Adherence to Combined Exercise and Dietary Intervention in Patients with Gastrointestinal Cancer Undergoing Neo-Adjuvant Therapy: An Open-Label, Pilot, Randomized Controlled Trial
Article Information
Velho S1, Moço S1, Capitão C1, Branco M1, Costa L2, Rodrigues S3, Abreu C4, Alves R4, Pires F4, Sousa P5, Agostinho L5, Cruz R5, Clemente S3, Borges A4, Lopes F2, Godinho J2, Faria A2, Teixeira JA2, Passos Coelho JL2, Maio R6, Baracos VE7, Cravo M8
1Dietetics and Nutrition Department, Hospital Beatriz Ângelo, Loures, Portugal
2Oncology Department, Hospital Beatriz Ângelo, Loures, Portugal
3Pneumology Department, Hospital Beatriz Ângelo, Loures, Portugal
4Physical Medicine and Rehabilitation Department, Hospital Beatriz Ângelo, Loures, Portugal
5Radiology Department, Hospital Beatriz Ângelo, Loures, Portugal
6Surgery Department, Hospital da Luz, Lisboa, Portugal
7Oncology Department, University of Alberta, Canada
8Gastroenterology Service, Hospital da Luz, Loures, Portugal
*Corresponding Author: Sónia Velho, Nutrition and Dietetics Department, Hospital Beatriz Ângelo, Loures, Portugal
Received: 30 August 2022; Accepted: 06 September 2022; Published: 11 October 2022
Supplementary File Table
Supplementary File Figures
Citation: Velho S, Moço S, Capitão C, Branco M, Costa L, Rodrigues S, Abreu C, Alves R, Pires F, Sousa P, Agostinho L, Cruz R, Clemente S, Borges A, Lopes F, Godinho J, Faria A, Teixeira JA, Passos Coelho JL, Maio R, Baracos VE, Cravo M. Adherence to Combined Exercise and Dietary Intervention in Patients with Gastrointestinal Cancer Undergoing Neo-Adjuvant Therapy: An Open-Label, Pilot, Randomized Controlled Trial. Journal of Food Science and Nutrition Research 5 (2022): 669-681.
Share at FacebookAbstract
Background: To assess adherence of gastrointestinal cancer patients to a Combined Exercise and Dietary Intervention (CEDI) during neo-adjuvant chemotherapy.
Methods: Parallel randomized controlled, open label, pilot trial. A table from a web based randomization system was used to allocate treatments. 46 patients were screened at diagnosis of esophageal, gastric, pancreatic and rectal cancer from June 2018 to November 2019 at a teaching hospital in Loures, 39 were randomized. A planned interim analysis was performed and results are herein presented. Patients were randomized to receive either 8 week individualized CEDI, with moderate aerobic and resistance training, dietary counseling and oral nutritional supplements or standard care. Follow up was conducted after neo-adjuvant treatment. Main outcome measures were adherence to CEDI, change in weight, body composition and functional status. Adherence to CEDI was analyzed with an intention to treat approach, other outcome measures were analyzed with a per protocol approach. Data analysis was conducted with Chi-square test or Fisher exact test and t-test or Mann Whitney U test. Effect size was computed with Cohen’s d for t tests and r for Mann-Whitney U tests. Paired-samples t test or Wilcoxon Signed Rank Test were used to analyze longitudinal data.
Results: 39 patients (CEDI n=19 or control n=20) were randomized and included in the intention to treat analysis (29 (74.3%) male, median age 63.5 (Interquartile Range (IQR):11.75)). 32 patients completed follow up. 13/19 (68.4%) were fully adherent to CEDI. CEDI patients maintained weight (Effect size (EF):0.457; 95% Confidence Interval (95%CI): [0.44,0.46]), waist circumference (EF:-0.56, 95%CI: [-1.08, -0.034]), had a lower skeletal muscle loss (EF:-0.79; [-1.77;0.18]) and improved 6 minute walking test distance (E
Keywords
Adhesion; Exercise, Diet, Oral nutritional supplements, Gastrointestinal cancer
Adhesion articles; Exercise articles; Diet articles; Oral nutritional supplements articles; Gastrointestinal cancer articles
Adhesion articles Adhesion Research articles Adhesion review articles Adhesion PubMed articles Adhesion PubMed Central articles Adhesion 2023 articles Adhesion 2024 articles Adhesion Scopus articles Adhesion impact factor journals Adhesion Scopus journals Adhesion PubMed journals Adhesion medical journals Adhesion free journals Adhesion best journals Adhesion top journals Adhesion free medical journals Adhesion famous journals Adhesion Google Scholar indexed journals Exercise articles Exercise Research articles Exercise review articles Exercise PubMed articles Exercise PubMed Central articles Exercise 2023 articles Exercise 2024 articles Exercise Scopus articles Exercise impact factor journals Exercise Scopus journals Exercise PubMed journals Exercise medical journals Exercise free journals Exercise best journals Exercise top journals Exercise free medical journals Exercise famous journals Exercise Google Scholar indexed journals Diet articles Diet Research articles Diet review articles Diet PubMed articles Diet PubMed Central articles Diet 2023 articles Diet 2024 articles Diet Scopus articles Diet impact factor journals Diet Scopus journals Diet PubMed journals Diet medical journals Diet free journals Diet best journals Diet top journals Diet free medical journals Diet famous journals Diet Google Scholar indexed journals Oral nutritional supplements articles Oral nutritional supplements Research articles Oral nutritional supplements review articles Oral nutritional supplements PubMed articles Oral nutritional supplements PubMed Central articles Oral nutritional supplements 2023 articles Oral nutritional supplements 2024 articles Oral nutritional supplements Scopus articles Oral nutritional supplements impact factor journals Oral nutritional supplements Scopus journals Oral nutritional supplements PubMed journals Oral nutritional supplements medical journals Oral nutritional supplements free journals Oral nutritional supplements best journals Oral nutritional supplements top journals Oral nutritional supplements free medical journals Oral nutritional supplements famous journals Oral nutritional supplements Google Scholar indexed journals Gastrointestinal cancer articles Gastrointestinal cancer Research articles Gastrointestinal cancer review articles Gastrointestinal cancer PubMed articles Gastrointestinal cancer PubMed Central articles Gastrointestinal cancer 2023 articles Gastrointestinal cancer 2024 articles Gastrointestinal cancer Scopus articles Gastrointestinal cancer impact factor journals Gastrointestinal cancer Scopus journals Gastrointestinal cancer PubMed journals Gastrointestinal cancer medical journals Gastrointestinal cancer free journals Gastrointestinal cancer best journals Gastrointestinal cancer top journals Gastrointestinal cancer free medical journals Gastrointestinal cancer famous journals Gastrointestinal cancer Google Scholar indexed journals advanced cancer articles advanced cancer Research articles advanced cancer review articles advanced cancer PubMed articles advanced cancer PubMed Central articles advanced cancer 2023 articles advanced cancer 2024 articles advanced cancer Scopus articles advanced cancer impact factor journals advanced cancer Scopus journals advanced cancer PubMed journals advanced cancer medical journals advanced cancer free journals advanced cancer best journals advanced cancer top journals advanced cancer free medical journals advanced cancer famous journals advanced cancer Google Scholar indexed journals cancer survivors articles cancer survivors Research articles cancer survivors review articles cancer survivors PubMed articles cancer survivors PubMed Central articles cancer survivors 2023 articles cancer survivors 2024 articles cancer survivors Scopus articles cancer survivors impact factor journals cancer survivors Scopus journals cancer survivors PubMed journals cancer survivors medical journals cancer survivors free journals cancer survivors best journals cancer survivors top journals cancer survivors free medical journals cancer survivors famous journals cancer survivors Google Scholar indexed journals fish diet articles fish diet Research articles fish diet review articles fish diet PubMed articles fish diet PubMed Central articles fish diet 2023 articles fish diet 2024 articles fish diet Scopus articles fish diet impact factor journals fish diet Scopus journals fish diet PubMed journals fish diet medical journals fish diet free journals fish diet best journals fish diet top journals fish diet free medical journals fish diet famous journals fish diet Google Scholar indexed journals protein intake articles protein intake Research articles protein intake review articles protein intake PubMed articles protein intake PubMed Central articles protein intake 2023 articles protein intake 2024 articles protein intake Scopus articles protein intake impact factor journals protein intake Scopus journals protein intake PubMed journals protein intake medical journals protein intake free journals protein intake best journals protein intake top journals protein intake free medical journals protein intake famous journals protein intake Google Scholar indexed journals
Article Details
Background
Body composition alterations, namely sarcopenia and sarcopenic obesity, are known to have a negative impact on cancer patients outcome [1-10], but the benefit of intervention strategies, remain unclear. Exercise has been associated with improved functional status and patient reported outcomes in cancer patients [11,12], but mostly in breast and colorectal cancer survivors [13,14]. In patients undergoing treatment a positive effect has also been observed and exercise has been considered safe and feasible even in advanced cancer [15]. However, optimal exercise frequency, intensity and duration is still open to debate. On the other hand, dietary intake is also relevant since it seems to have an important role in skeletal muscle maintenance. It has been suggested that cancer patients may experience an anabolic resistance to protein stimuli, but protein synthesis is not completely blunted and may respond to an elevated protein intake [16]. In fact, protein supplementation has proven to improve protein synthesis [17], body composition, muscle strength [18] and walking capacity [19] in cancer patients. Besides the effect of single nutrients, dietary patterns namely a high fat and fish diet, is associated with a reduced odds of sarcopenia [20] and simultaneous energy and protein intake seem to result in a more robust effect on muscle mass and strength [21].
Few studies have investigated the influence of a combined exercise and dietary intervention [22]. Solheim et al have reported on a phase II Multimodal Intervention Exercise, Nutrition and Anti-Inflammatory medication in cachexia (pre-MENAC) versus standard care, showing that this intervention is feasible and safe in patients with incurable lung and pancreatic cancer and may have a positive effect on patients weight [23]. This multimodal approach was designed to address cachexia which is known to be a multidimensional condition [24], and therefore is expected to be a more suitable approach for cancer patients. The aim of this randomized controlled, open label pilot study was to assess the adherence to a Combined Exercise and Dietary Intervention (CEDI) in patients with GI cancer submitted to neo-adjuvant chemo(radio)therapy, in order to pursue other outcome associated studies in the future. Bearing in mind that compliance is a limiting factor to the benefit provided from exercise and diet, assessing adherence to these interventions is paramount before pursuing further studies
Methods
Study design and participants
A parallel randomized controlled, open label pilot trial was conducted. This trial is registred at CinicalTrials.gov: NCT05237921 and conforms to CONSORT guidelines for randomized controlled trials. Study protocol is available online www.clinicaltrials.gov. Recruitment was conducted at the Oncology center of Hospital Beatriz Ângelo and patients were consecutively selected by Oncologists during the weekly multidisciplinary meeting. Patients with esophageal, gastric, pancreatic and rectal cancer, were enrolled at diagnosis provided that they were eligible for neo-adjuvant chemo/radiotherapy (ChT) and with age higher than 18 years and lower than 80 years. Before enrollment initiation, besides upper gastrointestinal cancer (as initially planned for), we decided to also include patients with rectal cancer to have a broader view of adherence to CEDI in patients with gastrointestinal cancer under neo-adjuvant treatment, which is in line with the exploratory nature of this study.
Combined Dietary and Exercise Intervention arm
The intervention group received a supervised combined moderate aerobic and resistance training, once a week with duration of 40-60 minutes plus daily home exercise. All patients were evaluated in respect to their physical condition by a physical medicine and rehabilitation physician, and exercise was administered by a physiotherapist. Exercise was planned within a “slow and low“ approach and was personalized according to patients’ age and functional status. The first exercise session was dedicated to full patient evaluation in order to perceive patients individual tolerance and to educate in regard to home exercises. Most common exercises were aerobic exercises as 10-15 minutes of walking and resistance exercises as squatting with theraband around knees, shoulder flexor strengthening in standing using theraband and stretching. Educational written and illustrated materials as well as therabands were provided to each patient for home based exercise.
Besides exercise, the intervention group received a one-on-one nutritional counseling, by a senior and research Dietitian (SV). In the first visit a dietary plan was designed and one daily oral nutritional supplement (Forticare®, Nutricia) was given to meet the European Society of Parenteral and Enteral Nutrition (ESPEN) recommended intake of 25-30kcal/kg/day and 1-1.5g of protein/kg/day [25]. Also, patients were recommended to maintain a fat intake of 30% of total daily calories, with mostly being provided by monounsaturated fat. Patients were suggested to drink the supplements after exercise. All dietary plans were created with Nutrium® software, in order to obtain personalized dietary plan prescriptions that conveyed nutritional needs targets. Nutrium is a Portuguese software that allows rigorous dietary planning, since it enables the user to set energy and nutrient estimated requirements and to create dietary plans with nutritional composition information determined for Portuguese foodstuffs [26]. Written materials were given to patients and/or caregivers. Follow up visits took place every week during exercise. Total duration of the intervention was set at 8 weeks, although patients with longer neo-adjuvant treatments, namely patients with rectal cancer, maintained the intervention for a longer period of time, with a maximum of 12 weeks. Patients were recommended to maintain the dietary plan and exercise during the whole ChT treatment plan. Due to possible symptoms after ChT, namely nausea and vomiting, patients were asked to intensify compliance on the week preceding ChT when there is a higher probability that patients are less symptomatic. Whenever patients did not attend the weekly exercise activity, they were contacted to provide support and to assess if any diet or exercise adjustment was needed in order to maximize adherence.
Control arm- Standard care
Patients allocated to the control arm received standard care, in which patients were referred to the dietitian only when the attending physicians felt there was a need for dietary intervention. Whenever relevant, exercise was recommended but without personalized training program, according to our current practice.
Outcome measures
The primary outcome was intervention adherence, that was evaluated according to five criteria: 1) proportion of patients willing to engage in CEDI; 2) adherence to dietary plan, patients were considered adherent if they have met ≥ 75% of their calorie and protein estimated requirements; 3) adherence to oral nutritional supplements, one supplement per day was prescribed, and supplement intake ≥ 4 weeks was considered acceptable; 4) adherence to exercise, were attendance to the exercise class for at least 4 consecutive weeks was considered acceptable; 5) adherence to CEDI, patients were considered adherent if they were able to meet more than 75% of their calorie and protein estimated requirements/oral nutritional supplementation and adhered to exercise, approximately one month after initiation of CEDI. Dropout rates and reasons for leaving the study were also recorded. The secondary outcomes included change in weight, waist circumference, CT derived body composition and functional status assessed with hand grip strength, 6MWT and functional score of EORTC quality of life questionnaire. Measurements were conducted before and after neo-adjuvant treatment.
Sample size
Sample size per group was calculated bearing in mind that according to data form the World Health Organization, 14% of Portuguese adults are compliant to moderate exercise, and in our study adherence was set as compliance ≥ 50%. Considering a power of 0.80 and an α set at 0.05, 25 patients will be needed per group. A planned interim analysis was performed to substantiate preparation of further study protocols using CEDI, and results are reported in this paper.
Randomization
A table was created by a web based randomization system to allocate treatments, with an allocation ratio of 1:1. Stratified block randomization using random block size (2, 4 and 6) was conducted to allocate patients to standard care and to intervention with CEDI. Stratification was performed according to disease location. Patients eligible to enter the study were referred by Oncologists, and after obtaining consent, patients were enrolled in the study by researcher (SV), which was responsible for allocation consignment.
Procedures
Clinical data
Demographic and clinical data as age, gender, tumor site, histological type, TNM staging, ChT toxicity, overall survival were prospectively recorded and retrieved from electronic records. ChT toxicity was graded according to National Cancer Institute Common Toxicity Criteria. Dose-limiting toxicity (DLT) was defined as any grade 3/4 toxicity associated with physician-ordered dose reduction or termination of therapy and ChT delay. This data was collected by Oncologists. The most common neoadjuvant treatments were: FLOT (5-Fluorouracil, Folinic acid, Oxaliplatin, Docetaxel) for gastric, XELOX (Oxaliplatine and Capecitabine) followed by Capecitabine plus radiotherapy for rectal, Carboplatine/Paclitaxel and radiotherapy for esophagus and FOLFIRINOX (5-Fluorouracil, Irinotecan and Oxaliplatin) for pancreatic cancer patients. Duration of neo-adjuvant therapies varied from 8 to 12 weeks.
Anthropometric measures and nutritional assessment
Anthropometric measures (AM) such as weight and height were obtained, and Body Mass Index was calculated. All AM were performed according to previously established protocols [27]. Patient Generated Subjective Global Assessment (PG-SGA) was conducted by an experienced dietitian and patients were classified as well nourished (SGA A), moderately or suspected of being malnourished (SGA B) or severely malnourished (SGA C). Assessments were conducted before and after neo-adjuvant treatments.
Body composition assessment
Cross-sectional imaging evaluation
Body composition analysis was conducted with Computed Tomography (CT) scan image analysis [5]. Images were selected at the third lumbar vertebra (L3) using a portal venous phase. CT scans were used opportunistically, as CT is performed at diagnosis and after neo-adjuvant treatment. Image thickness was 5mm and tube voltage was 100kv. Images were processed with Slice-o-Matic (Tomovison) and ABCS module that performs automatic segmentation of tissue cross-sectional areas, whereas posterior validation of image processing was done by the Radiologist, with manual corrections as necessary. Segmentation of tissue cross-sectional areas was conducted according to the following Hounsfield unit thresholds: -29 to 150 for skeletal muscle, -190 to -30 for subcutaneous and intramuscular adipose tissue, and -50 to -150 for visceral adipose tissue. Cross-sectional skeletal muscle, visceral fat, and subcutaneous fat was recorded in squared centimeters and mean muscle radiation attenuation in Hounsfield units. Skeletal muscle area (SMA) was normalized for stature to calculate the skeletal muscle index (SMI) - cm2/m2. Sarcopenia was defined as SMI lower than 41 cm2/m2 in women, lower than 43 cm2/m2 in men with body mass index (BMI) <25 Kg/m2 and lower than 53 in men with BMI > 25 Kg/m2 as described by Martin et al [5]. Visceral obesity was defined as visceral fat area >130cm2 [28]. An inter-reliability analysis was conducted and variance coefficients computed for two duplicate CT scans was 0.32%, 1.09%, 0.39% and 4.04%, for skeletal muscle, visceral adipose tissue, subcutaneous adipose tissue and intramuscular adipose tissue areas, respectively.
Dietary Intake assessment
Dietary intake was assessed with a Semi-quantitative Food frequency questionnaire to estimate dietary intake of both the intervention and control group before and after neo-adjuvant therapy and 24h recalls to assess dietary intake of patients undergoing CEDI at every 2 weeks in order to estimate compliance to established dietary goals. The Semi-quantitative Food Frequency Questionnaire (FFQ) used was developed for the Portuguese population [29] and is designed to evaluate usual dietary intake. This questionnaire includes 86 commonly-eaten food or drinks and participants were asked to estimate the amount and frequency of intake of each food/drink according to frequency and amount at baseline and before surgery. Conversion of foodstuffs to nutrients was conducted with software Food Processor Plus (ESHA Research, Salem, Oregon) which has been adapted to the Portuguese commonly-eaten food or drinks. The 24h recall using a modified USDA five-pass method consists in 5 steps where the first is to list all foods consumed on the previous 24h. On the second step the interviewer asks about possible forgotten food items. In the third step the interviewer clarifies the time and occasion of the consumed foods and on the fourth step clarifies portion size [30]. Conversion of foodstuffs to nutrients was conducted with Nutrium® software which has been developed for the Portuguese population [26].
Functional status assessment
Performance Status was assessed with Eastern Cooperative Oncology Group Performance Status scale. According to these criteria patients are classified from grade 0 (fully active) to grade 4 (bedridden). Prior to initiation and after neo-adjuvant treatment a 6 min walk test (6MWT) was conducted by cardiopulmonary technicians blinded to the intervention groups, were walking distance and percentage of predicted normal values were recorded. Handgrip strength was measured with a dynamometer (JAMAR®) and measurements were recorded in kg. Handgrip strength was measured 3 times with the non-dominant arm according to manufacturer’s instructions. Mean handgrip strength was analyzed with gender specific thresholds from the revised guidelines of the European Working Group on Sarcopenia in Older People (EWGSOP) (<27kg in men and <16kg in women) [31].
Patient Reported Outcome Measures
Quality of life was assessed before and after neo-adjuvant treatment with the European Organization for Research and Treatment of Cancer (EORTC) questionnaire. This questionnaire allows determination of functional, symptoms and overall quality of life score.
Statistical analysis
Adherence to CEDI was analyzed with an intention to treat approach, whereas anthropometric measures, body composition, functional status, quality of life and dietary intake were analyzed with a per protocol approach. Continuous variables were described as median and inter-quartile range, while categorical variables were expressed as frequency and percentage. Chi-square test or Fisher exact test were used to assess association between categorical variables. Differences in means from continuous variables were analyzed by t-test or Mann Whitney U test as appropriate, according to variables’ adjustment to a normal distribution. Shapiro-Wilk test was used to test for normality. Effect size was computed with Cohen’s d for t tests and r for Mann-Whitney U tests. Paired-samples t test or Wilcoxon Signed Rank Test were used to analyze longitudinal data within the control and intervention arm. Statistical analysis was conducted with R Studio Version 1.2.5042 software.
Results
Study population
From June 2018 to November 2019, 46 patients were screened resulting in 39 patients being randomly allocated either to the intervention (n=19) or to the control arm (n=20). All patients had indication for neo-adjuvant treatment. All patients had stages II/III disease, except for one patient randomly allocated to the intervention arm with gastric cancer and a single liver metastasis (stage IV disease) who was included since the patient was eligible for neo-adjuvant treatment. Figure 1 presents the trial profile and reasons for exclusion. A total of 32 patients completed follow up evaluations.
Baseline characteristics are shown on table 1. Patients in both groups were well matched in regard to age, sex, disease site, serum C-reactive protein, albumin and total proteins, Body Mass Index (BMI), nutritional assessment (PG-SGA and CT-derived body composition), functional status (handgrip strength and 6 minute walking test), quality of life score and dietary intake. In regard to ECOG scale we found a higher proportion of patients with ECOG 0 in the control arm and a lower proportion of patients ECOG 2, than in the intervention arm (p=0.026).
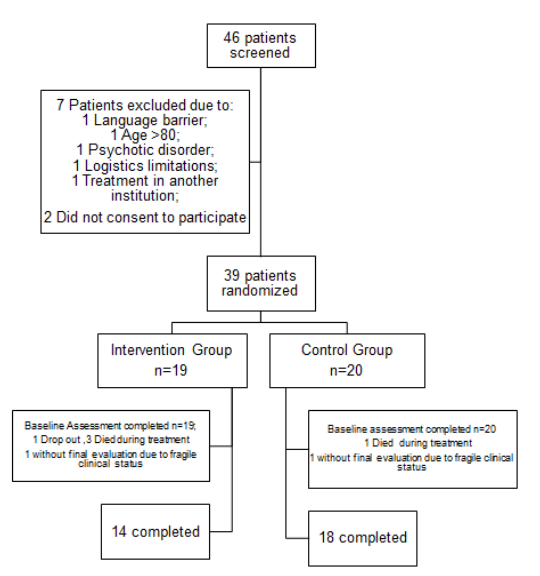
Figure 1: Trial profile
Table 1: Baseline characteristics. Med-Median; IQR-Interquartile Range; PG-SGA-Patient Generated Subjective Global Assessment; ECOG-Eastern Cooperative Oncology Group Performance Status scale; CT- Computed Tomography; 6MWT-6 Minute Walking Test; 6MWT-% Predicted-Percentage of predicted normal values; aSemi Quantitative Food Frequency questionnaire derived estimated daily calorie, protein, carbohydrates and fat intake for usual daily intake before disease.
|
Intervention n=19 |
Control n=20 |
||||||||
|
n |
% |
Med |
IQR |
n |
% |
Med |
IQR |
p |
|
|
Age |
64 |
9.5 |
20 |
64.5 |
19 |
0.978 |
|||
|
Male |
14 |
73.3 |
15 |
75 |
0.925 |
||||
|
Disease Site |
|||||||||
|
Esophagus |
2 |
10.5 |
1 |
5 |
0.899 |
||||
|
Gastric |
9 |
47.4 |
9 |
45 |
|||||
|
Pancreas |
2 |
10.5 |
3 |
15 |
|||||
|
Rectum |
6 |
31.6 |
7 |
35 |
|||||
|
C-Reactive Protein |
0.5 |
0.6 |
0.4 |
0.9 |
1 |
||||
|
Albumin |
4.2 |
0.5 |
4.1 |
0.7 |
0.67 |
||||
|
Total proteins |
6.8 |
0.8 |
6.7 |
1.1 |
0.665 |
||||
|
Body Mass Index |
24.9 |
6.5 |
26 |
5.8 |
0.737 |
||||
|
Body Mass Index Categories |
|||||||||
|
Underweight |
3 |
15.8 |
2 |
10 |
0.927 |
||||
|
Normal weight |
7 |
35 |
7 |
35 |
|||||
|
Overweight |
6 |
31.6 |
8 |
40 |
|||||
|
Obese |
3 |
15.8 |
3 |
15 |
|||||
|
PG-SGA |
|||||||||
|
Suspected Malnutrition |
6 |
31.6 |
11 |
55 |
0.324 |
||||
|
Malnourished |
2 |
10.5 |
1 |
5 |
|||||
|
ECOG |
0.026 |
||||||||
|
0 |
8 |
42.1 |
12 |
60 |
|||||
|
1 |
9 |
47.4 |
2 |
10 |
|||||
|
2 |
2 |
10.5 |
6 |
30 |
|||||
|
CT Body Composition |
|||||||||
|
Skeletal Muscle Area |
|||||||||
|
Male |
158.5 |
32.5 |
166.1 |
20.3 |
0.679 |
||||
|
Female |
99.8 |
11.8 |
101 |
22 |
0.739 |
||||
|
Skeletal Muscle Index |
|||||||||
|
Male |
54.2 |
13.4 |
56.5 |
8.4 |
0.431 |
||||
|
Female |
41.5 |
7.8 |
41.6 |
8.9 |
0.828 |
||||
|
Visceral Adipose Tissue |
|||||||||
|
Male |
133.4 |
102.4 |
175.4 |
153.9 |
0.2 |
||||
|
Female |
83.1 |
65.8 |
91.9 |
64.8 |
0.904 |
||||
|
Subcutaneous Adipose Tissue |
|||||||||
|
Male |
98.6 |
113.2 |
112.5 |
81.3 |
0.538 |
||||
|
Female |
137.3 |
50.5 |
249.2 |
60.4 |
0.246 |
||||
|
Total Adipose Tissue |
|||||||||
|
Male |
274.7 |
200.9 |
342.5 |
182.9 |
0.238 |
||||
|
Female |
248.7 |
67.8 |
362.4 |
145.4 |
0.433 |
||||
|
Intramuscle Adipose Tissue |
|||||||||
|
Male |
9.1 |
5.6 |
9.7 |
5.7 |
0.92 |
||||
|
Female |
9 |
6.7 |
16.2 |
8.3 |
0.109 |
||||
|
Sarcopenia |
4 |
23.5 |
3 |
21.4 |
0.889 |
||||
|
Low Muscle Attenuation |
8 |
47.1 |
6 |
42.9 |
0.815 |
||||
|
Visceral Obesity |
7 |
41.2 |
8 |
57.1 |
0.376 |
||||
|
Sarcopenic Obesity |
1 |
5.26 |
0 |
0 |
0.31 |
||||
|
HandGrip Strength |
33 |
18.5 |
29 |
14 |
0.713 |
||||
|
Low HandGrip Strength |
5 |
27.8 |
5 |
25 |
0.846 |
||||
|
6MWT-Distance (m) |
400 |
127.5 |
444 |
182 |
0.183 |
||||
|
6MWT-% Predicted |
76 |
21.2 |
81 |
24.9 |
0.326 |
||||
|
Low 6MWT-Distance (m) |
9 |
47.4 |
7 |
41.2 |
0.708 |
||||
|
Quality of life Global |
66.7 |
37.5 |
58.3 |
29.2 |
0.997 |
||||
|
Calorie Intake (kcal)a |
3847 |
1278 |
3208 |
1308 |
0.517 |
||||
|
Calorie Intake (kcal/kg)a |
55 |
26 |
47 |
25 |
0.158 |
||||
|
Protein (g)a |
159 |
59 |
131 |
59 |
0.515 |
||||
|
Protein (g/kg)a |
2.2 |
0.7 |
1.7 |
1.1 |
0.376 |
||||
|
Carbohydrates (g)a |
369 |
167 |
334 |
144 |
0.275 |
||||
|
Carbohydrates (g/kg)a |
5.3 |
1.5 |
4.9 |
1.5 |
0.289 |
||||
|
Fat (g)a |
172 |
71 |
131 |
60 |
0.463 |
||||
|
Fat (g/kg)a |
2.3 |
1.1 |
1.9 |
1.3 |
0.239 |
||||
Adherence analysis
Analysis was conducted for 19 patients that gave consent and completed baseline measurements. One patient dropped out on the second week of intervention because CEDI was viewed as an additional burden. During follow up 3 patients who entered CEDI, died and one refused to pursue further evaluations due to decline of performance status. In the control group 1 patient died and 1 patient refused to pursue further evaluations due to decline of performance status.
Adherence to estimated nutritional requirements
Globally, 17/19 (89.4%), 14/19 (73.6%) and 6/19 (31.5%) were able to meet a daily calorie intake above 50%, 75% and 100% of estimated total calorie daily requirements on at least one visit, respectively. In regard to protein intake, 17/19 (89.4%), 17/19 (89.4%) and 9/19 (47.3%) were able to meet a protein intake above 50%, 75% and 100% of estimated protein requirements, respectively. In regard to total fat intake, most patients were able to maintain fat intake within 25-30% of total calorie intake and 7/19 (36.8%) patients had a total fat intake exceeding 35% on at least one visit. As planned all patients had a monounsaturated fat intake above 30% of total fat intake on at least one visit. Details for each patient concerning percentage of nutritional requirements met are presented in figure S1 of supplementary material.
Adherence to oral nutritional supplements and exercise
A total of 13/19(68.4%) adhered to oral nutritional supplements and 13/19 (68.4%) to the exercise program. Patients that adhered to oral nutritional supplements (ONS) were found to have a significantly higher median daily calorie intake (ONS Adherent- 1781kcal/day, Interquartile Range (IQR)-633 vs. ONS non-Adherent- Median (Med)-1537kcal, IQR-332; p=0.022), but no difference in regard to protein intake (ONS Adherent-91g/day, IQR- 22 vs ONS non-Adherent-84g/day, IQR-25, p=0.707). Adherence to supplementation was not influenced by tumor location (esophagus-2/2(15.4%), gastric-6/8 (46.2%), pancreatic-1/2 (7.7%) and rectal-4/6 (30.8%); p=1). In regard to exercise, no differences were found in regard to daily calorie (Exercise Adherent: Med-1659kcal/day, IQR-452, Exercise non-Adherent: Med-1470kcal/day, IQR-319; p=0.208) and protein (Exercise Adherent: Med-91g/day, IQR-19, Exercise non-Adherent: Med-81g/day, IQR-25; p=0.593) intake. In respect to tumor location we found that all patients with gastric cancer adhered to exercise (1/2(7.7%) esophagus, gastric-8/8 (61.5%), pancreatic-0/2 (0.0%) and rectal-4/6 (30.8%) (p=0.02)).
Adherence to Combined Exercise and Dietary Intervention (CEDI)
At the second visit, approximately one month after CEDI initiation, 13/19 (68.4%) were able to meet more than 75% of their calorie and protein estimated requirements or maintained oral nutritional supplement intake and exercise for 1 month and thus were considered fully adherent to CEDI. Adhesion to all studied criteria is presented on figures 2 and 3.
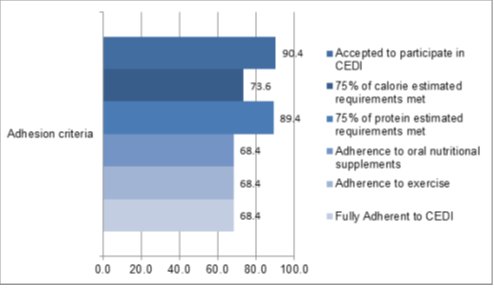
Figure 2: Adherence to Combined Exercise and Dietary Intervention: percentage of patients that accepted to participate in CEDI and patients who adhered to individual components such as 75% of estimated calories and protein requirements, oral nutritional supplements, exercise and fully adherent patients with 75% of calorie and protein estimated requirements met /maintained oral supplement intake and exercise after 1 month follow up.
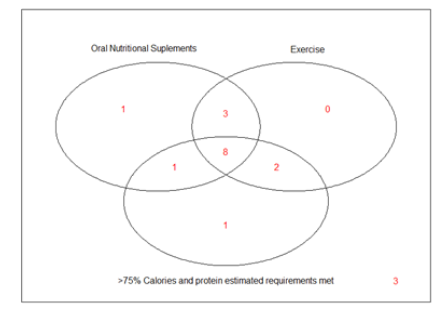
Figure 3: Venn diagram for the adherence to oral nutritional supplements, exercise and more than 75% of calorie and proteins estimated requirements met after 1 month of Combined Exercise and Dietary Intervention (CEDI) prescription.
Longitudinal analysis
CEDI vs. Control
Anthropometric, bioelectrical impedance, CT scan body composition measures, as well as grip strength, 5 minute walking distance, percentage of predicted normal values and quality of life score at baseline (before neo-adjuvant treatment) and at follow up (after neo-adjuvant treatment) are presented per trial arm on table 2. Further information regarding weight and CT-derived body composition change is available in figure S2 of supplementary material. Patients in the intervention arm were able to maintain weight during neo-adjuvant therapy, in contrast to patients in the control arm who lost a median weight of 3.34kg, which represents 5.10% of their initial weight. Similarly, patients in the intervention arm maintained waist circumference, whereas patients in the control arm lost a median 2.5 cm. In regard to CT scan derived body composition a near significant difference was found for skeletal muscle area, where patients in the control arm had a higher median loss of skeletal muscle area when compared with the intervention arm. In respect to visceral adipose tissue, we observed a significantly higher loss in the control group. There were no differences between study groups in bioelectrical impedance measurements. In regard to functional status, patients in the intervention group improved median walking distance and median percentage of predicted normal values from the 6 minute walking test. Also, functional score from quality of life questionnaire differed significantly between groups with a significant improvement for the intervention group. In respect to daily caloric and protein intake estimated with food frequency questionnaire, no differences were found between control and intervention arm in regard to the median difference before and after neo-adjuvant treatment (Calories-Intervention: Med:-1243, IQR: 1159 vs. Control: Med:-478, IQR:1216, p=0.483; Protein- Intervention: Med:-11, IQR: 69 vs. Control:Med:17, IQR:44, p=0.91).
Pairwise analysis-CEDI group
Patients in the intervention arm had a near significant skeletal muscle area loss (Baseline: Med: 151.30, IQR:56.0 vs. follow up: Med: 153.60, IQR:32.40, p=0.052), but probably clinically negligent, since they were able to improve significantly their 6MWT distance (Baseline:Med-400.0, IQR: 127.5; follow up: Med: 486.00, IQR:151.75, p=0.02), percentage of predicted normal value of 6MWT (Baseline:75.97, IQR: 21.24; follow up: Med: 89.54, IQR:23.07, p=0.08) and median functional score from quality of life questionnaire (Baseline:82.35, IQR:21.56; follow up: Med: 88.24, IQR:13.73, p=0.009). Improvement in symptoms was observed (intervention-Baseline: 18.8, IQR:30.3 ; follow up: Med: 9.09, IQR:12.12, p= 0.035).
Pairwise analysis-control group
Patients in the control arm had a significant reduction in in their median weight (Baseline:Med:68.7 (21.87), follow up: Med: 61.20 (25.55), p=0.017), waist circumference (Baseline: Med:94.5(13.5), follow up: Med:89.5, p=0.017), median skeletal muscle area (Baseline: Med: 147.25, IQR: 51.30 vs. follow up: Med: 128.15, IQR:51.30, p=0.0008) and visceral adipose tissue (Baseline: Med: 141.5, IQR: 136.67 vs. follow up: Med: 107.05, IQR:130.0, p=0.0097), as well as a near significant reduction in 6MWT distance (Baseline:444.0, IQR: 182.0; follow up: Med: 451.00, IQR:163.00, p=0.09). Also, there was a significant reduction in phase angle (baseline: Med: 6.30, IQR: 1.15 vs. follow up: Med: 5.29, IQR: 0.85, p=0.003). Improvement in symptoms was observed (Baseline:27.27, IQR:30.3; follow up: Med:18.18, IQR:18.18, p=0.010).
Chemotherapy toxicity and adverse events
A total of 20/39 (51.28%) experienced toxicity to neo-adjuvant treatment with no differences between groups (CEDI-8/19 (42.1%) vs. control-12/20 (60.0%); p=0.33). No between groups differences were found in regard to the percentage of patients that had to reduce dosage (CEDI-4/7 (57.1%); control-3/7 (42.9%), p=0.56), dose limiting toxicity (CEDI-2/4 (50.0%); control 2/4 (50.0%), p=0.92) or delay treatment (CEDI-1 (100%); control-0 (0%), p=0.28). There were 4 serious adverse events, 3 in the intervention and 1 in the control arm but none related to the intervention. Details regarding neo-adjuvant treatment can be found in table S1 of supplemental material.
Table 2: Anthropometric measures, bioelectrical impedance, computed tomography (CT) derived body composition, functional status and quality of life at baseline (before neoadjuvant treatment) and follow up (after neoadjuvant treatment). aBetween group differences- 2-sample t test or Mann-Whitney U test; b Effect size computed with Cohen’s d for t tests and r for Mann-Whitney U tests; 6MWT-6 Minute Walking Test; 6MWT-% Predicted-Percentage of predicted normal values.
|
Intervention arm Median (IQR) n=19 |
Control arm Median (IQR) n=20 |
Pa |
Effect Sizeb |
||
|
Anthropometric Measures |
|||||
|
Weight (kg) |
n=14 |
n=18 |
|||
|
Baseline |
67.0 (18.4) |
68.7(21.9) |
|||
|
Follow Up |
72.5(17.0) |
61.2(25.5) |
|||
|
Difference |
0.05 (2.9) |
-3.4 (6.3) |
|||
|
%Difference |
0.062(4.5) |
5.1(10.7) |
0.008 |
0.457 |
|
|
Waist Circumference (cm) |
n=14 |
n=18 |
[0.44,0.46] |
||
|
Baseline |
91.0(14.5) |
94.5(13.5) |
|||
|
Follow Up |
96.0(11.5) |
89.5(16.7) |
|||
|
Difference |
0(4.5) |
-2.5(9.5) |
0.028 |
-0.56 |
|
|
[-1.08, -0.034] |
|||||
|
Bioelectrical impedance |
|||||
|
Fat Free Mass (kg) |
n=13 |
n=16 |
|||
|
Baseline |
52.6(16.5) |
53.9(23.4) |
|||
|
Follow Up |
56.5(18.9) |
49.8(21.7) |
|||
|
Difference |
0.1 (1.8) |
-0.70(3.9) |
0.455 |
||
|
Fat Mass (kg) |
n=14 |
n=16 |
|||
|
Baseline |
17.10(6.0) |
16.30(7.4) |
|||
|
Follow Up |
15.20(7.2) |
15.15(6.8) |
|||
|
Difference |
1.80(5.2) |
-0.75(4.9) |
0.58 |
||
|
Phase angle |
n=13 |
n=16 |
|||
|
Baseline |
6.00(1.3) |
6.30(1.1) |
|||
|
Follow Up |
5.80(0.8) |
5.25(1.8) |
|||
|
Difference |
-0.60(1.1) |
-0.6(0.8) |
0.126 |
||
|
CT scan image analysis |
|||||
|
Skeletal Muscle tissue area (cm2) |
n=11 |
n=14 |
|||
|
Baseline |
151.3(56.0) |
147.25(51.3) |
|||
|
Follow Up |
153.6(32.4) |
128.15(45.4) |
|||
|
Difference |
-8.2(16.2) |
-12.15(15.7) |
0.09 |
-0.79 |
|
|
[-1.77;0.18] |
|||||
|
Visceral adipose tissue area (cm2) |
n=11 |
n=14 |
|||
|
Baseline |
115.4(132.1) |
141.50(136.7) |
|||
|
Follow Up |
108.1(103.3) |
107.(130.0) |
|||
|
Difference |
-4.0(38.6) |
-57.9(102.1) |
0.027 |
-1.1 |
|
|
[-2.10;-0.09] |
|||||
|
Subcutaneous adipose tissue area (cm2) |
n=11 |
n=14 |
|||
|
Baseline |
115.0(83.4) |
123.0(83.4) |
|||
|
Follow Up |
77.41(91.6) |
141.85(150.3) |
|||
|
Difference |
9.40(41.1) |
-20.93(23.4) |
0.5192 |
||
|
Intra Muscular Adipose Tissue area (cm2) |
n=11 |
n=14 |
|||
|
Baseline |
9.0(4.0) |
10.6(6.9) |
|||
|
Follow Up |
11.3(4.3) |
9.6(10.0) |
|||
|
Difference |
1.5(3.1) |
-0.5(4.6) |
0.311 |
||
|
Muscle Attenuation |
n=11 |
n=14 |
|||
|
Baseline |
39.6(8.5) |
37.6(10.3) |
|||
|
Follow Up |
37.4(5.6) |
37.4(13.9) |
|||
|
Difference |
1.5(1.7) |
0.12(3.2) |
0.725 |
||
Discussion
This open label randomized controlled trial demonstrated that a Combined Exercise and Dietary Intervention (CEDI) in patients with gastrointestinal cancer under neo-adjuvant treatment is feasible and has a reasonably high adherence. Also, CEDI patients were able to maintain their pre-treatment nutritional status and improve functional status. To our knowledge this is the first combined exercise and nutritional intervention program performed in cancer GI patients during neo-adjuvant treatment.
Recent studies have reported that adherence to behavioural interventions varies substantially, from 8 to 93% [23,32-34]. It is noteworthy that this high adhesion variability, may be attributed to heterogeneity in the type of intervention, namely the time of implementation (pre-treatment, post-treatment, survivors), aim (ex: weight loss in overweight survivors, nutritional status optimization preoperatively, implementation of specific dietary recommendations as high fiber diet, etc), duration, type (dietary intervention, supplements and exercise), disease stage, site and treatment, etc. Another challenge that further adds to the complexity of studying adherence rates is the inexistence of specific criteria to define optimal adherence, although some studies have defined an adherence equal or higher to 50% as acceptable [23]. In our study 68.4% of patients were fully adhered to CEDI, which we consider as reasonably high, comparing with previously reported adherence rates as low as 48% for oral nutritional supplements and 60% for exercise [23], and bearing in mind that these patients had locally advanced disease, were under neo-adjuvant treatment and therefore may be more symptomatic. This adhesion study was deemed by us as crucial, since adhesion rates are variable and we are aiming to pursue further studies to explore the influence of CEDI in patients under neo-adjuvant treatments, and thus it would be imprudent to tackle this issue before knowing if these patients were willing to participate in CEDI. Although cancer cachexia is known to impair anti-cancer treatments, cause distress in patients and families and decreased survival, it remains to date without standard care, and therefore strategies to deal with this condition are highly warranted [35]. During the past decades a multimodal intervention has been advocated, due to the existing knowledge that cancer cachexia is a multidimensional condition [22,36,37]. Still, further increasing the complexity is the uncertainty of the most appropriate endpoint regarding cancer cachexia, were besides weight, muscle mass quantity and quality, measures of function such as 6MWT, hand grip strength, quality of life and activities of daily living are at present considered equally or even more relevant [35]. When addressing multimodal interventions in cancer patients the MENAC study clearly stands out. Solheim T et al. [23] have reported on an intervention Exercise, Nutrition and Anti-Inflammatory medication in cachexia (pre-MENAC) versus standard care, conducted with patients with stage III/IV small cell lung cancer or inoperable pancreatic cancer with indication for ChT, showing a positive effect on weight. Indeed our results are consistent with those of MENAC, since patients in CEDI group were also able to maintain weight, and in addition we were able to show that these patients loose less muscle mass and improve functional status. In contrast, patients in the control arm lost skeletal muscle, visceral adipose tissue and worsened functional status. Visceral adipose tissue loss could seem like a positive characteristic of the control group, however it is important to note that 1) concomitant reduction of skeletal muscle and visceral adipose tissue is inherent to dietary restriction [38], meaning that these patients probably did not meet their nutritional requirements during treatment; 2) evidence supports a survival advantage for patients with higher content in skeletal muscle mass in obese patients with cancer [39], showing that muscle mass is presumably a key component. Still, this finding further supports the use of sophisticated and reliable body composition techniques for the optimization of dietary intervention, since besides calculating nutritional requirements with established calories and macronutrients per kg, body composition should also be considered in the estimation of nutritional requirements. Indeed we are aware that sample size is one of the limiting aspects of generalizability of results and cautious interpretation is therefore needed. The open label nature of our study design is also a limitation, but we did address this issue providing an intervention individualized for each patient, that would be difficult to mimic and all professionals involved except for Nutritionists and Physiotherapist were blinded to the study intervention.
Conclusions
Our study has allowed us to understand that CEDI is feasible, and that most patients are willing to participate even under neo-adjuvant ChT, resulting in potential benefits regarding nutritional and functional status. To our knowledge there are no studies evaluating intervention programs with the characteristics of CEDI in patients with gastrointestinal cancer undergoing neo-adjuvant treatment. We are aware that due to sample size interpretation of results should be conscious, however we feel that the encouraging results of this study are a starting point to pursue further well powered studies namely to investigate the role of CEDI in post-operatory complications, cancer cachexia and inflammation.
Abbreviations
AM- Anthropometric measures
CEDI- Combined Exercise and Dietary Intervention
ChT- neo-adjuvant chemo/radiotherapy
CT- Computed Tomography
DLT- Dose-limiting toxicity
EF- Effect size
EORTC- European Organization for Research and Treatment of Cancer
EWGSOP- European Working Group on Sarcopenia in Older People
FFQ- Food Frequency Questionnaire
GI-Gastrointestinal
PG-SGA- Patient Generated Subjective Global Assessment
6MWT-6 min walk test
SMA- Skeletal muscle area
SMI- skeletal muscle index
Declarations
Ethics approval
Approval was obtained from the Scientific and Ethics Committee of Hospital de Santa Maria and Hospital Beatriz Ângelo, Portugal. The procedures used in the study adhere to the tenets of Declaration of Helsinki. Informed written consent was obtained from all individual participants included in the study. Clinical data was prospectively collected from electronic charts, however data was coded in order to maintain anonymity.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding sources
No funds, grants, or other support was received.
Authors’ contributions
MC,VB study conceptualization; SM, CC, MB, LC, FL, JG, AF collected data and created the study database; CA, FP, AB, SC, SR exercise intervention conceptualization and implementation; PS, LA, RC validation of computed tomography image analysis for body composition; SV nutritional intervention conceptualization and implementation; data analysis, original draft preparation; JAT, JLPC resources and review; RM, MC, VB supervision, review and editing.
Acknowledgements
The authors would like to thank Andre Santos, CEO of Nutrium, who provided access to Nutrium free of charge. The Oral Nutritional Supplements (Forticare®) were provided from Nutricia free of charge.
References
- Peng PD, Van Vledder MG, Tsai S, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. Hpb 13 (2011): 439-446.
- Lieffers JR, Bathe OF, Fassbender K, et al. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br. J. Cancer 107 (2012): 931-936.
- Joglekar S, Mezhir JJ. The Impact of Sarcopenia on Survival and Complications in Surgical Oncology: A Review of the Current Literature. J Surg Oncol 112 (2015): 503-509.
- Prado CMM, Baracos VE, McCargar LJ, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin. Cancer Res. 13 (2007): 3264-3268.
- Martin L, Birdsell L, MacDonald N, et al. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol 31 (2013): 1539-1547.
- Kazemi-Bajestani SMR, Mazurak VC, Baracos V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin. Cell Dev. Biol 54 (2016): 2-10.
- Chu MP, Lieffers J, Ghosh S, et al. Skeletal muscle density is an independent predictor of diffuse large B-cell lymphoma outcomes treated with rituximab-based chemoimmunotherapy. J. Cachexia. Sarcopenia Muscle 8 (2017): 298-304.
- Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9 (2008): 629-635.
- Palmela C, Velho S, Agostinho L, et al. Body composition as a prognostic factor of neoadjuvant chemotherapy toxicity and outcome in patients with locally advanced gastric cancer. J. Gastric Cancer 17 (2017): 17-35.
- Velho S, Costa Santos MP, Cunha C, et al. Body Composition Influences Post-Operative Complications and 90-Day and Overall Survival in Pancreatic Surgery Patients. GE - Port. J. Gastroenterol 12 (2020): 1-13.
- Jones LW, Alfano CM. Exercise-oncology research: Past, present, and future. Acta Oncol. (Madr) 52 (2013): 195-215.
- Cormie P, Zopf EM, Zhang X, et al. The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol. Rev 39 (2017): 71-92.
- Kim JY, Lee MK, Lee DH, et al. Effects of a 12-week home-based exercise program on quality of life, psychological health, and the level of physical activity in colorectal cancer survivors: a randomized controlled trial. Support. Care Cancer 27 (2019): 2933-2940.
- Burgess A, Shah K, Hough O, et al. Focused ultrasound-mediated drug delivery through the blood-brain barrier. HHS Public Access 15 (2016): 477-491.
- Heywood R, McCarthy AL, Skinner TL. Safety and feasibility of exercise interventions in patients with advanced cancer: a systematic review. Support. Care Cancer 25 (2017): 3031-3050.
- Antoun S, Raynard B. Muscle protein anabolism in advanced cancer patients: response to protein and amino acids support, and to physical activity. Ann. Oncol 29 (2018): 10-17.
- Deutz NEP, Safar A, Schutzler S, et al. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin. Nutr 30 (2011): 759-768.
- Cereda E, Turri A, Klersy C, et al. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med 8 (2019): 6923-6932.
- Gillis C, Loiselle SE, Fiore JF, et al. Prehabilitation with Whey Protein Supplementation on Perioperative Functional Exercise Capacity in Patients Undergoing Colorectal Resection for Cancer: A Pilot Double-Blinded Randomized Placebo-Controlled Trial. J. Acad. Nutr. Diet 116 (2016): 802-812.
- Velho S, Moço S, Cruz R, et al. Dietary patterns and its relationship to sarcopenia in Portuguese patients with gastrointestinal cancer: An exploratory study. Clin. Nutr 37 (2018): S203-S204.
- Zanetti M, Cappellari GG, Barazzoni R, et al. The impact of protein supplementation targeted at improving muscle mass on strength in cancer patients: A scoping review. Nutrients 12 (2020): 1-16.
- Solheim TS, Vagnildhaug OM, Laird BJ, et al. Combining optimal nutrition and exercise in a multimodal approach for patients with active cancer and risk for losing weight: Rationale and practical approach. Nutrition 15 (2019): 67-68.
- Solheim TS, Laird BJA, Balstad TR, et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J. Cachexia. Sarcopenia Muscle 8 (2017): 778-788.
- Solheim TS, Laird BJA, Balstad TR, et al. Cancer cachexia: Rationale for the MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support. Palliat. Care 8 (2018): 258-265.
- Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 36 (2017): 11-48.
- Nutrium (2020).
- Rosa G, Palma A. Avaliação nutricional do paciente hospitalizado. Rio de Janeiro: Guanabara Koogan SA (2008).
- Ribeiro-Filho FF, Faria AN, Azjen S, et al. Methods of estimation of visceral fat: Advantages of ultrasonography. Obes. Res 11 (2003): 1488-1494.
- Lopes C, Aro A, Azevedo A, et al. Intake and adipose tissue composition of fatty acids and risk of myocardial infarction in a male Portuguese community sample. J Am Diet Assoc 107 (2007): 276-286.
- Johnson RK. Dietary Intake-How do we measure what people are really eating?. Obes. Res 10 (2002): 63S-68S.
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 48 (2019): 16-31.
- Grabenbauer A, Grabenbauer AJ, Lengenfelder R, et al. Distel, Feasibility of a 12-month-exercise intervention during and after radiation and chemotherapy in cancer patients: Impact on quality of life, peak oxygen consumption, and body composition. Radiat. Oncol 11 (2016): 5-11.
- Djuric Z, Ellsworth J, Weldon A et al. A diet and exercise intervention during chemotherapy for breast cancer. Obesity 3 (2011): 87-97.
- McCahon D, Daley AJ, Jones J, et al. Enhancing adherence in trials promoting change in diet and physical activity in individuals with a diagnosis of colorectal adenoma; a systematic review of behavioural intervention approaches. BMC Cancer 15 (2015): 189-193.
- Laird B, Fallon M. Treating cancer cachexia: An evolving landscape. Ann. Oncol 28 (2017): 2055-2056.
- Bosaeus I. Nutritional support in multimodal therapy for cancer cachexia. Support. Care Cancer 16 (2008): 447-451.
- Fearon KCH. Cancer cachexia: Developing multimodal therapy for a multidimensional problem. Eur. J. Cancer 44 (2008): 1124-1132.
- Doucet E, St-Pierre S, Alméras N, et al. Reduction of visceral adipose tissue during weight loss. Eur. J. Clin. Nutr 56 (2002): 297-304.
- Caan BJ, Meyerhardt JA, Kroenke CH, et al. Explaining the obesity paradox: The association between body composition and colorectal cancer survival (c-scans study). Cancer Epidemiol. Biomarkers Prev 26 (2017): 1008-1015.
