A flow Cytometry Gating Strategy for Profiling CD4+CD25+/highCD127-/low Regulatory T Cells in the Rheumatoid Arthritis Scenario
Article Information
Martínez Rosales Ricardo Ulises*, Crespo Oliva Celia del Carmen, Chávez Valdés Sheila, Vazquez Arteaga Amalia, Coizeau Rodríguez Edelgis, Ávila Díaz Lismary, Freyre Corrales Giselle, Gonzalez Formental Hany Lianet, Chacón Quintero Yahima, Lemos Perez Gilda
Center for Genetic Engineering and Biotechnology, Cuba
*Corresponding author: Martínez Rosales Ricardo Ulises, Center for Genetic Engineering and Biotechnology, Cuba.
Received: 07 November 2022; Accepted: 11 November 2022; Published: 10 May 2023
Citation: Martínez Rosales Ricardo Ulises, Crespo Oliva Celia del Carmen, Chávez Valdés Sheila, Vazquez Arteaga Amalia, Coizeau Rodríguez Edelgis, Ávila Díaz Lismary, Freyre Corrales Giselle, Gonzalez Formental Hany Lianet, Chacón Quintero Yahima, Lemos Perez Gilda. A flow Cytometry Gating Strategy for Profiling CD4+CD25+/highCD127-/low Regulatory T Cells in the Rheumatoid Arthritis Scenario. Journal of Biotechnology and Biomedicine. 6 (2023): 197-201.
Share at FacebookAbstract
Regulatory T cells (Tregs) has been consider as a potential biomarker in rheumatologic clinical trials as this cell population has been shown to be involved in the autoimmune diseases outcome. Human Tregs are defined as CD4+CD25+/highCD127-/low cells; however its accurate identification by flow cytometry has been argued, especially as a result of different gating strategies. A flow cytometry gating strategy was settled to measure human Tregs from PBMC isolated via density gradient using CPT tubes. With this method we performed a standardized procedure for Treg profiling, useful for immunomonitoring in clinicals assessment.
Keywords
Tregs; Flow cytometry; Rheumatoid Arthritis (RA)
Tregs articles Tregs Research articles Tregs review articles Tregs PubMed articles Tregs PubMed Central articles Tregs 2023 articles Tregs 2024 articles Tregs Scopus articles Tregs impact factor journals Tregs Scopus journals Tregs PubMed journals Tregs medical journals Tregs free journals Tregs best journals Tregs top journals Tregs free medical journals Tregs famous journals Tregs Google Scholar indexed journals Flow cytometry articles Flow cytometry Research articles Flow cytometry review articles Flow cytometry PubMed articles Flow cytometry PubMed Central articles Flow cytometry 2023 articles Flow cytometry 2024 articles Flow cytometry Scopus articles Flow cytometry impact factor journals Flow cytometry Scopus journals Flow cytometry PubMed journals Flow cytometry medical journals Flow cytometry free journals Flow cytometry best journals Flow cytometry top journals Flow cytometry free medical journals Flow cytometry famous journals Flow cytometry Google Scholar indexed journals Rheumatoid Arthritis articles Rheumatoid Arthritis Research articles Rheumatoid Arthritis review articles Rheumatoid Arthritis PubMed articles Rheumatoid Arthritis PubMed Central articles Rheumatoid Arthritis 2023 articles Rheumatoid Arthritis 2024 articles Rheumatoid Arthritis Scopus articles Rheumatoid Arthritis impact factor journals Rheumatoid Arthritis Scopus journals Rheumatoid Arthritis PubMed journals Rheumatoid Arthritis medical journals Rheumatoid Arthritis free journals Rheumatoid Arthritis best journals Rheumatoid Arthritis top journals Rheumatoid Arthritis free medical journals Rheumatoid Arthritis famous journals Rheumatoid Arthritis Google Scholar indexed journals Chronic articles Chronic Research articles Chronic review articles Chronic PubMed articles Chronic PubMed Central articles Chronic 2023 articles Chronic 2024 articles Chronic Scopus articles Chronic impact factor journals Chronic Scopus journals Chronic PubMed journals Chronic medical journals Chronic free journals Chronic best journals Chronic top journals Chronic free medical journals Chronic famous journals Chronic Google Scholar indexed journals homeostasis articles homeostasis Research articles homeostasis review articles homeostasis PubMed articles homeostasis PubMed Central articles homeostasis 2023 articles homeostasis 2024 articles homeostasis Scopus articles homeostasis impact factor journals homeostasis Scopus journals homeostasis PubMed journals homeostasis medical journals homeostasis free journals homeostasis best journals homeostasis top journals homeostasis free medical journals homeostasis famous journals homeostasis Google Scholar indexed journals autoimmunity articles autoimmunity Research articles autoimmunity review articles autoimmunity PubMed articles autoimmunity PubMed Central articles autoimmunity 2023 articles autoimmunity 2024 articles autoimmunity Scopus articles autoimmunity impact factor journals autoimmunity Scopus journals autoimmunity PubMed journals autoimmunity medical journals autoimmunity free journals autoimmunity best journals autoimmunity top journals autoimmunity free medical journals autoimmunity famous journals autoimmunity Google Scholar indexed journals Blood collection articles Blood collection Research articles Blood collection review articles Blood collection PubMed articles Blood collection PubMed Central articles Blood collection 2023 articles Blood collection 2024 articles Blood collection Scopus articles Blood collection impact factor journals Blood collection Scopus journals Blood collection PubMed journals Blood collection medical journals Blood collection free journals Blood collection best journals Blood collection top journals Blood collection free medical journals Blood collection famous journals Blood collection Google Scholar indexed journals granulocytes articles granulocytes Research articles granulocytes review articles granulocytes PubMed articles granulocytes PubMed Central articles granulocytes 2023 articles granulocytes 2024 articles granulocytes Scopus articles granulocytes impact factor journals granulocytes Scopus journals granulocytes PubMed journals granulocytes medical journals granulocytes free journals granulocytes best journals granulocytes top journals granulocytes free medical journals granulocytes famous journals granulocytes Google Scholar indexed journals lymphocytes articles lymphocytes Research articles lymphocytes review articles lymphocytes PubMed articles lymphocytes PubMed Central articles lymphocytes 2023 articles lymphocytes 2024 articles lymphocytes Scopus articles lymphocytes impact factor journals lymphocytes Scopus journals lymphocytes PubMed journals lymphocytes medical journals lymphocytes free journals lymphocytes best journals lymphocytes top journals lymphocytes free medical journals lymphocytes famous journals lymphocytes Google Scholar indexed journals cellular articles cellular Research articles cellular review articles cellular PubMed articles cellular PubMed Central articles cellular 2023 articles cellular 2024 articles cellular Scopus articles cellular impact factor journals cellular Scopus journals cellular PubMed journals cellular medical journals cellular free journals cellular best journals cellular top journals cellular free medical journals cellular famous journals cellular Google Scholar indexed journals
Article Details
1. Introduction
Chronic autoimmune disorders, including Rheumatoid Arthritis (RA), are characterized by a disturbed balance between effector and regulatory immune responses (1). In RA, an extended inflammatory reaction leads to severe tissue damage and further physical function disability (2). Here is the importance of enhancing autologous immunosuppressive mechanisms to promote immune homeostasis. In this regard, several authors remark on the key role of Treg cells in autoimmune disease outcomes(3) . This cell population is well-known for modulating the immune system by repressing an exacerbated inflammatory response toward self-antigens(4). Previous data report the bad prognosis in collagen-induced arthritis (CIA) mouse model disease course, in a context of Tregs deprivation. In this model, a reduction in the proportion of Tregs following treatment with anti-CD25 antibody aggravated arthritis, whilst injection of Tregs had a contrary effect(5). Additionally, Avdeeva et al reported that responsiveness to therapy with Methotrexate (MT), the “Gold Standard” of RA pharmacotherapy was related to augmentation of Treg population in patients with early-onset, suggesting Treg as a decisive biomarker for determination of treatment effectiveness in disease resolution(4). Therefore, given the role of Tregs in autoimmunity, monitoring this subset of T lymphocytes in therapeutics trials could be a promising tool in predicting the course of RA treatment. Human Treg cells are defined as CD4+ T cells with a high expression of CD25 (interleukin [IL]-2 receptor α-chain). CD25+CD4+ Treg cells also express the Foxp3 gene, a member of the Forkhead/winged-helix family of transcriptional regulators, recognized as the master regulator of the suppressive capability of Treg cells (6). Since Foxp3 identification requires intracellular staining which is rather time-consuming, as an alternative staining strategy many authors recommend the CD127 surface marker (the α-chain of the IL-7 receptor), inversely correlated to FOXP3 expression. Consequently, CD4+CD25+CD127low T cell is the phenotype identified for Treg cells with suppressive activity (7). Although this phenotype is the most commonly used in Treg isolation through flow cytometry, identification of Treg cells can be controversial as a consequence of different gating strategies. This lack of homogenization strongly influences interassay discrepancies in flow cytometry-based Treg analysis. As a result, this hampers the accurate interpretation of the data and further clinical deductions (4). Thus, to improve the precision and reliability of Treg evaluation, we implemented a gating strategy protocol for flow cytometry-based Treg isolation. The application of this method in our lab made a gating strategy possible and suitable for current and further clinical immunological evaluations.
2. Materials and Methods
2.1 Blood collection
In this study, a total of 41 patients classified with RA were included according to the ACR / EULAR classification criteria (8). Patients were recruited from the Hermanos Amejeiras Hospital in Havana, Cuba. Additionally, 12 healthy volunteers were also incorporated. All the individual´s data were anonymously recorded to ensure confidentiality. Blood samples were collected following informed consent as approved by the Helsinki Declaration for research in humans (9). Peripheral blood samples from volunteers were obtained using 8 mL Cell Preparation Tubes (CPT) with sodium heparin (BD Biosciences). Each sample was processed within the first two hours after extraction and processed for PBMCs isolation via density gradient in CPT tubes (see below). For blood samples collection, each donor signed informed consent.
2.2 Isolation of PBMC
Peripheral whole blood was collected into an 8 mL sodium heparinized CPT tube and inverted multiple times to ensure homogenization of the sodium heparin anticoagulant and blood. The sample was centrifuged at 1800 x g for 30 min at 21 °C, resulting in the segregation of the homogenized into four visible layers: the upper layer corresponding to plasma with a cloudy band of PBMCs, the middle layer containing a thick polyester gel, and the bottom layer containing erythrocytes and granulocytes. After centrifugation, PBMCs were extracted and transferred into a 15 mL conical polystyrene tube, and then PBS 1X (Gibco, USA) was added to fill the 15 mL tube. The capped 15 mL conical tube was mixed by inversion and centrifuged at 300 x g for 15 min at 4 °C. The supernatant was decanted and the cell pellet was resuspended in 15 mL of OpTmizer T-Cell Expansion SFM and centrifuged for a subsequent 10 min at 300 x g at 4 °C. Following this centrifugation step, the supernatant was decanted by inversion without disturbing the cell pellet, and the pelleted PBMCs were resuspended in 2 mL of OpTmizer T-Cell Expansion SFM for further cell counting assessment on the cytometer.
2.3 Flow cytometry staining and acquisition
For FACS analysis, aliquots of 85 µL containing 104-108 cells/mL were set per condition prior to addition of 5 µL of the selected antibody. Each condition was assessed by triplicate. The full stained panel set-up was performed with: CD4-FITC (clone RPA-T4, 1 mL), CD25-PE (clone 2A3, 1 mL) and CD127-APC (clone HIL-7R-M21, 1 mL) from eBioscience, Ireland. Samples were incubated for 30 min at room temperature in the dark before centrifugation for 5 min at 250 x g at 4 °C. Subsequently, the supernatant was removed to leave the pellet dried and the cells were resuspended in 1 mL PBS 1X and acquired on cytometer. All the experimental analysis was performed in a 13 multicolor CyFlow Space cell cytometer analyzer (SYSMEX-PARTEC, Germany) equipped with FloMax 3.0 software (SYSMEX-PARTEC, Germany). This device is equipped with a 50 mW blue solid state laser emitting at a wave length of 488 nm and also with a 25 mW red solid state laser emitting at a wave length of 640 nm. Data collection of Fluorescein isothiocyanate (FITC) and Phycoerythrin (PE) was accomplished in the photomultiplier with a band pass filter of 530 ± 30 nm, while the fluorescence of Allophycocyanin (APC) was determined in the photomultiplier with a band pass filter of 640 ± 30 nm. To avoid spillover of one fluorochrome´s emission into other fluorochrome detection, fluorescence compensation controls were set on single color controls using healthy donor cells stained with FITC and PE.
2.4 Gating strategy for Treg definition
Identification of the total viable cell count of lymphocytes was assessed firstly by eliminating cellular debris and doublets on FSC-W and FSC-A gating parameters (Fig1A). Sequentially, the live cells were gated based on forward (FSC) and side (SSC) Light scatter parameters profiles designating size and granularity respectively (Fig1B). Within single, live, lymphocytes population, CD4+ lymphocytes were selected (Fig1C). This CD4+ cell population was further gated into CD4+ cells with the highest level of CD25 fluorescence defined as CD4+CD25high cells (Fig1D) and CD4+ cells the lowest levels of CD127 defined as CD4+ CD127-/low (Fig1E). Lastly, in lymphocytes population, those with CD4+ CD25+/highCD127-/low profile were defined as a Treg cell population (Fig1F).
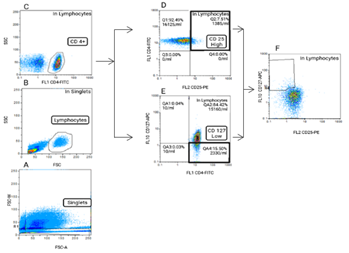
Figure 1: After removal of the doublets and cellular debris, on the FSC-W/ FSC-A (A) and FSC/SSC (B) respective graphs, a lymphocyte population were defined. This population was assessed for CD 4 cell surface expression (C). CD 4+ lymphocytes with high expression of CD 25 were gated (D) and also with low or no expression of CD 127 (E). Treg cells were defined as lymphocyte with a CD4+ CD25+/highCD127-/low profile (F).
2.4 Statistical analysis
Statistical analyses were performed using Graph Pad Prism version 8.0 GraphPad Software Inc, EE.UU).The normal distribution of each set of variables was analyzed using the D’Agostino & Pearson omnibus normality test. Paired data were compared using Mann-Whitney test. Correlation tests were performed using the Pearson test. A P value<0.001 was considered significant.
3. Results
3.1 Isolation of PBMCs by gradient centrifugation
PBMCs were isolated by density gradient and then analyzed on FACS by removal of the debris and doublets on the FCS/SSC graph (Fig 1). For further evaluations, we compared data obtained from healthy donors and RA patients analyzing the final cell concentration. In this analysis the mean concentration of PBMC obtained in healthy volunteers was statistically higher than the one obtained in RA patients.
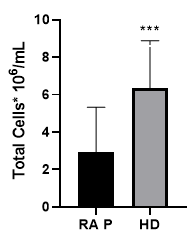
Figure2: After removal of the doublets and cellular debris, on the FSC-W/ FSC-A and FSC/SSC respective graphs, a population defined as the lymphocytes population was established and further compared between RA P: RA patients (n=41) and HD: healthy donors (n=12) using the Mann-Whitney test. Total cell concentration in healthy donors [6,33 ± 2,55], was significantly higher than the one observed in RA patients [2,91 ± 2,41], P value<0.001.
3.2 Treg cell analysis
Treg cell identification by Flow cytometry was assessed by gating a lymphocyte population with a CD4+ CD25+/highCD127-/low profile. With our gating strategy we compared the frequency of Treg cells obtained from healthy donor samples to the ones obtained from RA patients. With this method a clearly defined population was determined in both cases and the frequency of Treg in healthy donors was significantly higher than the one obtained in RA patients.
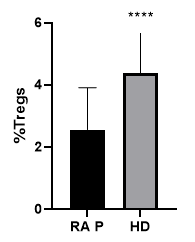
Figure3: Treg cell percentage comparative analysis between RA P: RA patients (n=41) and HD: healthy donors (n=12) using the Mann-Whitney test. Treg cell percentage in healthy donors [4.38 ± 1,31], was significantly higher than the one observed in RA patients [2.55 ± 1,35], P value<0.001.
Conclusion and Discussion
In the light of immunomodulatory therapies, the investigation of treatment effects on T cell subsets as part of the clinical monitoring is pivotal, specifically Tregs, since this population in particular has a main role in the immune homeostasis (6)(7). Earlier studies revealed the prognostic importance of Tregs as biomarkers in clinical outcomes of immune mediated diseases. For example, Avdeeva et al characterized the abundance of Treg in peripheral blood of healthy donors compared to untreated early RA patients, demonstrating a significant decrease in blood Treg percentage in comparison to healthy control. Furthermore, in this study MT treatment of patients with early untreated RA increased the proportion of Treg suggesting their prognostic role for a positive course of RA treatment (4). In addition Kanjana et al assessed the number and inhibitory activity of Treg between active RA and clinical remission patients, concluding that this parameters were higher in the clinical remission as compared to the active RA and Treg inhibitory activity was inversely correlated with disease severity (10). Further investigations related to autoimmune disorders including systemic lupus (11), juvenile idiopathic arthritis(12) and inflammatory bowel disease (13) also infer the association of Treg with disease outcome and its predictive value in clinical trials. Herein we performed a flow cytometry gating strategy for profiling Treg cells. We identified and sorted Tregs within a CD4+ lymphocyte population based upon high expression levels of CD25. Although FoxP3 is considered to be the central marker for quantitative identification of Treg cells lineage, as an intracellular antigen, it is not appropriate for sorting viable Tregs that can be used in downstream studies. As an alternative method we further isolated CD127low events as a surrogate of FOXP3 phenotype, within the CD4+CD25+ lymphocyte population. This gating approach offered a suitable method for large scale immunological evaluation, and a Treg population with high recoveries for subsequent evaluations. Several authors have reported the discrepancies in Treg frequencies between different methods. In our research, the proportion of Treg obtained in PBMC of RA patients was in accordance with other studies based on CD4+CD25+/highCD127-/low gating strategy [2.55 ± 1,35] (14) and this population was less abundant in comparison with the one obtained in healthy individuals [4.38 ± 1,31].
Acknowledgments
Assistance provided by the rheumatologist and nurses of the Hermanos Amejeiras hospital was greatly valued in the diagnosis and further classification of RA patients included in this study.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- Rosenblum MD, Remedios KA, Abbas AK. Mechanisms of human autoimmunity. J Clin Invest 125 (2015): 2228-2233.
- Guo Q, Wang Y, Xu D, et al. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res 6 (2018).
- Romano M, Fanelli G, Albany CJ, et al. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front Immunol 10 (2019).
- Avdeeva A, Rubtsov Y, Dyikanov D, et al. Regulatory T cells in patients with early untreated rheumatoid arthritis: Phenotypic changes in the course of methotrexate treatment. Biochimie 174 (2020): 9-17.
- Rodríguez-Perea AL, Arcia ED, Rueda CM, et al. Phenotypical characterization of regulatory T cells in humans and rodents. Clin Exp Immunol 185 (2016): 281-291.
- Zeng G, Jin L, Ying Q, et al. Regulatory t cells in cancer immunotherapy: Basic research outcomes and clinical directions. Cancer Manag Res 12 (2020): 10411-10421.
- Charlotte A, De Wolf MT, Herberts CA, et al. Dawn of monitoring regulatory T cells in (Pre-)clinical studies: Their relevance is slowly recognised. Front Med 7 (2020).
- Kay J, Upchurch KS. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatol (United Kingdom) 51 (2012): 5-9.
- Association WM. World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. Jama 310 (2013): 2191-2194.
- Kanjana K, Chevaisrakul P, Matangkasombut P, et al. Inhibitory activity of FOXP3+ regulatory T cells reveals high specificity for displaying immune tolerance in remission state rheumatoid arthritis. Sci Rep 10 (2020): 1-11.
- Silva-Neta HL, Brelaz-De-Castro MCA, Chagas MBO, et al. CD4+CD45RA-FOXP3low Regulatory T Cells as Potential Biomarkers of Disease Activity in Systemic Lupus Erythematosus Brazilian Patients. Biomed Res Int 2018 (2018).
- Hoeppli RE, Pesenacker AM. Targeting Tregs in juvenile idiopathic arthritis and juvenile dermatomyositis-insights from other diseases. Front Immunol 10 (2019): 1-9.
- Smids C, Horje CSHT, Drylewicz J, et al. Intestinal T cell profiling in inflammatory bowel disease: Linking T cell subsets to disease activity and disease course. J Crohn’s Colitis 12 (2018): 465-475.
- Morita T, Shima Y, Wing JB, et al. The proportion of regulatory T cells in patients with rheumatoid arthritis: A meta-Analysis. PLoS One 11(2016): 1-17.
