Achalasia-like Motility Disorder in a Kidney Transplant Recipient
Article Information
Sascha T Bender1, Wilfried Obst2, Peter R Mertens1, Christian Gross1
1Clinic for Nephrology and Hypertension, Diabetology and Endocrinology, Otto-von-Guericke University, Magdeburg, Germany
2Clinic for Gastroenterology, Hepatology and Infectious Disease, Otto-von-Guericke University, Magdeburg, Germany
*Corresponding author: Sascha T Bender, Clinic for Nephrology and Hypertension, Diabetology and Endocrinology Otto-von-Guericke University Magdeburg Leipziger Str. 44 39120 Magdeburg, Germany
Received: 27 April 2021; Accepted: 06 May 2021; Published: 25 May 2021
Citation: Sascha T Bender, Wilfried Obst, Peter R Mertens, Christian Gross. Achalasia-like Motility Disorder in a Kidney Transplant Recipient. Archives of Clinical and Biomedical Research 5 (2021): 396-399.
Share at FacebookAbstract
We present a case of tacrolimus intoxication with an achalasia-like esophageal motility disorder in a long term-term renal recipient, who was admitted to hospital because of an acute on chronic kidney transplant injury and newly onset dysphagia. Esophageal manometry secured the diagnosis. Unfortunately despite recovered kidney function the clinical outcome of our index patient was fatal. He deceased in consequence of an extensive esophageal rupture. Our case demonstrates a rare but potentially serious complication of post-transplant patients on calcineurin inhibitors. Calcineurin inhibitor-induced achalasia should be considered in this population when newly onset dysphagia occurs with otherwise negative workup.
Keywords
Calcineurin inhibitor; CNI; Achalasia; Transplantation; Acute kidney injury
Article Details
Presentation of the Case
A 82-year-old kidney transplant recipient for 17 years, who developed end-stage kidney disease due to membranous glomerulonephritis, complained of acute onset dysphagia with difficulties in swallowing fluids since two weeks. Solid food was still ingested, vomiting did not occur. Signs of hypovolemia were apparent, with secondary oliguria and arterial hypotension (RR 100/60mmHg). Furthermore a strong finger tremor became apparent on physical examination. Lab values revealed acute kidney injury with a fall of eGFR from 28 to 13ml/min. The immunosuppressive regimen included 1.5mg tacrolimus twice daily. Unexpectedly, trough tacrolimus serum level was 65ng/ml (target range 4 to 7ng/ml).
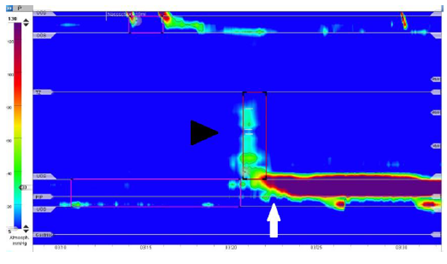
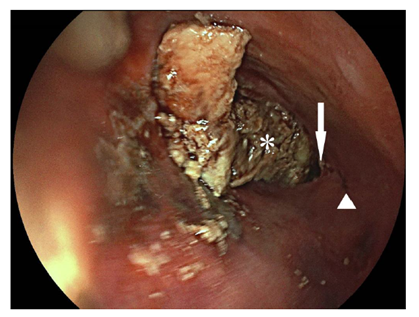
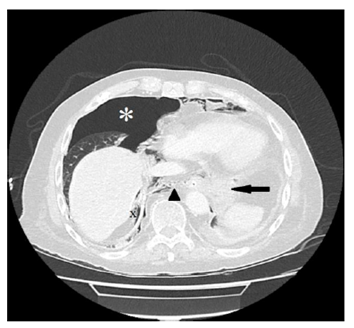
Endoscopy ruled out common causes of dysphagia, such as peptic or malignant stenoses, infections or eosinophilic esophagitis. However the lumen of the esophagus was enlarged and the lower esophagus sphincter (LES) was only passable by application of slight pressure. High resolution esophageal manometry revealed hypertensive contraction of the LES (quantified mean resting pressure of 152mmHg [normal range <5-18mmHg]) with inadequate relaxation after swallowing of liquids (mean integrated relaxation pressure [IRP] of 35,5mmHg [normal range <15mmHg]) (Figure 1). The peristalsis of the tubular esophagus was nearly completely absent. The diagnosis of hypertensive LES with transition to achalasia type I due to tacrolimus-intoxication was made. The most likely reason being false tablet intake regimen. Besides intravenous fluid substitution tacrolimus administration was paused until the original target levels of 4-7ng/ml were attained. Kidney function recovered to prevalues. Balloon dilatation was planned as next therapeutic step, however the patient ingested a large amount of food at a family celebration and experienced acute retrosternal pain. An emergency esophagogastroscopy revealed esophageal rupture (Figure 2), which was also confirmed by computed tomography (Figure 3), with extending to the esophagogastric junction. Due to the patient will, an operation was not performed and he deceased.
Figure 1. High resolution esophageal manometry. Swallow test shows a hypertensive lower esophageal sphincter with a mean pressure of 152mmHg (5-18mmHg) in rest (white arrow), an inadequate postdeglutitive relaxation quantified with a mean integrated relaxation pressure of 35,5mmHg (<15mmHg) and an almost aperistaltic tubular body (black arrowhead) with a detected distal contractile integral of 105mmHg x cm x s (450-8000mmHg x cm x s). These findings are compatible with an achalasia type I.
Figure 2. Esophagoscopic view into the distal esophagus. The true lumen (white arrowhead) is on the right side, whereas the extensive perforation of approximately 10cm is on the left side including the gastroesophageal junction (white arrow). Behind the lesion on the left side is the adjoining pericard depicted (white star).
Figure 3. Esophageal perforation diagnosed by computed tomography scan. A distal esophageal perforation is diagnosed due to a 5.5 x 5.8cm intrathoracal enhancement (indicated by bold black arrow) with involvement of the esophagogastric junction, consecutive pneumothorax (white star), tension component on the right, intraabdominal free air dorsomedial to the liver (indicated by black x) and pneumomediastinum (black arrowhead).
2. Conclusion
The index case is diagnosed with achalasia due to a rare, however severe complication of calcineurin inhibitor (CNI) intoxication. Regarding pathophysiology calcineurin inhibition mediates nitric oxide synthase inhibition [1,2]. Balloon dilatation, botulinum toxin injection, Gottstein-Heller myotomy and switching to another CNI are considered appropriate therapeutic options [3,4].
References
- Saco TV, Ledford DK, Shah S, et al. Tacrolimus: A Heart pill to swallow. Journal of Allergy and Clinical Immunology 137 (2016): AB25.
- Koch R, Zoller H, Graziadei I, et al. Cyclosporine A-induced achalasia-like esophageal motility disorder in a liver transplant recipient: successful conversion to tacrolimus. Transplantation 76 (2003): 744-745.
- Goklemez S, Curtis LM, Hawwa A, et al. Achalasia in a Patient undergoing hematologic stem cell transplant after exposure to Tacrolimus. Mayo Clin Proc Innov Qual Outcomes 1 (2017): 198-201.
- Campos M, Matlock R. Endoscopic Botulinum toxin injection for tacrolimus-induced Achalasia in a renal transplant recipient. Gastroenterology Res 12 (2019): 171-173.