Alkaline Extraction of Protein from Sacha Inchi Oil Press-cake: Effect of pH, Temperature, and Extraction Time
Article Information
Monika Mich2, Rong Phaltevy Ung2, Sereytevin Chab2, Marinich Net2, Sela Kong1,2*, Reasmey Tan1,2, Manit Say1, Yukleav Nat1,2, Chin Ping Tan3
1Research and Innovation Center, Institute of Technology of Cambodia, Russian Federation Blvd., P.O. Box 86, Phnom Penh, Cambodia
2Faculty of Chemical and Food Engineering, Institute of Technology of Cambodia, Russian Federation Blvd., P.O. Box 86, Phnom Penh, Cambodia
3Department of Food Technology, Faculty of Food Science and Technology, Universiti Putra Malaysia, 43400 UPM Serdang Selangor, Malaysia
*Corresponding Author: Sela Kong, Institute of Technology of Cambodia, Russian Federation Blvd., P.O. Box 86, Phnom Penh, Cambodia.
Received: 09 December 2023; Accepted: 21 December 2023; Published: 14 March 2024
Citation: Monika Mich, Rong Phaltevy Ung, Sereytevin Chab, Marinich Net, Sela Kong, Reasmey Tan, Manit Say, Yukleav Nat, Chin Ping Tan. Alkaline Extraction of Protein from Sacha Inchi Oil Press-cake: Effect of pH, Temperature, and Extraction Time. Journal of Food Science and Nutrition Research. 7 (2024): 74-79.
Share at FacebookAbstract
Sacha inchi (Plukenetia volubilis L.) has been used for decades due to its rich in oil and protein. Within the oil extraction, a by-product known as oil press-cake is produced and it is typically wasted even though it contains a high protein content. Therefore, this study aimed to valorize Sacha inchi oil press-cake by extraction of protein using an alkaline extraction method. The effects of the alkaline extraction conditions, including pH (10, 11, and 12), temperature (40, 50, and 60°C), and extraction time (30 and 60 min) were evaluated. The proximate composition of Sacha inchi oil press-cake was also determined using AOAC standard method, while the extracted protein recovery yield was analyzed using Lowry method. As a result, the proximate composition of sacha inchi oil press-cake contained protein (59.5%), total carbohydrates (22.38%), fat (3.59%), ash (5.22%), and moisture (9.32%). The highest protein recovery using alkaline extraction from oil press-cake was 83.57 ± 0.37% with the following conditions: pH 12, 60°C of temperature, and 30 min of extraction time. These results demonstrate that the sacha inchi oil press-cake is a good source of proteins for human nutrition. The conventional alkaline extraction method can be applied to extract protein from the sacha inchi oil press-cake with high efficiency.
Keywords
Sacha inchi, Oil press-cake, By-product, Protein recovery, Extraction
Sacha inchi articles; Oil press-cake articles; By-product articles; Protein recovery articles; Extraction articles
Article Details
Introduction
Sacha Inchi (Plukenetia volubilis) is a native plant from the Amazon region of South America. It is globally recognized as a functional food due to its high nutritional value including 35-60% lipids, 25-30% proteins and other Vitamin E and polyphenols [1,2]. After the mechanical extraction of oil from Sacha Inchi using an oil press machine, a residue known as Sacha inchi oil press-cake (SIOPC) is produced and it is often improperly underutilized or not valorized. However, SIOPC is actually rich in beneficial compounds including fatty acids, amino acids, polyphenols, polysaccharides, dietary fiber, and organic acids [3]. Additionally, SIOPC has been recognized as a significant source of protein. In fact, it contains more crude protein than tea seed press cake, which is primarily rich in carbohydrates [4]. Therefore, this residue has the potential to be a unique food commodity, presenting an innovative way of turning waste into a valuable food source [5]. The alkaline extraction method is a traditional technique extensively employed to extract proteins from diverse industrial resources. It principally involves the use of alkaline solutions which typically with a pH range of 8-11, like sodium hydroxide (NaOH), at different concentrations. This method also requires varying the ratio of solvent to sample, temperatures, and extraction time to optimize results [6].
Therefore, the objective of this study is to extract protein from SIOPC and to evaluate the effect of alkaline extraction conditions such as pH and extraction temperature and extraction times on protein yield and protein recovery.
Methodology
Chemicals and reagents
The N-hexane with 99% purity (RIC labscane, Thailand) was used to analyze crude oil content, and NaOH (0.1M) (Merck, Germany) was used to extract protein from the SIOPC. Total protein test kit (micro-lowry) and protein standard (Sigma Aldrich, Germany) were used to determine the quantitation of soluble protein.
Sample preparation
Sacha inchi seeds were purchased from Rattanakiri, Cambodia. The seeds were manually de-shelled and ground before being defatted with a hydraulic press machine. Sacha inchi oil was extracted by using cold press extraction with a hydraulic press machine (BY-180, China) under pressure 50MPa for 40 min at a temperature of 25 °C. The oil residues from the oil extraction were SIOPC. The SIOPC was ground using a dry grinder machine and was sieved into average particle sizes of approximately 0.5 mm for further extraction.
Experimental design
The protein was extracted using the combinations of temperature and pH and the experimental design as shown in table 1.
|
Fixed variables |
|
|
Solvent type |
Water |
|
Ratio of sample to solvent |
5g/50mL |
|
Sample size |
0.5mm |
|
Independent variables |
|
|
pH |
10, 11, and 12 |
|
Temperature |
40, 50, and 60°C |
|
Extraction time |
30 and 60 min |
Table 1: Experimental design of variables.
Protein extraction of sacha inchi oil press-cake
Protein extraction from sacha inchi was conducted according to the alkaline extraction and acid precipitation method defined by KIM et al. (1992), with minor modifications. Five grams of SIOPC flour samples were mixed with 50 ml of distilled water at a 1:10 (w/v) ratio. These mixtures were then extracted using selected combinations of independent variables from the experimental design in table 1. The pH was adjusted to 10, 11, and 12 using 1 M of NaOH, and the mixtures were constantly stirred and heated at various temperatures (40, 50, and 60°C) for 30 and 60 min of extraction time. After extraction, the solutions were immediately centrifuged at 4000×g for 30 min at 25°C (HERML Z326K, German). The resulting suspension was collected, and both the soluble protein and protein yield (%) were measured using Lowry method. And the experiments were conducted in duplicate.
Protein quantification
Lowry method: The protein yield of extraction suspension was analyzed by Total Protein Kit using micro-Lowry, Peterson’s modification [8]. Firstly, the standard tubes were prepared by diluting the 400 µg/mL protein standard solution in water to a volume of 1.0 mL. These standard tubes were appropriately prepared according to table 2, whereas blank was prepared by adding 1.0 mL of water to a labeled test tube. After that, the sample was diluted with water by a factor of 115, and then added 1 ml to the appropriately labeled test tube. The Lowry reagent solution of 1.0 ml was added to the standards, blank, and sample tubes, and mixed well. The solutions were allowed to stand at room temperature for approximately 20 minutes. Then, 0.5 ml of Folin & Ciocalteu’s Phenol reagent working solution was added to each tube with rapid and immediate mixing. The color was allowed to develop for 30 minutes. Next, the solutions were carefully transferred to cuvettes, and measured the absorbance of the standards and sample tubes versus the blank at 750 nm (Agilent Cary 60, Australia). The absorbance readings were completed within 30 minutes. To prepare the calibration curve, the absorbance values of the standards were plotted versus their corresponding protein concentrations.
|
Protein standard solution (ml) |
Water (ml) |
Protein concentration (µg/ml) |
|
0.125 |
0.875 |
50 |
|
0.25 |
0.75 |
100 |
|
0.5 |
0.5 |
200 |
|
0.75 |
0.25 |
300 |
|
1 |
0 |
400 |
Table 2: Dilution of protein standard solution
Protein calculation: The protein concentration in the original SIOPC sample was determined by multiplying the measured results with the dilution factors. The protein yield and protein recovery of SIOPC were calculated using the following equations.
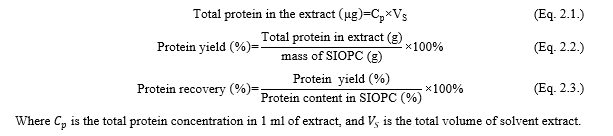
Proximate composition of oil-press cake analysis: The standard method of AOAC were used to analyze the proximate composition of sacha inchi seeds, including moisture content (AOAC method 925.10), ash content (AOAC method 942.05), fat content (AOAC method 920.39), and protein content (AOAC method 960.52)[9]. The total carbohydrate, moisture content, and crude fat content were calculated as percentage in wet basis with the following formula:
Data analysis
The proximate compositions, protein content, protein concentration, and protein recovery were presented as mean ± SD, calculated using SPSS Statistics 25 (IBM, Armonk, NY, USA). To examine the effect of pH and temperature on protein recovery at each extraction time, the data were subjected to an analysis of variance (two-way ANOVA). Duncan’s multiple-range test was used for mean comparison to investigate significant differences (p<0.05). The correlation coefficient (R2) was applied to measure the strength of a linear relationship between protein concentration and absorbance of protein standards.
Results and Discussion
Proximate composition of sacha inchi seed (SIS): The proximate composition of SIS in this study compared to the previous study is presented in table 3. As seen in this table, the proximate composition of SIS was obtained including 4.87% of moisture, 48.80% of total fat, 16.93% of total carbohydrate, 28.02% of protein, and 2.39% of ash content. The result of this proximate composition was similar to the result reported by Ruiza et al. (2013). This data revealed that the SIS seeds are distinctive due to their higher fat content when compared to other oilseeds such as soybean (19%), peanut (45%), cotton (16%), and sunflower (48%) [11]. These differences in seed composition could be attributed to various factors such as diverse subspecies, differences in geographical and climatic conditions, the timing of seed harvesting, and the treatment of seeds after harvest. The content of total carbohydrates in SIS is relatively low, comprising only 16.93%, in comparison to the values reported by. This could be due to the high concentrations of crude oil and protein in SIS [1].
|
Compositions (%) |
This study |
Gutiérrez et al. (2011) |
Ruiza et al. (2013) |
|
Moisture |
4.87 ± 0.08 |
3.30 ± 0.30 |
- |
|
Total fat |
48.80 ± 0.50 |
42.0 ± 1.10 |
49.0 ± 0.10 |
|
Total carbohydrate |
16.93 ± 0.82 |
30.90 ± 0.60 |
18.7 ± 1.3 |
|
Protein |
28.02 ± 0.63 |
24.70 ± 0.50 |
29.6 ± 0.50 |
|
Ash |
2.39 ± 0.04 |
4.0 ± 0.70 |
2.7± 0.20 |
Table 3: Proximate compositions of sacha inchi seeds (SIS)
Proximate composition of SIOPC
The proximate composition of SIOPC flour after hydraulic pressing is depicted in table 4. The results revealed that SIOPC flour primarily consists of three main components including protein at 59.5%, total carbohydrates at 22.38%, and fat at 3.59%, while the minor components include ash (5.22%) and moisture content (9.32%). These findings align closely with the results reported by Chirinos et al. (2017) and Rawdkuen et al. (2018). However, a significantly lower of fat content in the flour was recognized. This can be attributed to the process and conditions used to extract oil from the SIS. In addition, the protein content proved to be significantly higher at 59.5%, compared to a typical range of 53% to 59% found in similar studies [11,14]. According to Hamaker et al. (1992), it should be noted that the protein content in SIOPC flour, produced from de-hulled oilseeds, can fluctuate based on the variety of seed, with the range typically falling between 35 and 60% (dry weight basic) [15].
|
Compositions (%) |
This study |
Chirinos et al. (2017) |
Rawdkuen et al. (2018) |
|
Moisture |
9.315 ± 0.08 |
7.9 |
5.3 |
|
Total fat |
3.59 ± 0.06 |
8.9 |
6.7 |
|
Total carbohydrate |
22.38 |
22.7 |
36.1 |
|
Protein |
59.5 |
58.4 |
45.9 |
|
Ash |
5.215 ± 0.02 |
5.7 |
5.9 |
Table 4: Proximate compositions of SIOPC
Furthermore, the protein content in SIOPC is higher than the protein in SIS due to the significant quantities of oil and protein present in SIS. When the oil is extracted, the protein content in SIOPC is increased. These studies indicate that protein content in SIOPC can fluctuate based on the effectiveness of the oil extraction process, hence making SIOPC a significant protein source.
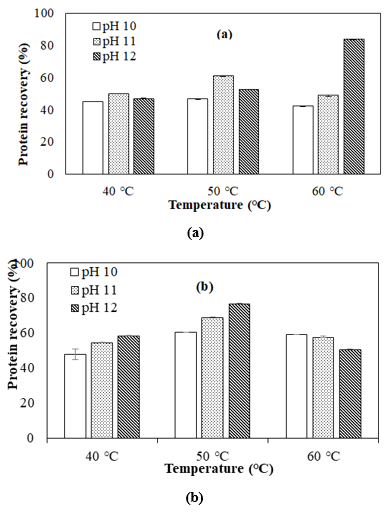
Effect of pH on the protein yield at different extraction times
The effect of pH on protein recovery yield using different temperatures at 30 and 60 min is shown in figure 1 (a) and 3 (b), respectively. At these extraction times, the experimental result revealed that the factor of extraction pH had a significant effect (p<0.05) on protein recovery.
At 30min, the lowest protein recovery (42.11%) was found at pH 10, while the highest protein recovery (83.57%) was found at pH 12. This finding is similar to Pezo and M. (2018), who reported that the protein recovery of SIOPC obtained by extraction with alkaline water at pH 12.0 presented 84.4%.
At 60 min, the lowest protein recovery (47.79%) was found at pH 10, while the highest protein recovery (76.88%) was found at pH 12. According to figure 3.1(b), the higher pH extraction increased the protein recovery at 40°C and 50°C. This result was symmetry to Ahlström et al. (2022) who reported that the extraction yield was highly influenced by the extraction pH, with higher pH values corresponding to larger yields. Thus, one approach to obtain a high protein recovery was to degrade the cell wall for protein diffusion into the solvent. However, at a condition of 60°C, the higher pH decreased the protein yield of extraction, which shows that the longer time extraction with higher temperature and higher pH caused the solubility of protein and the protein recovery was decreased. Based on the result of Ibrahim A. et al. (2012), the modest decrease in average extracted protein for extraction times more than 60 minutes could be due to soluble protein agglomeration with other food components such as phytate [19], which can be solubilized after a long extraction time. Accordingly, this result was attributed to increased protein denaturation at higher pH, which is highly relied on the cell wall and protein structure. Overall, the higher pH with the long extraction time provided more time for the diffusion of the protein into the solvent.
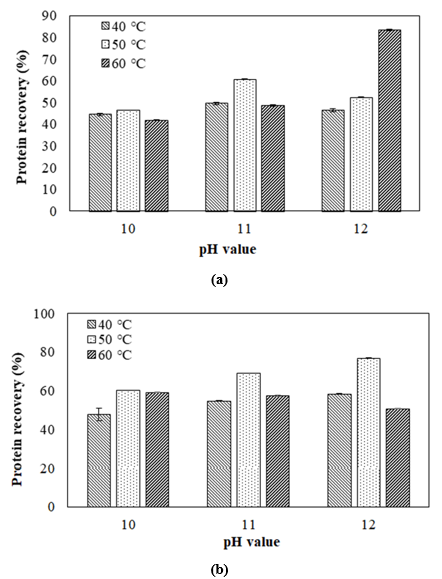
Effect of extraction temperature on protein yield at different extraction times
Figure 2 shows the effect of temperature on protein recovery using different extraction pH and time. According to the ANOVA analysis, the temperature was significantly affected (p< 0.05) on protein recovery. According to figure 2(a) at pH 12, the protein recovery was increased at higher temperatures. This result was supposed that during 30 min the high temperatures with the higher pH extraction increased the protein recovery of extraction. This is due to the modification of the protein structure and solubility of SIOPC in the alkali phase. Furthermore, the protein recovery at the conditions of pH 10 and 11 was high at 50°C. This result is consistent with the findings of Chirinos et al. (2017), who conducted a similar study for optimizing protein extraction parameters from SIOPC through alkaline and enzyme-assisted extraction techniques. The results were lower than this study due to the utilization of pH and temperature during the extraction, solid-to-solvent ratio, and type of solvent. The findings have also proven that temperature is one of the extraction factors influencing the extractability of SIOPC protein. Therefore, it can be used as a variable parameter to optimize the protein recovery.
Figure 2(d) shows the effect of temperature on protein recovery using different extraction pH at 60 min. The optimum temperature for protein extraction is 50°C, while at pH 12 and 60°C the protein recovery was decreased. Long-time extraction using a high temperature of 60°C or more could denature the protein and precipitate out of the solution [20,21]. The decreased protein recovery at 60°C could be attributed to the fact that increasing temperature reduces the viscosity of the mixture. This reduction in viscosity minimizes the diffusion resistance of the molecules, thereby leading to an increased mass transfer rate. However, the increase in temperature also diminishes the extraction rate [22]. As a result, an extraction temperature range of 40-60°C was selected to achieve the optimal extraction conditions.
Efficiency of protein extraction
The protein recovery yield from de-oiled cakes, made from oilseeds through an alkaline extraction process, varied for different seeds. The ranges were 10.9g to 32.6g of protein/100g for flaxseed, 3.3g to 5.7g for pigeon pea, 12.3g to 16.5g for soybean seed, and 40.8g to 58.7g for lentil [23–25]. As shown in table 4, the protein yield resulting from a 30 minutes extraction time ranged from 25.06% to 49.72%, with protein recovery ranging from 42.11% to 83.57%. However, when the extraction time was increased to 60 minutes, the protein yield ranged from 28.43% to 45.74%, and protein recovery ranged from 47.79% to 76.88%. The experimental finding showed that optimal protein recovery of 83.57% at 30 minutes of extraction was accomplished at a pH of 12.0 and a temperature of 60°C. Meanwhile, the highest protein recovery for a 60 minutes extraction, yielding 76.88%, was observed under the same pH, but with a lower temperature of 50°C. This finding results show a higher yield than Chirinos et al. (2017), who obtained a 29.7% yield using alkaline extraction which used the alkaline extraction was obtained 29.7% at 54.2°C, a solvent-to-solid ratio of 42:1 (v/w), a NaCl concentration of 1.65 M, a pH of 9.5, and 30 minutes of extraction time. However, when using the enzyme-assisted method the optimal yield of 44.7% was significantly higher at 50°C, a solid-to-solvent ratio of 1:50 (w/v), an enzyme concentration of 5.6%, a pH of 9.0, and 40.4 minutes of extraction time. Rawdkuen et al. (2022) reported the extraction protein yield and protein recovery of protein isolates obtained from the Peru SIS and Thai SIS were (7.0 ± 0.1%, 59.3 ± 2.5%) and (5.0 ± 0.7%, 49.2 ± 7.5%), respectively. These results were obtained using the extraction conditions of pH 11.0 at a temperature of 50°C and 60 minutes of extraction time. Furthermore, this result could be compared to the result of Sathe et al. (2012) who reported that the extraction yield of 47% was obtained.
|
Sample |
SIOPC |
|
Extraction method |
Alkaline extraction |
|
Optimum conditions |
Solvent/sample ratio 10/1 (v/w), extraction time 30 min, temperature 30°C, and pH 12 |
|
Protein yield (%) |
49.72% |
|
Protein recovery (%) |
83.57% |
Table 4: Results of protein yield and protein recovery at the optimum conditions
Conclusions
The study has made significant insights into the proximate compositions of sacha inchi seeds and oil press-cake, as well as the efficacy of the alkaline extraction method for protein recovery. The key findings of the research highlight the high fat and protein content in sacha inchi seeds, while its oil press cake contains protein as its key component. Furthermore, the extraction factors including pH and temperature demonstrably impact the protein recovery, indicating the need for specific parameters to ensure maximum protein extraction. Importantly, this study underscores the potential of protein derived from this by-product, not only as a source of dietary protein in fortified foods and supplements, but also in various industrial applications due to its inherent techno-functional characteristics.
Funding
The work was funded by the Cambodia Higher Education Improvement Project (Credit No. 6221-KH).
References
- Gutiérrez LF, Rosada LM, Jiménez Á. Chemical composition of sacha inchi seeds and characteristics of their lipid fraction. Grasas y Aceites 62 (2011): 76-83.
- Wang S, Zhu F, Kakuda Y. Sacha inchi (Plukenetia volubilis L): Nutritional composition, biological activity, and uses. Food Chem 265 (2018): 316-328.
- Kodahl N, Sørensen M. Sacha Inchi (Plukenetia volubilis L.) Is an underutilized crop with a great potential. Agronomy 11 (2021): 1066.
- Rawdkuen S, Murdayanti D, Ketnawa S, et al. Chemical properties and nutritional factors of pressed-cake from tea and sacha inchi seeds. Food Biosci 15 (2016): 64-71.
- Singh R, Langyan S, Sangwan S, et al. Protein for Human Consumption From Oilseed Cakes: A Review. Front Sustain Food Syst 6 (2022): 63-72.
- Souza D de, Sbardelotto AF, Ziegler DR, et al. Characterization of rice starch and protein obtained by a fast alkaline extraction method. Food Chem 191 (2016): 36-44.
- Kim N, Kim YJ, Nam YJ. Characteristics and functional properties of protein isolates from various peanut (Arachis hypogaea L.) cultivars. J Food Sci 57 (1992): 406-410.
- Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83 (1977): 346-356.
- Official methods of analysis. (15th edtn), Washington, DC, USA: Association of Official Analytical Chemists (1995).
- Ruiza C, Díazb C, Anayac J, Rojasa R. Análisis proximal, antinutrientes, perfil de ácidos grasos y de aminoácidos de semillas y tortas de 2 especies de Sacha inchi (Plukenetia volubilis y Plukenetia huayllabambana). Rev La Soc Química Del Perú 79 (2013): 29-36.
- Hamaker B, Valles C, Gilman R, et al. Amino acid and fatty acid profiles of the Inca peanut (Plukenetia volubilis). Cereal Chem 69 (1992): 461-463.
- Chirinos R, Aquino M, Pedreschi R, et al. Optimized methodology for alkaline and enzyme-assisted extraction of protein from sacha inchi (Plukenetia volubilis) kernel cake. J Food Process Eng 40 (2017): e12412.
- Rawdkuen S, Rodzi N, Pinijsuwan S. Characterization of sacha inchi protein hydrolysates produced by crude papain and Calotropis proteases. LWT 98 (2018): 18-24.
- Sathe SK, Kshirsagar HH, Sharma GM. Solubilization, fractionation, and electrophoretic characterization of Inca peanut (Plukenetia volubilis L.) proteins. Plant Foods Hum Nutr 67 (2012): 247-255.
- Hamaker BR, Valles C, Gilman R, et al. Amino Acid and Fatty Acid Profiles of the Inca Peanut (Plukenetia volubilis). Cereal Chem 69 (1992): 461-463.
- Pezo CMC. Obtención y caracterización de un aislado proteico a partir de la torta desengrasada de sacha inchi (Plukenetia volubilis L.). Universidad Nacional de San Martín. Fondo Editorial (2018).
- Ahlström C, Thuvander J, Rayner M, et al. The Effect of precipitation pH on protein recovery yield and emulsifying properties in the extraction of protein from cold-pressed rapeseed press cake. Molecules 27 (2022): 29-57.
- Ibrahim A, Akasha, Lydia C, et al. Extraction and characterisation of protein fraction from date palm fruit seeds. World Academy of Science (2012).
- Alli I, Baker BE. Constitution of leguminous seeds. A note on protein-phytic acid interactions during isolation of acid-soluble protein from phaseolus beans. J Sci Food Agric 32 (1981): 588-592.
- Massoura E, Vereijken JM, Kolster P, Derksen JTP. Protein from Crame abyssinica oilseed. Isolation procedure. J Am Oil Chem Soc 75 (1998): 323-327.
- Kwon KS, Bae D, Park KH, et al. Aqueous extraction and membrane techniques improve coconut protein concentrate functionality. J Food Sci 61 (1996): 753-756.
- Wu X, Sun Y, Shi Y, et al. Study on kinetics of protein forward extraction from Quinoa by AOT/ isooctane reverse micelles system. Proc. 2018 3rd Conf. Model. Simul. Appl. Math. (MSAM 2018), Paris, France: Atlantis Press (2018).
- Jarpa-Parra M, Bamdad F, Wang Y, et al. Optimization of lentil protein extraction and the influence of process pH on protein structure and functionality. LWT - Food Sci Technol 57 (2014): 461-469.
- Oomah BD, Mazza G, Cui W. Optimization of protein extraction from flaxseed meal. Food Res Int 27 (1994): 355-361.
- Tan ES, Ying-Yuan N, Gan CY. A comparative study of physicochemical characteristics and functionalities of pinto bean protein isolate (PBPI) against the soybean protein isolate (SPI) after the extraction optimisation. Food Chem 152 (2014): 447-455.
- Rawdkuen S, D’Amico S, Schoenlechner R. Physicochemical, functional, and in vitro digestibility of protein isolates from Thai and Peru sacha inchi (Plukenetia volubilis L.) oil Press-Cakes. Foods 11 (2022): 1869.