determine the functional role of PTX3 in MetS patients, cultured human visceral adipocytes were treated with hr-PTX3 in various conditions. As shown in Figure 1, the growth media-induced hydrogen peroxide production from differentiated adipocytes detected by DCFDA was markedly reduced by the treatment of hr-PTX3 for 15 min (44% reduction, P < 0.01).
Figure 1: Effect of PTX3 on the growth media-induced ROS production in human adipocytes.
Cultured human visceral adipocytes were differentiated and treated with hr-PTX3 in various conditions (in the basal media or the differentiation media; treated with hr- PTX3 0-80 ng/mL). The growth media-induced hydrogen peroxide production was detected by DCFDA. Values are means ± SE. * P < 0.05. ROS, reactive oxygen species; DCFDA, 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA); hr-PTX3, human recombinant pentraxin 3.
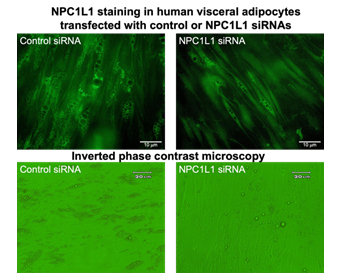
Differentiated human adipocytes were transfected with siRNA of human NPC1L1, a cholesterol transporter, to reduce lipids contents in cultured adipocytes (right panels). Control siRNA was also transfected as the same procedure (left panels). To check the efficacy of NPC1L1 siRNA transfection, NPC1L1 staining (green) was shown in upper panels. In NPC1L1 siRNA transfected adipocytes (right panels) were less intracellular accumulation of adipose droplets, which were observed as brightly light circular accumulations.
Furthermore, to examine the relationship between ROS production and the amount of lipid contents, differentiated human adipocytes were transfected with siRNA of human NPC1L1, a cholesterol transporter, to reduce lipids contents in cultured adipocytes. As shown in Figure 2, NPC1L1 siRNA decreased on intracellular lipid droplets in cultured human visceral adipocytes. The reduction of lipid contents induced by NPC1L1 siRNA was measured by AdipoRed (average 42% decrease, P < 0.05). The ROS-reducing effect of hr-PTX3 was attenuated by the decrease of intracellular lipid contents with functional knock down of NPC1L1 using siRNA transfection (P < 0.05).
4. Discussion
The present study showed that growth factors-induced ROS production in cultured human differentiated visceral adipocytes was significantly reduced by hr-PTX3. The inhibition was dependent on the intracellular lipid contents. PTX3-induced ROS reduction in human adipocytes suggest that athero-protective role of PTX3 in atherogenic patients, such as MetS patients. On the other hand, research of recent years revealed a role of adipose tissue beyond energy storage harboring inflammatory cells which are believed sustain inflammation and impair adipocyte function [4, 5]. The weight gain was associated with significantly increased body fat, food intake, glucose, insulin, glucagon, cholesterol, and leptin. Histological analysis of various organs showed only increased size of pancreatic islets in mutant mice.
According to a recent study, circulating PTX3 and CRP antagonistically participate in the development of obesity and MetS [6]. Circulating PTX3 levels are very low in normal subjects but are rapidly and dramatically increased by inflammation, acute coronary syndrome [7], and autoimmune diseases. PTX3 concentrations are increased in patients with type 2 diabetes, obesity, or polycystic ovary syndrome [8-10]. Using PTX3 knockout mice, it is revealed that PTXX3 regulates anti-inflammatory miR-21 expression and secretion in brown adipocytes during lipopolysaccharide (LPS)-induced inflame-mation [11]. Besides, protective role of PTX3 in LPS-induced sustained inflammation in adipose tissue using PTX3 knockdown 3T3-L1 cells was also examined [12]. Ptx3 gene promoters contain activator protein-1 (AP-1), nuclear factor kappa B (NF-kB), and selective promoter 1 (SP1). These molecules are important to regulate inflammation, and PTX3 could play a central role in the control of inflammation and metabolism [12]. Further studies are needed to clarify roles of PTX3 in the progress of atherosclerosis. PTX3-induced ROS reduction in human adipocytes suggested that MetS patients with reduced levels of PTX3 are high-risk patients for cardiovascular disease. In summary, circulating levels of PTX3 may be a novel biomarker to help predict the effectiveness of total risk management for cardiovascular prevention.
Competing Interests
The authors declare no conflict interest.
Acknowledgements
This work was partly supported by JSPS KAKENHI grant number JP19K11371.
References
- Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol 28 (2008): 629-636.
- Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120 (2009): 1640-1645.
- Savchenko A, Imamura M, Ohashi R, et al. Expression of pentraxin 3 (PTX3) in human atherosclerotic lesions. J Pathol 215 (2008): 48-55.
- Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112 (2003): 1821-1830.
- Jung C, Gerdes N, Fritzenwanger M, et al. Circulating levels of interleukin-1 family cytokines in overweight adolescents. Mediators Inflamm (2010): 958403.
- Ogawa T, Kawano Y, Imamura T, et al. Reciprocal contribution of pentraxin 3 and C-reactive protein to obesity and metabolic syndrome. Obesity Silver Spring 18 (2010): 1871-1874.
- Latini R, Maggioni AP, Peri G, et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation 110 (2004): 2349-2354.
- Netea MG, Joosten LA, Lewis E, et al. Deficiency of interleukin- 18 in mice leads to hyperphagia, obesity and insulin resistance. Nature medicine 12 (2006): 650-656.
- Fischer CP, Perstrup LB, Berntsen A, et al. Elevated plasma interleukin-18 is a marker of insulin-resistance in type 2 diabetic and non-diabetic humans. Clinical immunology Orlando, Fla 117 (2005): 152-160.
- Aso Y, Okumura K, Takebayashi K, et al. Relationships of plasma interleukin-18 concentrations to hyperhomocysteinemia and carotid intimal-media wall thickness in patients with type 2 diabetes. Diabetes care 26 (2003): 2622-2627.
- Lin T, Guo H, Chen X. Pentraxin 3 Regulates miR-21 Expression and Secretion in Brown Adipocytes During Lipopolysaccharide-Induced Inflammation. Obesity (Silver Spring 28 (2020): 323-332.
- Guo H, Qiu X, Deis J, et al. Pentraxin 3 deficiency exacerbates lipopolysaccharide-induced inflammation in adipose tissue. Int J Obes (Lond) 44 (2020): 525-538.