Adherence of health care practitioners to WHO management guidelines for malaria in Ijebu Ode, Nigeria
Article Information
Owolabi Emmanuel Sokefun1,2, Fisayo Tolulope Afuye2, Olabode Onileere1,, Onikepe Folarin2,3,*
1Department of Biological Sciences, Covenant University, Ota, Ogun State, Nigeria
2Department of Biological Sciences, Redeemer’s University, Ede, Osun State, Nigeria
3African Centre of Excellence for Genomics of Infectious Diseases (ACEGID), Redeemer’s University, Ede, Osun State, Nigeria
*Corresponding author: Onikepe Folarin, African Centre of Excellence for Genomics of Infectious Diseases (ACEGID), Redeemer’s University, Ede, Osun State, Nigeria.
Received: 07 June 2022; Accepted: 15 June 2022; Published: 22 June 2022
Citation:
Owolabi Emmanuel Sokefun, Fisayo Tolulope Afuye, Olabode Onileere,, Onikepe Folarin. Adherence of health care practitioners to WHO management guidelines for malaria in Ijebu Ode Nigeria. Fortune Journal of Health Sciences 5 (2022): 321-333
Share at FacebookAbstract
Adherence to treatment guidelines is pivotal to malaria control, and ultimately the actualization of the malaria eradication agenda in Africa. This study assessed malaria management practices among health care practitioners in Ijebu Ode community, Ogun State, Nigeria. Structured questionnaires measuring treatment practices were administered to consenting health care practitioners in the study area. Responses were scored and compared to national and World Health Organization (WHO) guidelines for malaria management. Fifty-one (51) responses comprising mostly of doctors (56.86%) were utilized out of 54 participants enrolled. Consecutive adults (>18 years old) had the greatest susceptibility to malaria from the study. Presumptive diagnosis (82.33%) with (74.49%) or without (7.84%) the combination of other methods was the most reported form of diagnosis while Arthemether-Lumefantrine (88.0%) was the most prescribed drug for treating uncomplicated malaria. The correct Intermittent Prevention Therapy (IPT) of Sulfadoxine-Pyrimethamine (SP) administered at least twice during pregnancy was followed by only 45.10% of the practitioners. Mean practice score in the study was 2.3±1.0 out of a total obtainable score of 4. Nurses had the highest practice score (72.5%), followed by doctors (55.0%). Average practice scores were equal across private (2.3±0.9) and government-owned (2.3±1.4) hospitals with the only difference observed in the standard deviation. Lack of funding and necessary equipment was reasons reported to hinder performance. Management practices of health care institutions in the study area are average, and a multifaceted approach is needed to ensure strict compliance with national and WHO malaria management guidelines to achieve the malaria eradication agenda.
Keywords
Malaria, Malaria Treatment, Malaria Eradication, World Health Organization, Ijebu Ode
Article Details
1. Introduction
Malaria remains a major public health challenge in sub-Saharan Africa. The World Health Organization (WHO) estimates that there were over 200 million cases of malaria with 409,000 deaths in 2019, 90% of which were recorded in sub-Saharan Africa [1]. Stakeholders from around the world met in 2015 and adopted the sustainable development goals (SDG) which has as one of its targets, the complete eradication of malaria globally. In the absence of an effective vaccine, the WHO identified accurate diagnosis and prompt treatment of malaria as being crucial in achieving this target [2]. The effectiveness of accurate and prompt diagnosis is in turn dependent on compliance of health care practitioners in affected areas to stipulated guidelines.
The widespread parasite resistance to Sulphadoxine-Pyrimethamine (SP) and Chloroquine (CQ) in most malaria-endemic countries including Nigeria, resulted in the switch to the Artemisinin Combination Therapies (ACTs) in 2005 [3]. There have been recent reports of reduced susceptibility and delayed parasite clearance to these ACTs. It is therefore important to ensure and protect the effectiveness of the ACTs in the treatment of acute, uncomplicated malaria infection if eradication and elimination goals are to be achieved. Thus, scaling up on the distribution of the rapid diagnostic tests (RDTs) was ensured, in addition to the availability of the ACTs [20].
Clinical audits assessing adherence to new malaria management guidelines have however shown that CQ is still being prescribed in health care facilities, and that health care practitioners still adopt presumptive diagnosis as the first line [3, 4]. This non-compliance found in some communities in Nigeria necessitates the need for renewed efforts informed by constant monitoring of malaria management practices across the country. This study assessed malaria management practices of health care-practitioners in Ijebu Ode, a major town in southwestern Nigeria.
2. Methodology
2.1 Study site and sampling
The study was conducted from April to July, 2015, in Ijebu Ode, Ogun State, Nigeria. Convenience sampling was adopted in the selection of health care facilities and participants across government and private health care institutions. Health care practitioners in selected facilities were approached and enrolled into the study after obtaining informed consent. Participants comprised doctors, nurses, matrons, and other members of staff, such as clinic assistants, in the health care facilities where the data was gathered. Participants in this study included workers in primary, secondary and tertiary health care institutions in the town.
2.2 Study instrument
The study instrument used in this study was a close ended questionnaire consisting of 5 sections designed to collect demographic information and measure the various aspects of malaria management. General information of the respondents, including their designation, the number of years they had spent working in the particular institution, and daily patient count visiting the health care facility, were recorded in the first section. Respondents were also asked whether or not they carry out malaria awareness to their patients, advising them on the judicious use of insecticide-treated nets, mosquito repellent creams, insecticide sprays, and other barriers that keep the disease vector away. The second section included patients’ information, including the malaria symptoms exhibited by the patients, frequency of malaria and other illnesses, the genotype(s) mostly infected with malaria in their facility, frequency by age group and severity of infection. In determining the age group most often treated for malaria, respondents answered how often a particular age group was treated for malaria, and the responses were scored as follows: Never-1; Seldom- 2; Often- 3; Always- 4. Data from applicants who indicated “No idea” or unavailable data were discarded from the study and given a score of zero (0). The different age groups were: Toddlers ≤5 old; Children 6-12 years old; teenagers 13-18 years old; > 18 years old; and pregnant women. The scores were added and the average scores were determined by dividing the cumulative score by the number of respondents.
The third section included the method of diagnosis of malaria by the practitioners and/or the health care facility where the participants work, the reasons for selecting their chosen method and whether plans were afoot for upgrading their techniques. Respondents were also asked for the specie that the encounter most often. The fourth section contained information on the drug prescription, treatment failure and the frequency of complicated malaria from treatment failure. The fifth section captured data from malaria in pregnancy, including cases, treatment, prevention, symptoms and prescription, as well as frequency of vertical transmission of malaria. In total, 54 questionnaires were distributed across all health care facilities.
2.3 Data analysis
The WHO guidelines for the treatment of malaria [5] were used as a baseline for measuring correct practice. A subset of 3 questions relating to malaria management techniques (correct diagnosis, treatment and IPT recommendation) were used to measure management practice. Respondents were scored one (1) point if response was in line with WHO guidelines and zero (0) points if otherwise. In addition, we scored respondents one (1) point if they indicated that they carry out malaria awareness to patients, advising them on vector decimating and screening methods, and zero (0) points if they did not. Their total score out of 4 was converted to percentage and this was their final grade in the assessment of their conformity to the national and WHO standards for the management of malaria. Chi-square, student’s t-test and ANOVA were used to analyze the various subgroups of data.
3. Results
3.1 Respondent demographic characteristics
From the 54 questionnaires distributed, 3 questionnaires were excluded because the respondents did not indicate their designation with the health care facility, i.e. whether they are doctors, nurses, attendants, etc. In total, we worked with 51 questionnaires. Study participants were mostly doctors (56.86%) followed by nurses (29.41%). Only a few (5.88%) had spent more than 5 years in their facility. Majority of the health care facilities involved were private (82.35%) and the respondents majorly had spent less than one year (49.02%) in their current health care facilities, and the facilities mostly give secondary treatment (47.06%). Majority (58.82% of the respondents indicated that they organize malaria sensitization campaigns to the public (table 1). Most health care facilities (43.14%) receive at least 10 outpatients in a day, 13.73% receive less than 10 patients, and 5 (9.80%) had no records for outpatient count and 1 (1.96%) did not indicate.
Table 1: Demographic characteristics of respondents
|
Count % |
|||
|
Type of health care services rendered |
Primary health care |
19 |
37.26 |
|
Secondary health care |
24 |
47.06 |
|
|
Tertiary health care |
5 |
9.8 |
|
|
Unindicated |
3 |
5.88 |
|
|
Ownership |
Private |
40 |
78.43 |
|
Government |
11 |
21.57 |
|
|
Designation |
Doctor |
29 |
56.86 |
|
Matron |
5 |
9.8 |
|
|
Nurse |
15 |
29.41 |
|
|
Other |
2 |
3.92 |
|
|
Participant’s years of experience in current facility (Yrs) |
<1 |
25 |
49.02 |
|
5-Jan |
20 |
39.22 |
|
|
>6 |
3 |
5.88 |
|
|
Unindicated |
3 |
5.88 |
|
|
Organize awareness programs |
Yes |
30 |
58.82 |
|
No |
21 |
41.18 |
|
3.2 Patient Presentation
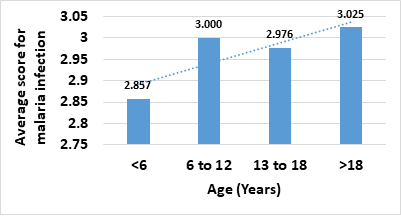
Patients visiting the health care facility exhibited fever alone (66.67%), nausea alone (7.84%), fever and nausea (11.77%), fever and diarrhea (1.96%), and fever and at least 2 other symptoms (11.77%). Average malaria cases of >20 in a month occurred the most (62.75%), followed by 10-20 malaria cases monthly (13.73%), 6-10 cases (7.84%), and <5 cases (11.77%). Consecutive adults (above 18 years old) are mostly infected with malaria in the study area with a mean score of 3.025 based on our grading system. Children <6 years old had the least score of 2.857 (Fig. 1). Pregnant women had a score of 2.511. Patients with malaria were mostly of the genotype: AA (78.05%); AS (14.63%); SS (4.88%); AA/AS co-occurring frequency (2.44%); and other genotypes (2.44%).
3.3 Disease diagnosis
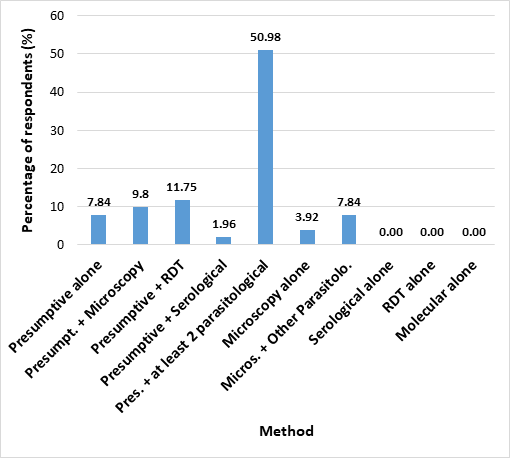
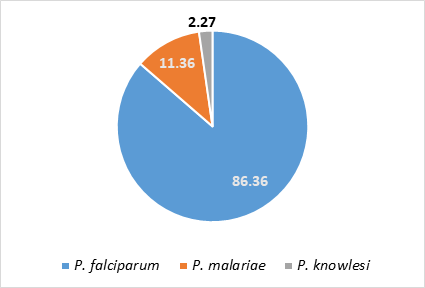
Of all the respondents, 7.84% (6) indicated that they rely solely on patient symptoms (presumptive diagnosis) without carrying out any tests, in diagnosing malaria, 11.75% combine presumptive diagnosis with rapid diagnostic test (RDT), 9.80% (5) combine presumptive diagnosis with microscopy, and 1.96% (1) combine symptomatic evaluation with serological tests. Combination of presumptive diagnosis and at least two parasitological methods was reported by 50.98% (26) of the respondents in their health care facility, while 9.80% (5) do not rely on symptoms at all (Fig 2). We found no significant difference (p>0.05) in the diagnostic techniques between private and government owned facilities. The reasons given for the diagnostic method of choice were available equipment (48.72%), available funds (15.39%), technical know-how (15.39%). Some respondents (15.39%) highlighted a combination of all three reasons as the reason for their diagnostic method, 2.56% said it was due to a combination of financial reasons and available equipment. Others (1.96%) cited other reasons. We found no significant difference (p>0.05) between the response from respondents in government facilities compared to those in private facilities as regards plans to upgrade or modify their methods of testing for malaria. Majority of the respondents (73.81%) indicated plans are being made to upgrade, but 26.19% have no upgrade plans. Among the respondents who carry out any form of laboratory tests, 86.36% reported Plasmodium falciparum as the most common species. (Fig. 3).
3.4 Treatment of uncomplicated malaria
Arthemeter-Lumefantrine (AL) was reported by 88.00% of the respondents as a prescribed drug for the treatment of malaria (Table 2). Chloroquine was prescribed by 28.00% of the respondents while 22.00% also prescribed Sulfadoxine-Pyrimethamine as part of the treatment regimen (Table 2).
3.5 Malaria in pregnancy
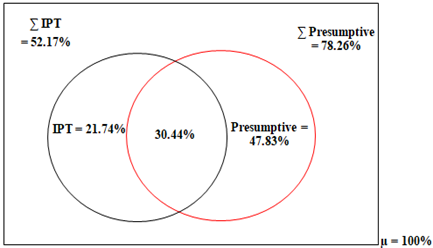
Malaria in pregnancy cases were mostly treated when symptoms were exhibited (Fig. 4). Following symptoms, 52.17%, 19.57% and 15.22% of the respondents prescribed AL, CQ and SP respectively for treatment of malaria in pregnancy (Table 2). Intermittent preventive therapy (IPT) using SP was recommended by 84.78% of respondents to pregnant women visiting their health care facilities and its recommended for at least twice in the pregnancy. SP (68.42%) was selected as the main drug of choice for IPT. Pyrimethamine (PY) was chosen by 5.26% of the participants, while 13.16% selected both SP and PY. IPT was mainly administered twice during pregnancy (76.19%).
Table 2: Drugs used for malaria treatment in the health care facilities
|
Drug |
Uncomplicated malaria |
Malaria in pregnancy |
||
|
Count |
% |
Count |
% |
|
|
Amodiaquine |
8 |
16 |
6 |
13.04 |
|
Artemether/Lumefantrine |
44 |
88 |
24 |
52.17 |
|
Chloroquine |
14 |
28 |
9 |
19.57 |
|
Sulfadoxine/Pyrimethamine |
11 |
22 |
7 |
15.22 |
|
Metronidazole |
0 |
0 |
N/A |
N/A |
|
IPT |
2 |
4 |
4 |
8.7 |
|
Quinine |
9 |
18 |
7 |
15.22 |
|
Trimethoprim/Sulfamethoxazole |
1 |
2 |
3 |
6.52 |
|
Other |
8 |
16 |
8 |
17.39 |
3.6 Practice scores for the management of malaria by respondents
Table 3: Responses to practice questions
|
S/N |
Question |
N=51 |
Mean Score |
|||
|
Correct |
Incorrect |
|||||
|
Count |
% |
Count |
% |
|||
|
1 |
Does the health care institution organize malaria awareness to the patients? |
30 |
58.82 |
21 |
41.18 |
0.59 |
|
Correct Response: Yes |
||||||
|
2 |
What diagnostic procedure(s) does this health care centre employ in confirming malaria infection in the patients? |
44 |
86.28 |
7 |
13.72 |
0.86 |
|
Correct Response: Microscopy/Serological test/RDT |
||||||
|
3 |
What medication is normally prescribed for malaria patients in this health care centre? |
21 |
41.18 |
30 |
58.82 |
0.41 |
|
Correct Response: Artemether Lumefantrine (alone) |
||||||
|
4 |
IPT recommendation during pregnancy. |
23 |
45.1 |
28 |
54.9 |
0.45 |
|
Correct Response: IPT-SP at least twice during the pregnancy |
||||||
Table 4: Practice scores of the different groups of respondents
|
Count (N=51) |
Mean score (Max=4) |
Grade (%) |
P Value |
||
|
Designation |
Doctors |
29 |
2.2±0.9 |
55 |
|
|
Matrons |
5 |
1.4±1.3 |
35 |
||
|
Nurses |
15 |
2.9±0.7 |
72.5 |
0.767 |
|
|
Other |
2 |
2.0±1.4 |
50 |
||
|
Ownership |
Private |
40 |
2.3±0.9 |
57.5 |
|
|
Government |
11 |
2.3±1.4 |
57.5 |
0.439 |
|
|
Years spent in current facility |
<1 |
14 |
2.0±0.8 |
50 |
|
|
5-Jan |
20 |
2.6±1.2 |
65 |
||
|
>5 |
14 |
2.4±0.9 |
60 |
1.051 |
|
|
Unindicated |
3 |
1.7±0.6 |
42.5 |
||
|
Type of service |
Primary health care |
19 |
2.1±1.0 |
52.5 |
|
|
Secondary health care |
23 |
2.52±0.9 |
63 |
||
|
Tertiary health care |
5 |
2.2±0.8 |
55 |
0.359 |
|
|
Unindicated |
3 |
1.7±1.1 |
41.75 |
||
|
Total |
N/A |
51 |
2.3±1.0 |
57.5 |
|
Figure 5: Histogram of practice score
4. Discussion
The prevention, management and control of any disease are critical in curtailing its spread in any community. As time ticks down on the Sustainable Development Goals, there is a need to revisit the knowledge and practices of health care personnel in communities with incidence of malaria, to assess whether they are working in line with recommended national [5, 6] guidelines. Our study shows an average score in management practices for malaria infection. There is therefore a need to monitor the practices of health care practitioners to ensure adherence to WHO guideline in order to achieve elimination and eradication of the disease
Almost half (41.18%) of the respondents indicated not carrying out malaria awareness and sensitization to the patients, focusing solely on diagnosis and treatment instead. This approach may be a financially favorable one for the practitioners but it represents a major drawback in the fight against malaria eradication, which increases cases and places a heavier burden on drugs. Many malaria cases would be averted if more people are aware of ways in which the infection can be averted, such as the use of insecticide-treated nets, draining swamps and destroying mosquito breeding places and use of window and door nets. Ironically, most of these techniques are cheaper than the cost of malaria treatment.
Respondents indicated that the genotype mostly infected with malaria is the AA genotype while the least is the SS genotype This tallies with conventional knowledge and other studies that persons with the hemoglobin genotype group AA, are more susceptible to malaria due to the absence of the sickle cells than those with the heterozygous sickle cell hemoglobin group AS [7]. This report runs contrary to other studies which show that the SS population is the most susceptible to malaria [8]. However, the AA genotype constitutes majority of the Nigerian population, followed by the AS and SS [9]. This greatly influences the number of patients with the respective genotypes visiting the health care facilities and will skew the malaria data infections incidence towards persons with the AA genotype.
Children aged 0-5, whose immunity has not developed fully, and pregnant women, whose immunity has decreased due to pregnancy, are most susceptible to malaria (Centers for Disease Control and Prevention, 2020; WHO, no date). However, in this study consecutive adults (more than 18 years old) are mostly infected with malaria in the study area with a mean score of 3.03 next to children aged 6-12 (3.00), teenagers aged 13-18 (2.98), pregnant women (2.51) and children aged 0-5 years old (2.86). The results, which defy convention, may have been influenced by the small sample size but could also be due to certain peculiarities about the study area with respect to lifestyle, for example, increased exposure of the prevalent groups, or older adults getting stressed out from work thereby decreasing immunity. A change in epidemiology of susceptible groups may be a likely cause, as children above 5 years of age were seen to be more susceptible in a previous study on malaria prevalence among children [10].
The majority (82.33%) of respondents indicate that malaria diagnosis is done by presumptive evaluation in their health care facility. From this number, 74.49% combine symptomatic evaluation with parasitological methods of diagnosis, and 7.84% rely absolutely on presumptive diagnosis. Inquest was not made as to whether the practitioners who diagnose by a combination of presumptive and parasitological techniques do so interchangeably or simultaneously. In this present time one would not expect health care practitioners to rely solely on presumptive diagnosis. Although the dependence on presumptive diagnosis alone may be explained by the low willingness of the patients to pay for the cost of diagnosis which is common in low-mid income regions such as our study setting [11], but the respondents also cited lack of available resources and equipment to perform the job optimally. One downside of presumptive diagnosis of malaria is a high chance of misdiagnosis, considering that numerous infectious diseases have similar signs and symptoms such as fever. Considering the ongoing COVID-19 pandemic, presumptive diagnosis of fever as malaria may actually be COVID-19 infection thus misdiagnosing and allowing for continuous spread of the infection. In addition misdiagnosis may contribute to indiscriminate use of antimalarial drug which can ultimately contribute to emergence of resistance to the antimalarial drug.
The recommended first and second line treatment for acute uncomplicated Plasmodium falciparum malaria in Nigeria are Arthemeter-Lumefantrine (AL) and Artesunate-Amodiaquine (AA). Only 41.18% of the respondents selected the AL. Others either selected entirely different drugs from the recommended AL or selected AL as well as other drugs, which is not the the approved first line treatment in the country. Our study also showed some cases of CQ prescription, despite an official change to the ACTs since 2005, necessitated by the high rate of CQ resistant parasites in the region [12, 13]. We suspect that the continued prescription of CQ in health care facilities in Nigeria is mediated by cost as CQ is cheaper than most antimalarials, including the ACTs. Moreover, CQ is a very popular drug known by the average Nigerian and health care practitioner. Lack of strong enforcement of the removal of CQ still encourages its availability and prescription. The implication of the availability and prescription of CQ is persistence and increasing CQR parasites within the country. This may be one of the contributing factor why there is persistence of parasite with the mutant allele of pfcrt gene in the country even after about 20 years of change to the ACTs in the country [14]. In countries such as Malawi where the use of CQ was totally banned (no over the counter), the mutant allele pfcrt gene in the P. falciparum was completely reversed to the wild type allele 10 years after the change [15]. It is therefore important to ensure that CQ though cheaper, should be completely removed from circulation should we plan that it can be re-introduced for use as antimalarial drug based on the reversal phenomenon..
Management of malaria in pregnancy was similar to the management of uncomplicated malaria, with most prescribing AL and a few prescribing CQ among the respondents. This is especially noteworthy as pregnant women are among the population at high risk [16]. Prescription of CQ could be harmful for both the mother and the foetus [13]. More concerningly, less than half of the respondents indicated that Sulfadoxine/Pyrimethamine (SP) was the drug given for Intermittent Prevention Therapy (IPT) at least twice during pregnancy. Others either do not recommend IPT, do not give it up to twice, for instance, only at the beginning of the pregnancy, or they give a different drug than the officially recommended one. This practice may negatively influence vertical malaria transmission, as well as mother and fetal health.
The average practice score among the health care practitioners in this study was 2.33 out of 4, or 57.5%. This score is just average hence the need for constant monitoring of the practices and campaigning in order to achieve elimination. We infer from our findings that the patterns of malaria management practice found in this study are likely due to factors external to the health care workers. Factors such as lack of necessary drugs in store, and lack of equipment, as found in this study, have been shown to influence malaria management practice among health care professionals [17, 18]. Scores were the same (2.3 or 57.5%) across private and government-owned hospitals, with only the standard deviation differing (±0.9 for private and ±1.4 for government-owned). This hints that the culture, practices and challenges in health care facilities in the study town are similar, irrespective of the ownership of the facility.
Achieving the set mark of complete eradication of malaria by 2030 would require strict compliance with stipulated guidelines. The availability and prescription of CQ has serious implications for the malaria eradication agenda. Continued use of CQ could potentially roll back some of the progress made so far, especially in terms of morbidity and mortality rate, resistance reversal or effectiveness of the ACTs. Presumptive diagnosis alone needs to be totally abolished. The practice is indicative of malaria overdiagnosis and overtreatment [18, 19]. Overtreatment of malaria puts pressure on the current drugs and is often a precursor for the emergence of resistance. Moreover, given that malaria may share the same symptoms with other infections, it may mean that other infections are not being properly treated, which may have its own negative effects on the health of residents in the study town. It is important that all individuals and institutions involved in health care management increase awareness on the ways to intercept transmission by decimating the disease vector. The government has to increase the quota for the health sector in the annual budget, and periodic retraining of personnel on the best practices in the management of certain diseases in recommended. In the face of the ongoing pandemic, practices on management of malaria infection has to according to the WHO guideline in order to enable prompt control of the COVID-19 pandemic. Treating all fever as malaria may mean missing some COVID_19 infections thus increasing the number of cases in a particular community.
The main limitation of this study is the small sample size. More studies need to be carried out on a larger sample size in the study area. In conclusion, this study showed that the mean malaria management practices in the study area area are average mostly due to sub-optimal diagnosis and drug prescription habits. There is therefore an urgent need for multifaceted interventions for the national and WHO recommended guidelines for malaria management to be implemented, as a step closer to the actualization of the target of malaria eradication in the country. In addition there is a need to carry out periodic evaluation of the malaria management in the country in general as this will contribute greatly to achieving elimination of malaria in particular and control of other similar infectious diseases.
Conflicts of Interest:
The authors declared no conflicts of interest.
Funding:
The authors received no funding.
Data Availability Statement:
The data used to support the findings of this study are available from the corresponding author upon request.
References
- World Health Organization. World Malaria Report, Geneva (2020).
- World Health Organization. Malaria: Diagnostic Testing (2018).
- Udoh E, Oyo-Ita A, Odey F, Effa E, Esu E, Oduwole E et al. Management of uncomplicated malaria in underfives in private and public health facilities in South-eastern Nigeria: A clinical audit of current practices. Malaria Research and Treatment 575080 (2013).
- Bamiselu OF, Ajayi I, Fawole O, Dairo D, Ajumobi O, Oladimeji A et al. Adherence to malaria diagnosis and treatment guidelines among health care workers in Ogun State, Nigeria. BMC Public Health 16 (2016): 828.
- World Health Organization. Guidelines for the treatment of malaria, 3rd Edition (2015).
- Federal Ministry of Health. National Guidelines for the Diagnosis and Treatment of Malaria, 3rd Edition. (2015).
- Opara KN, Atting IA, Ukpong IG, Nwanbueze AA, Inokon II. Susceptibility of genetic indices to falciparum malaria in infants and young children in Southern Nigeria. Pakistan Journal of Biological Sciences 9 (2006): 452-456.
- Ito EE, Egwunyenga OA, Ake JEG. Prevalence of malaria and human blood factors among patients in Ethiope East, Delta State, Nigeria. International Journal of Medicine and Biomedical Research 3 (2014):191-201.
- Luzzato L. Sickle cell anemia and malaria. Mediterranean Journal of Hematology and Infectious Diseases 4 (2012): e2012065.
- Mawili-Mboumba DP, Akotet MKB, Nzamba J, Medang MO, Mbina JRM, Kombila M et al. Increase in malaria prevalence and age of at risk population in different areas of Gabon. Malaria Journal 12 (2013): 3.
- Uzochukwu BS, Onwujekwe OE, Uguru NP, Ughasoro MD, Ezeoke OP. Willingness to pay for rapid diagnostic tests for the diagnosis and treatment of malaria in southeast Nigeria: ex post and ex ante. International Journal for Equity in Health 9 (2010): 1.
- Efunshile M, Runsewe-Abiodun T, Ghebremedhin B, König W, König B. Prevalence of the molecular marker of chloroquine resistance (pfcrt 76) in Nigeria 5 years after withdrawal of the drug as first-line antimalarial: a cross-sectional study. South African Journal of Child Health 5 (2011): 2.
- Folarin OA, Gbotosho GO, Sowunmi A, Olorunsogo OO, Oduola AMJ, Happi TC. Chloroquine resistant Plasmodium falciparum in Nigeria: relationship between pfcrt and pfmdr1 polymorphisms, in-vitro resistance and treatment outcome. The Open Tropical Medicine Journal 1 (2008): 74–82.
- Kayode AT, Akano K, Ajogbasile FV, Uwanibe JV, Oluniyi PE, Bankole BE, et al. Polymorphisms in Plasmodium falciparum chloroquine resistance transporter (Pfcrt) and multidrug-resistant gene 1 (Pfmdr-1) in Nigerian children 10 years post-adoption of artemisinin-based combination treatments. International Journal of Parasitology Dec 24 (2020): S0020-7519(20)30318-0.
- Kublin JG, Cortese JF, Njunju EM, Mukadam RAG, Wirima JJ, Kazembe PN, et al. Re-emergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. Journal of Infectious Diseases 187 (2003):1870–5
- World Health Organization. Malaria: malaria in pregnant women 2017 (2019).
- Riley C, Dellicour S, Ouma P, Kioko U, ter Kuile FO, Omar A et al. Knowledge and adherence to the National Guidelines for Malaria Case Management in pregnancy among health careproviders and drug outlet dispensers in rural, Western Kenya. PloS One 11 (2016): e0145616.
- Awoleye OJ, Thron C. Improving access to malaria Rapid Diagnostic Test in Niger State, Nigeria: An assessment of implementation up to 2013. Malaria Research and Treatment 2016:1–13.
- Oladosu OO, Oyibo WA. Overdiagnosis and overtreatment of malaria in children that presented with fever in Lagos, Nigeria, ISRN Infectious Diseases 2013: 1–6.
- Nigerian Centers for Disease Control and Prevention. Malaria Five-Year Operation Plan (2009–2013).
- Centers for Disease Control and Prevention. Malaria’s impact worldwide (2021).
- World Health Organization. Population’s atrisk of malaria (high).
- Gwer S, Newton CJRC, Berkley JA. Over-diagnosis and co-morbidity of severe malaria in African children: A guide for clinicians. The American Journal of Tropical Medicine and Hygiene 77 (2007): 6–13.