A Pseudo-Meig Syndrome Associated with Malignant Brenner Tumor and Small Cell Carcinoma of the Ovary (FIGO IIIC)
Article Information
Chi-Shang Wei1, Chi-Feng Su1, Horng-Jyh Tsai1 *, Chien-Kuan Lee2
1Department of Obstetrics and Gynecology, Kuan Tien General Hospital, Taichung, Taiwan
2Department of Pathology, Kuan Tien General Hospital, Taichung, Taiwan
*Corresponding author: Horng-Jyh Tsai, Department of Obstetrics and Gynecology, Kuan Tien General Hospital, 117 Sha-Tian Road, Sha-Lu, Taichung, Taiwan 433.
Received: 29 July 2021; Accepted: 06 August 2021; Published: 13 August 2021
Citation: Chi-Shang Wei, Chi-Feng Su, Horng-Jyh Tsai, Chien-Kuan Lee. A Pseudo-Meig Syndrome Associated with Malignant Brenner Tumor and Small Cell Carcinoma of the Ovary (FIGO IIIC). Fortune Journal of Health Sciences 4 (2021): 437-440.
Share at FacebookKeywords
Small Cell Carcinoma; Ovary; Pseudo-Meig Syndrome
Syndrome articles, Small Cell Carcinoma articles Small Cell Carcinoma Research articles Small Cell Carcinoma review articles Small Cell Carcinoma PubMed articles Small Cell Carcinoma PubMed Central articles Small Cell Carcinoma 2023 articles Small Cell Carcinoma 2024 articles Small Cell Carcinoma Scopus articles Small Cell Carcinoma impact factor journals Small Cell Carcinoma Scopus journals Small Cell Carcinoma PubMed journals Small Cell Carcinoma medical journals Small Cell Carcinoma free journals Small Cell Carcinoma best journals Small Cell Carcinoma top journals Small Cell Carcinoma free medical journals Small Cell Carcinoma famous journals Small Cell Carcinoma Google Scholar indexed journals Ovary articles Ovary Research articles Ovary review articles Ovary PubMed articles Ovary PubMed Central articles Ovary 2023 articles Ovary 2024 articles Ovary Scopus articles Ovary impact factor journals Ovary Scopus journals Ovary PubMed journals Ovary medical journals Ovary free journals Ovary best journals Ovary top journals Ovary free medical journals Ovary famous journals Ovary Google Scholar indexed journals Pseudo-Meig Syndrome articles Pseudo-Meig Syndrome Research articles Pseudo-Meig Syndrome review articles Pseudo-Meig Syndrome PubMed articles Pseudo-Meig Syndrome PubMed Central articles Pseudo-Meig Syndrome 2023 articles Pseudo-Meig Syndrome 2024 articles Pseudo-Meig Syndrome Scopus articles Pseudo-Meig Syndrome impact factor journals Pseudo-Meig Syndrome Scopus journals Pseudo-Meig Syndrome PubMed journals Pseudo-Meig Syndrome medical journals Pseudo-Meig Syndrome free journals Pseudo-Meig Syndrome best journals Pseudo-Meig Syndrome top journals Pseudo-Meig Syndrome free medical journals Pseudo-Meig Syndrome famous journals Pseudo-Meig Syndrome Google Scholar indexed journals immunopositivity articles immunopositivity Research articles immunopositivity review articles immunopositivity PubMed articles immunopositivity PubMed Central articles immunopositivity 2023 articles immunopositivity 2024 articles immunopositivity Scopus articles immunopositivity impact factor journals immunopositivity Scopus journals immunopositivity PubMed journals immunopositivity medical journals immunopositivity free journals immunopositivity best journals immunopositivity top journals immunopositivity free medical journals immunopositivity famous journals immunopositivity Google Scholar indexed journals malignant Brenner tumor articles malignant Brenner tumor Research articles malignant Brenner tumor review articles malignant Brenner tumor PubMed articles malignant Brenner tumor PubMed Central articles malignant Brenner tumor 2023 articles malignant Brenner tumor 2024 articles malignant Brenner tumor Scopus articles malignant Brenner tumor impact factor journals malignant Brenner tumor Scopus journals malignant Brenner tumor PubMed journals malignant Brenner tumor medical journals malignant Brenner tumor free journals malignant Brenner tumor best journals malignant Brenner tumor top journals malignant Brenner tumor free medical journals malignant Brenner tumor famous journals malignant Brenner tumor Google Scholar indexed journals
Article Details
1. Introduction
Ovarian Brenner tumors are rare epithelial tumors that account for 1%-2% of all ovarian neoplasm’s; only about 1% of Brenner tumors are malignant [1]. Pseudo-Meig syndrome mimics the Meig syndrome triad (benign ovarian tumor, ascites, and pleural effusion); however, the ovarian tumor usually represents a primary malignancy or metastases. We report a rare encounter of Pseudo-Meig syndrome with malignant Brenner tumor (MBT) and small cell carcinoma of the left ovary.
A 71-year-old woman, para 3, with menopause at 56 years of age, had a medical history of hypertension, type 2 diabetes mellitus, and dementia well control for 10 years, presented with progressive abdominal swelling for a month. Her surgical history showed a total knee replacement many years before and a gastrojejunostomy/enterolysis 5 years ago for duodenal ulcer. A left pleural effusion was found exact 12 months prior and the result of effusion culture showed no growth; cell block/cytology was unable to detect malignant cells. A pleural biopsy revealed granulation tissue with chronic inflammation and old hemorrhage. Physically, the abdomen was soft, markedly distended, with hypoactive bowel sounds. An abdominal ultrasonography showed prominent ascites, a large left ovarian tumor, irregular of 15 cm in size with calcification part. An abdominal computerized tomography confirmed the complex tumor (Fig. 1a) with some small nodules on the peritoneum, also.
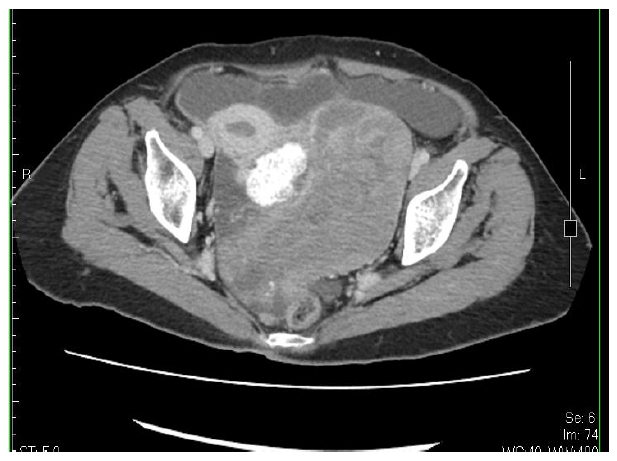
Figure 1(a): An abdominal computerized tomography showing a complex tumor of left ovary, most solid, with calcification part
Tumor markers of CA19-9 at 170.7 U/mL and CA-125 at 358 U/mL were reported. She underwent cancer staging/debulking; ascites of 4800 mL was removed. Pathological diagnosis showed ruptured left ovarian tumor, weighing 524 gm; malignant Brenner tumor 95% and small cell carcinoma component 5% (Fig. 1b,c);
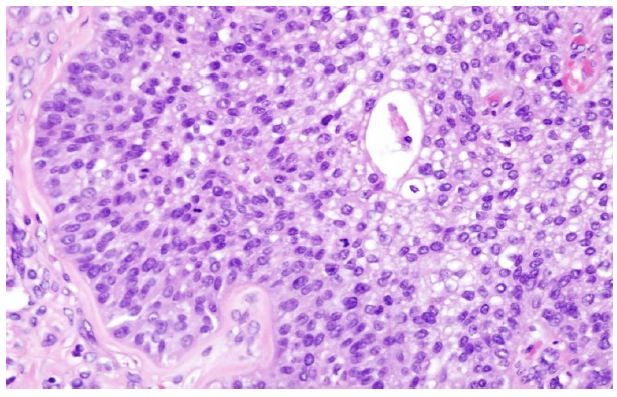
Figure 1(b): Microscopically, malignant Brenner tumor (95%) (H&E 400X)
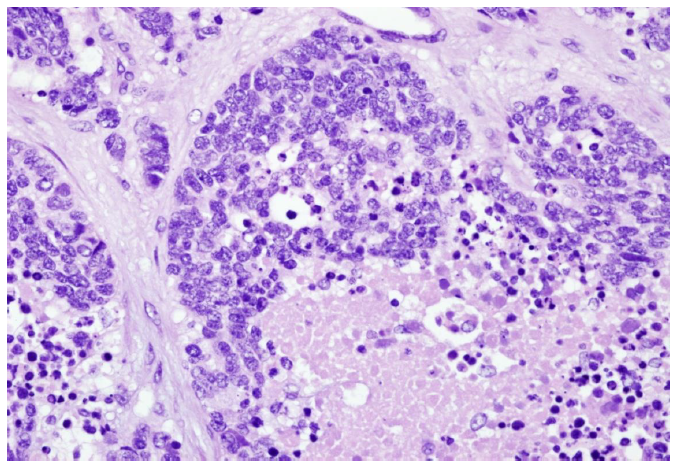
Figure 1(c): Microscopically, small cell component (5%) (H&E 400X)
Metastatic small cell carcinoma in right fallopian tube, uterine serosa and retroperitoneum ; (FIGO IIIC). The MBT shows immunopositivity for GATA-3 (focal) and p63, negative for PAX-8. Shared decision making offered, we agreed on symptomatic treatment, and she died 3 months later.
In a population-based study of MBT, between 1988 and 2012 from the National Cancer Institute Surveillance and Epidemiology End Results database, 207 patients were identified [2]. Median patient age was 65 years; the majority presented with unilateral, high grade tumors with a median size of 10 cm. Stage I, II, III and IV disease was noted for 55.4%, 14.4%, 18%, and 12.2% respectively. Only 5.1% had positive lymph nodes for metastatic disease; the authors concluded that lymphadenectomy was not associated with an improved five-year disease-specific survival (p=0.2) [2]. Of 13 cases of MBT at Selçuk University, Turkey, from 2004 to 2010, the median age was 55.69 years; the mean size of the tumors was 9.19 cm [3]. A total of 10 patients of MBT were found between 1991 and 2013 at Asan Medical Center, Korea. The median age was 55.5; the median size of the tumors was 10.5 cm [4]. Another 10 cases between 1999 and 2018 at University of North Carolina showed median age was 64; the median tumor stage IIa/IIb; 7 patients with adjuvant chemotherapy, and median progression-free survival of 37months [5]. Therefore, clinical stages of patients of MBT when detected vary, and are closely related to the prognosis, the same as with other malignancies.
2. Authors Conflicts:
The authors declare no conflicts of interest.
3. References
- ArnogiannakiN, Grigoriadis C, Zygouris D, Terzakis E, Sebastiadou M, Tserkezoglou Proliferative Brenner tumor of the ovary. Clinicopathological study of two cases and review of the literature. Eur J Gynaecol Oncol 32 (2011): 576-8.
- Nasioudis D, Sisti G, Holcomb K, Kanninen T, Witkin SS. Malignant Brenner tumors of the ovary; a population-based analysis. Gynecol Oncol 142 (2016): 44-49.
- Gezginç K, Karatayli R, Yazici F, Acar A, Çelik Ç, Çapar M, et al. Malignant Brenner tumor of the ovary: analysis of 13 cases. Int J Clin Oncol 17 (2012): 324-9.
- Han JH, Kim DY, Lee SW, Park JY, Kim JH, Kim YM, et al. Intensive systemic chemotherapy is effective against recurrent malignant Brenner tumor of the ovary: An analysis of 10 cases within a single center. Taiwan J Obstet Gynecol 54 (2015): 178-82.
- Zhang Y, Staley SA, Tucker K, Clark LH. Malignant Brenner tumor of the ovary: Case series and review of treatment strategies. Gynecol Oncol Rep 28 (2019): 29-32.
