Analysis of Antibody Responses of Vaccinated Persons with Coronavac
Article Information
Aydin BALCI*, 1, Muhammed Emin DÜZ2, Sibel Günay3
1Afyonkarahisar University, Medical Faculty, Department of Pulmonology, Afyon, Turkey
2Amasya University, Sabuncuoglu Serefeddin Training and Research Hospital, Medical Biochemistry, Amasya, Turkey
3Ankara City HospitalDepartment of Pulmonology Ankara, Turkey
*Corresponding author: Aydin BALCI, Afyonkarahisar University, Medical Faculty, Department of Pulmonology, Afyon, Turkey
Received: 03 July 2023 Accepted: 11 July 2023 Published: 19 October 2023
Citation:
Aydın BALCI, Muhammed Emin DÜZ, Sibel Günay. Analysis of Antibody Responses of Vaccinated Persons with Coronavac. Archives of Microbiology and Immunology. 7 (2023): 242-245
Share at FacebookAbstract
Introduction: Vaccination is the most efficient method available to combat the COVID-19 pandemic. However, vaccine production and logistics problems bring the dose-comparing approach to mind to protect the most people in the shortest time.
Method: Day one (believed to be first dose-dependent), day 14, day 21, and 3 months after 2nd dose anti-SARS-COV-2 anti-spike IgG and IgM responses of one and two doses of CoronaVac (Sinovac Life Sciences, Beijing, China) COVID-19 vaccine in 80 healthcare workers without a history of COVID-19 were investigated.
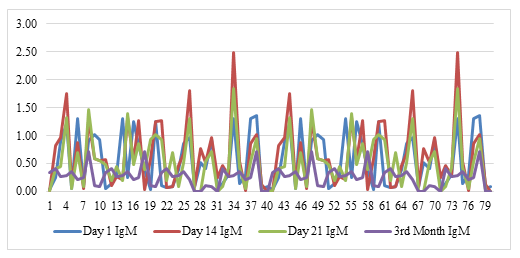
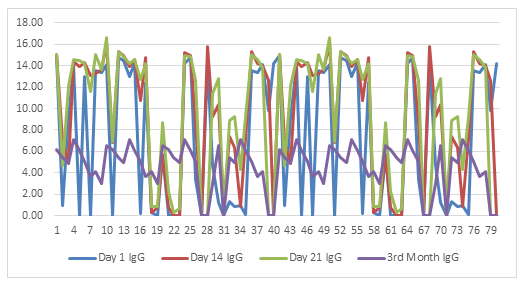
Result: There was a statistically significant difference between day one (median:7.13) and day 14 (median:10.18) and day 21 (median:11.75) and between day 14 and day 21 in terms of mean IgG values (p<0.0001, p<0.0001, and p=0.005, respectively). Also, a significant correlation was found between day 14 and day 21 in terms of IgM values (r=0.888, p<0.001), and a robust correlation were found between day one and day 14 and between day 14 and day 21 in IgG values (r=0.798, p< 0.001 and r=0.947, p<0.001). Considering the 3rd-month data (IgG median:5.235, and IgM median:0.301), we observed that the antibody levels decreased significantly compared to the 21st and 14th days (p:0.031 for IgG, and p:0.042 for IgM).
Conclusion: Dose-comparing strategy can provide a certain level of protection and slow down the pandemic by delaying viral mutations at least until the second dose of vaccine, especially those living and working in crowded places. T and B cell memory efficiency should be kept in the foreground instead of thinking that individuals with low antibody responses are vulnerable to COVID-19. The long-term protection of the Coronavac vaccine is questionable, and a booster may be required at certain intervals until the pandemic is over.
Keywords
COVID-19 Vaccines, SARS-CoV-2 Virus, Coronavac, Humoral Immunity, Cellular Immunity, Dose-comparing
COVID-19 Vaccines articles, SARS-CoV-2 Virus articles, Coronavac articles, Humoral Immunity articles, Cellular Immunity articles, Dose-comparing articles
Article Details
1. Introduction
The world has not yet recovered from the COVID-19 crisis due to the lack of a cure. Nevertheless, despite inequalities in vaccine distribution, the availability and widespread use of vaccines in a concise time is crucial, at least in preventing severe cases and deaths. Today, various vaccines developed with different methodologies are used globally to protect human life against the SARS-COV-2 virus [1]. Also, various vaccine candidates are waiting to be used by completing their phase studies [2]. Although vaccination studies are promising, there are no definitive data on neutralizing antibody levels (Nab). Besides, the inability of companies to produce vaccines at sufficient speed and delays in distribution brought the option of single-dose vaccination to scientists hoping to protect as many people as possible, at least until the probable second dose of vaccine is reached [3-5]. Turkey was the first European country to give emergency use approval for Coronavac (Sinovac Life Sciences, Beijing, China) inactivated the COVID-19 vaccine for the vaccination program after phase 3 results. It was started primarily from the beginning health care workers who have been in intensive contact with patients [6]. As a different factor, worldwide production and stock problems have led to vaccine wars between countries [7,8]. The difficulties experienced in the production and supply points promised by the companies may continue. At this point, the problem of using limited resources may come to the fore in a short time.
Based on these points, we investigated the antibody responses on the first day (day 1) of the second dose, which we believe to be dependent on the first dose, and on the fourteenth (day 14) and twenty-first (day 21) days, where we can evaluate the effectiveness of the second dose. Thus, we aim to question the first dose's effectiveness and have information about antibody response kinetics after two doses of the Coronavac vaccine. In addition, we aimed to examine whether the vaccine continued to be protective by comparing the antibody responses three months after the 2nd dose.
2. Materials and Methods
2.1 Study Participants
From eighty-five health care workers vaccinated with double-dose CoronaVac (Sinovac Life Sciences, Beijing, China) COVID-19 vaccine applied with an interval of 28 days, seventy-nine individuals with 19-57 ages and %55 females and who had no history of COVID-19 were included in the study. One individual whose SARS-COV-2 virus genetic material was detected on day 10 by reverse transcriptase-polymerase chain reaction (RT-PCR) in the nasal swab sample was excluded from the day 14, day 21, and 3rd-month phases of the study. Four participants did not come to provide samples for the day 21 and 3rd-month analysis. Using drugs or having diseases that cause immunosuppression, organ transplant patients, pregnant women were omitted. The individuals volunteered for the study by signing the informed consent form. After the pre-approval from the Republic of Turkey Ministry of Health study, ethical approval was obtained from the Afyon Health Sciences University School of Medicine ethical board.
2.2 Study Design
Anti-spike protein IgG and IgM antibody analyses were performed on days one, fourteen, twenty-one, and 3rd-month of the second vaccine dose between January 14, 2021, and June 14, 2021. In addition, antibody responses developed by individuals between days were investigated. Blood samples taken from the participants were centrifuged at 1500G for 15 minutes in tubes without additives to obtain serum samples, and analyses were performed. The tests were analyzed using the Standard F2400, CE-approved rapid POCT device (S.D. Biosensor, Gyeonggi-do, Republic of Korea), with lateral flow immunoassay method and card tests containing two-dimensional square code under the manufacturer's product insert. According to the manufacturer, the results were reported as calculated luminescence units per mL (A.U./mL); values ≥ 1.00 AU/mL are considered positive, while values < 1.00 AU/mL are considered negative.
2.3 Statistics
The data distributed normally was investigated using Excel (Microsoft Inc, Redmont, Washington, USA). The paired sample t-test test was used to compare group means according to days. Antibody responses expected to change over time were analyzed using Pearson's correlation analysis and the Wilcoxon rank test. We summarized variables as mean ± standard error (S.E.), mean ± standard deviation (S.D.). P-values below 0.05 were considered significant. Statistical analyses were assessed via Minitab 19 statistical software (State College, Pennsylvania, USA).
3. Results
The mean age was 30.5 (95% CI 26.88; 34.12). Male volunteers (mean:26.05) were older than (mean:35.94, 55% of total) females (p=0.005). While there was no difference between day one (median:0.54) and day 14 (median:0.62) and day 21 (median:0.56) in terms of IgM mean values (p=0.192 and p=0.973, respectively), day 14 IgM mean values were slightly higher than day 21 (p=0.045). There was a significant difference between day one (median:7.13) and day 14 (median:10.18) and day 21 (median:11.75) and between day 14 and day 21 in terms of mean IgG values (p<0.0001, p<0.0001, and p=0.005, respectively). Also, a significant correlation was found between day 14 and day 21 in terms of IgM values (r=0.888, p<0.001), and a robust correlation were found between day one and day 14 and between day 14 and day 21 in IgG values (r=0.798, p< 0.001 and r=0.947, p<0.001). Correlation analyzes are demonstrated in figure 1 for IgM and figure 2 for IgG. The time series plots are displayed in figure 3 for IgM and figure 4 for IgG. Considering the 3rd-month data (IgG median:5.235, and IgM median:0.301), we observed that the antibody levels decreased significantly compared to the 21st and 14th days (p:0.031 for IgG, and p:0.042 for IgM). IgM levels are demonstrated in Figure 1 and IgG levels in Figure 2.
4. Discussion
Although IgM type antibody analyzes are usually performed in clinical laboratories to help diagnose acute or reactivated infection; for several viral infections, including Epstein-Barr virus (EBV), measles, and parvovirus B19, when patients admit clinically, for diagnosis in the presence of low viral load, nonspecific symptomatology and known arboviral infection with relatively short viremic periods, in serological diagnosis. It also helps the clinic in acute infections of the challenged herpes simplex virus types 1 and 2 (HSV-1/2), enterovirus, adenovirus, chickenpox zoster virus (VZV) [10]. After vaccination, the expected protective antibody responses are of the IgG type, and an increase in infection-like IgM levels is not likely. Our study results in this context are consistent with basic immunology knowledge. IgG type Nab responses are expected after vaccination, and in Coronavac vaccine studies, Nab geometric mean IgG titers that can neutralize live SARS-COV-2 virus were evaluated [11]. The same study also stated that neutralizing antibody responses developed in all subjects on the 14th day after two doses of vaccine administered with an interval of 14 days [11]. Our study group showed high IgG responses both on the 1st day, where the second dose of vaccine could not yet affect antibody production, and on the 14th and 21st days. Even a single dose of the vaccine that provides such high IgG responses is likely related to healthcare workers having certain protective antibody levels even before vaccination due to asymptomatic infection and constantly encountering COVID-19 patients. The Ad5 vectored COVID-19 vaccine expressing the spike glycoprotein of SARS-CoV-2 by Beijing Institute of Biotechnology (Beijing, China) and CanSino Biologics (Tianjin, China) ensured a sufficient amount of Nab production even with a single dose of small amounts [12].
Johnson & Johnson company claimed that the vaccine they produced would provide adequate protection against COVID-19 after at least 28 days with a single dose administration and prevent hospitalizations and deaths to a great extent [13]. In Sao Paolo, where COVID-19 continues to be the most severe, authorities have put the single-dose Coronavac vaccine strategy in the foreground to use the limited vaccine dose more efficiently [14]. Considering that most of society is asymptomatic or carriers, single-dose vaccination can be life-saving to protect more people until they reach the second dose of vaccine. People who live in communal areas agree with a large number of people. One might think that adequate immunity may not be achieved with a single dose vaccination, and viral escape from the vaccine may be possible. Nevertheless, various modeling studies have shown that dose sparing strategies will reduce the disease burden from COVID-19 [15-17]. As a general approach to vaccination, it is accepted that even with a single dose of vaccination, protection levels will be reached more than half compared to those who have an infection; thus, the rate of spread of the disease and, therefore, the possibility of mutation will decrease [18,19]. We believe that dose comparing could reduce disease burden while production and logistics problems continue in COVID-19 vaccines.
Another point that draws our attention in our study data was that the levels were generally low on day 14 and day 21 in patients with low IgG levels at day one and high in those with high levels. We think these responses may be related to biological variations, genetic and epigenetic factors, and exposure to fewer or more COVID-19 patients depending on the healthcare facility department. However, we also know that the only goal in vaccine applications is not to generate high Nab responses. It is aimed at the humoral and cellular immune systems to recognize the microbiological factor together. It has been stated that in response to COVID-19 infection, the production of virus-specific T cells increases in correlation with antibody responses in the defense system [20]. Besides, researchers demonstrate that infection generates both IgG and IgG memory B cells against the novel receptor binding domain and the conserved S2 subunit of the SARS-CoV-2 spike protein [21]. A preprint article interim report indicated that a significant T cell response induction characterized by IFN-gamma secretion upon stimulation was observed with mega peptide pools derived from SARS-CoV-2 proteins in those vaccinated with Coronavac [22]. We believe that people with low Nab responses are less likely to have a viral infection when they encounter SARS-COV-2, thanks to their T and B cell memory and that severe illness or death will not occur. Low antibody levels at three months may indicate that the protection of the Coronavac vaccine is rapidly diminishing. Although we need to vaccinate many people quickly to get rid of the pandemic, it is also desirable that the protection of vaccines should not be reduced immediately. Regular repeat doses may be required for vaccines.
5. Conclusion
While there are problems in vaccine production and logistics, the road covered in a short time in the fight against the pandemic is vital. In this context, single-dose vaccination can provide a certain level of protection and slow down the pandemic by delaying viral mutations at least until the second dose of vaccine, especially those living and working in crowded places. Therefore, T and B cell memory efficiency should be kept in the foreground instead of thinking that individuals with low antibody responses are vulnerable to COVID-19. Although long-term protection of vaccines is currently questionable, more data is needed.
Funding
No financial support has been received from any person or organization for this study. There is no conflict of interest.
References
- World Health Organisation. Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process.
- World Health Organisation. Draft landscape and tracker of COVID-19 candidate vaccines.
- Hung, Ivan FN, and Gregory A Poland. “Single-dose Oxford-AstraZeneca COVID-19 vaccine followed by a 12-week booster.” Lancet (London, England) 397 (2021): 854-855.
- Iacobucci, Gareth. “Covid-19: Single dose of Pfizer and Oxford vaccines cuts risk of hospital admission by 80% in over 80s, data suggest.” BMJ (Clinical research ed) 372 (2021).
- Ledford, Heidi. “J&J’s single-dose COVID vaccine raises hopes for faster rollout.
- Sinovac Commences Phase III Clinical Trials for COVID-19 Vaccine Candidate in Turkey.
- Biochemistry wins the Covid19 battle but Logistics and Supply Chain will win the war.
- Wong WKO. “Sino-Western rivalry in the COVID-19 “vaccine wars”–A race to the bottom?”, Asian Education and Development Studies (2021).
- Zhu, Feng-Cai et al. “Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial.” Lancet (London, England) 395 (2020): 1845-1854.
- Mayo Clinic Laboratories. Testing for IgM-class Antibodies to Determine Acute Infection: Clinical and Diagnostic Considerations.
- Zhang, Yanjun et al. “Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial.” The Lancet. Infectious diseases 21 (2021): 181-192.
- Food & Drug Administration. FDA briefing document: Janssen Ad26.COV2.S vaccine for the prevention of COVID-19 (2021).
- Ledford, Heidi. “J&J’s single-dose COVID vaccine raises hopes for faster rollout.” Nature.
- Olhar Digital. Coronavirus. Out of the ordinary: São Paulo is considering applying a single dose of CoronaVac.
- Tuite, Ashleigh R et al. “Alternative Dose Allocation Strategies to Increase Benefits From Constrained COVID-19 Vaccine Supply.” Annals of internal medicine (2021): M20-8137.
- Barnabas, Ruanne V, and Anna Wald. “A Public Health COVID-19 Vaccination Strategy to Maximize the Health Gains for Every Single Vaccine Dose.” Annals of internal medicine (2021): M20-8060.
- Paltiel, A David et al. “Speed Versus Efficacy: Quantifying Potential Tradeoffs in COVID-19 Vaccine Deployment.” Annals of internal medicine (2021): M20-7866.
- Riley, Steven et al. “Optimizing the dose of pre-pandemic influenza vaccines to reduce the infection attack rate.” PLoS medicine 4 (2007): e218.
- Wu, Joseph T et al. “Fractional dosing of yellow fever vaccine to extend supply: a modeling study.” Lancet (London, England) 388 (2016): 2904-2911.
- Ni, Ling et al. “Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals.” Immunity 52 (2020): 971-977.e3.
- Nguyen-Contant, Phuong et al. “S Protein-Reactive IgG and Memory B Cell Production after Human SARS-CoV-2 Infection Includes Broad Reactivity to the S2 Subunit.” mBio 11 (2020): e01991-20.
- Susan M Bueno, Katia Abarca, Pablo A Gonzalez. ‘’Interim Report: Safety And Immunogenicity Of An Inactivated Vaccine Against Sars-Cov-2 In Healthy Chilean Adults In A Phase 3 Clinical Trial’’ (2021).