Women are More Protected than Men against HHcy (Hyperhomocysteinemia) during their Reproductive Life: Gender-Related Differences in One- Carbon and Folate Cycles Metabolism
Article Information
Arthur Clement1, Silvia Alvarez2, Laetitia Jacquesson-Fournols3, Edouard Amar4, Charles Brami5, Dominique Cornet6, Marc Cohen7, Patrice Clement1, Edouard Servy8, Kay Elder9& Yves Menezo1
1Laboratoire Clement, 17 avenue d’Eylau 75016 Paris
2Cabinet Medical, 15 Avenue Raymond Poincaré, 75116 Paris
3cabinet medical d endocrinologie 40 Boulevard de Courcelles 75008 Paris
4Cabinet medical, 17 avenue Victor Hugo, 15016 Paris
5Cabinet 16 Avenue Paul Doumer 75116 Paris
6Cabinet medical 10 Rue Jean Richepin, 75016 Paris
7Clinique Natecia Lyon
8IVF Georgia, Augusta Georgia USA
9Bourn Hall Clinic, Cambridge UK
*Corresponding author: Yves Menezo. Laboratoire Clement, 17 avenue d’Eylau 75016 Paris.
Received: 24 April 2023; Accepted: 03 May 2023; Published: 17 May 2023
Citation: Arthur Clement, Silvia Alvarez, Laetitia Jacquesson-Fournols, Edouard Amar, Charles Brami, Dominique Cornet, Marc Cohen, Patrice Clement, Edouard Servy, Kay Elder & Yves Menezo. Women are More Protected than Men against HHcy (Hyperhomocysteinemia) during their Reproductive Life: Gener-Related Differences in One-Carbon and Folates Cycles Metabolism. Archives of Clinical and Biomedical Research. 7 (2023): 345-352.
Share at FacebookAbstract
Methylation is a crucially important ubiquitous biochemical process: It relies on 2 cycles, the folates and the one carbon cycles, which activity is affected by MTHFR SNPs (Methylene TetraHydrofolate reductase, Single nucleotide polymorphisms); These SNPs affect spermatogenesis, oocyte maturation anomalies and cytologic/chromosomic anomalies. The two main MTHFR SNP variants C677T also called 677CTor c.6777C>T and A1298C also called 1298AC or c.1298A>C and homocysteine, a recognized inhibitor of methylation, have been tested in >4500 men and women suffering long lasting infertility (>3years) including repeat miscarriages and/or having failed several ART attempts with the same partner. The genetic status with highest prevalence is, for both sexes, the heterozygous C677T isoform, followed by the combined Heterozygous C677T/A1298C, and then heterozygous A1298C background. Only 14% of our population is Wild Type (WT). The T677T isoform, induces hyperhomocysteinemia (HHcy) especially in men. HHcy is always much more frequent in men. We confirm that HHCy is strongly gender related and that steroid hormones are active regulators of Hcy homeostasis.
Determination of these 2 SNPs and homocysteine should not be overlooked for patients with infertility of long duration, including repeat miscarriages. Patients must also be informed about pleiotropic medical implications relevant to their own health, as well as to the health of future children (especially boys)
Keywords
Methylation; Homocysteine; MTHFR SNPs; Sex difference; One carbon cycle (OCC); Folates cycle
Methylation articles; Homocysteine articles; MTHFR SNPs articles; Sex difference articles; One carbon cycle (OCC) articles; Folates cycle articles
Article Details
1. Introduction
Methylation, the covalent addition of methyl groups to a wide variety of molecules, is a crucial biochemical process that is necessary for the transmission of life. During gametogenesis, methylation of DNA and histone targets ensures the completion of regulatory processes that are necessary for epigenesis and imprinting mechanisms during all steps of embryo development. DNA repair is also supported by a number of methyl transferases, via the synthesis of purines. The process of methylation relies exclusively on S Adenosyl methionine (SAM) as methyl donor. After release of a methyl group, the demethylation product of SAM is S- Adenosyl Homocysteine (SAH), which is hydrolysed to homocysteine (Hcy) [1-4]. Both SAH and Hcy are potent inhibitors of DNA methylation. Moreover, Hcy is a cause and a consequence of oxidative stress, known to induce an associated mechanism of de-methylation [5]. Cellular transport of Homocysteine is carried out via the classical amino-acid transporters L, A and ?+L; Hcy competes with methionine for these molecules: Homocysteine has an affinity for the transporters that is on average 4.5x less than that of Methionine [1]. Homocysteine accumulates in carriers of MTHFR SNPs, with a major negative impact on the female gamete. A deleterious effect on oocyte quality has been demonstrated in ART treatment cycles [6-9]. Homocysteine also has toxic effects on mitochondria, at least in part due to its competition with methionine [1,10-13]: embryonic mitochondria are completely dependent upon maternal inheritance. During the final stages of oocyte maturation, a jeopardized methylation process will affect the stability of DNA and cause chromosomal instability. This has been clearly demonstrated in carriers of MTHFR SNPs [14], where the acquisition of methylation tags during the final stage of maturation is inhibited [15-18] and maintenance of methylation driven by DNMT1 (DNA methyltransferase 1) is jeopardized. There is a fine-tuned equilibrium between de-methylation, methylation maintenance, and, to a lesser extent, de novo methylation; All of these misdirected processes lead to poor developmental capacity for the embryo [15,16] Methylation of mRNAs, at N6-methyladenosine (m6A), catalysed by methyltransferase-like (METTL 3, expressed in human oocyte) is the most prevalent mRNA epigenetic modification and should not be overlooked. This process allows a correct balance between epigenic regulation and mRNA translation [19-20], allowing a biochemically/timely correct maternal to zygotic transition (MZT).
A similar negative impact can also be seen in men [21-24], where Hcy is also toxic for sperm mitochondria: a correlation between DNA methylation and sperm quality is no longer a matter for debate. Methylation stabilizes DNA [23] and minimizes sperm DNA fragmentation.
Due to increasing concern about epigenetic transmission in children born following assisted reproduction [26-30], we now test both male and female partners who present with a history of long-standing infertility for MTHFR SNPs and homocysteine. The 2 MTHFR SNPs studied here, the C677T and A1298C are known to negatively impact male and female gametes, early embryos and induce miscarriages. Based on preliminary results [31] and bypassing concerns related to methylation and the question of UMFA (unmetabolized folic acid) [31-35], we compared circulating levels of homocysteine in relation to MTHFR genetic background as a strategy toward solving cases that are often classified as “idiopathic infertility”. Circulating unmetabolized folic acid competes with the natural folate 5MTHF: it binds and saturates the natural folate receptors, SLC 19A1 and SLC 46A1, impairing the activity of methionine synthase which allows the regeneration of Methionine from Homocysteine
We present here data documenting homocysteine and MTHFR SNP genetic background for more than 4500 men and women presenting with infertility of long duration, with or without a history of recurrent miscarriage.
2. Materials and Methods
Ethical considerations: Testing for Hcy and MTHFR SNPs has been standard practice in our units for patients with a history of ART failures and recurrent miscarriage since 2019. The study was carried out according to the classical ethics regulations recommended by the French “Agence de Biomedecine”. This is an observational study which was part of a clinical therapeutic program, and the “Agence de Biomedecine” has confirmed that no ethical approval is required. Testing must be prescribed by an andrologist, gynecologist/obstetrician or an endocrinologist, for patients seeking fertility treatments; signed informed consent is mandatory. Testing for the purpose of building a control group in a fertile population is not permitted. Research was performed in accordance with the Declaration of Helsinki; all of the tests must be performed in licensed laboratories. Based upon our first preliminary data [31] testing is recommended for both partners, but either one or both members of the couples may decline testing.
Genetic testing: The techniques have been previously described [36,37]. Tests are carried out on 5 µL venous blood samples, using the LAMP human MTHFR mutation kit (LaCAR, MDx, Belgium) based on selective hybridization. Amplification is performed at 65°C, using several sets of primers simultaneously. Six specific primers covering the locus of the mutation are used for the 677CT SNP. The same protocol was applied for 1298AC SNP, with 6 specific primers covering the region of the mutation. Two loop primers are used in both, and the probes used simultaneously amplify the wild type gene. The results were evaluated by comparing the curves obtained by fluorescence.
Homocysteine: The protocol has been previously described [36,37]. Briefly: fasting blood samples were collected in the morning, and serum Hcy measured using the VYTROS kit, which allows determination of homocystine and homocysteine. The assay is linear from 1 to 90 µM-homocysteine. Homocystine is reduced to homocysteine with tris(2-carboxyethyl), and total homocysteine is then transformed into cystathionine in the presence of cystathionine beta synthase (CBS). Cystathionine is hydrolyzed by cystathionine lyase to form Hcy, ammonia, and pyruvate. The pyruvate released is reduced to form lactate; the amount of NAD+ produced by lactic acid dehydrogenase is proportional to all the homocysteine present in the sample, and this is measured by spectrophotometry at 340 nm.
There is no real consensus for minimal Hcy cut-off values. With respect to dementia and cardiovascular diseases, a level of 10 μmoles/L appears to be a healthy baseline [38,39]. A 5 μM increase in plasma Hcy concentrations over 14 μM is considered to increase the risk of disease by 40%. We chose a level of 15 μmoles/L as the cut-off for increased risk.
Statistics: Three methods of calculation were used.
*Hcy values and MTHFR SNP background within sex groups; Hcy concentrations were compared between the different MTHFR SNP backgrounds in separate male and female groups.
*Comparison between sexes for HHcy (>15 µMolar) for each MTHFR SNP combination: Hcy values were compared in men and women for each of the MTHFR SNP combinations vs WT. The t test (Student) for mean comparisons has been also calculated
3. Results
Seven of the 2127 men tested were found to carry a triple mutation; one of the seven has a circulating homocysteine >15 micromolar. Eleven out of 2439 women tested also carried a triple mutation, with no elevated Hcy concentration detected. Altogether,18/4566 = 0.4% were affected by a triple mutation; these patients are not included in the tables.
The “normal physiological” baseline levels of Hcy can be estimated as 8.5 µM in women and 11.5 µM in men (as observed for the WT patients). In total 18.2% of the men and 3.4% of the women tested have a HHcy >15 µM
- *Comparison of the circulating Hcy between men and women according to the MTHFR SNP background (Table 1)
|
Men |
Women |
Significance |
|||||
|
N |
Hcy (SD) |
[%>15 µM] |
N |
Hcy (SD) |
[%>15 µM] |
||
|
677TT |
278 |
19.7 (13.6) |
[58.7%] |
270 |
10.6 (5.9) |
[13.3%] |
**** |
|
Range |
6.7-86.5 |
4.3-54.7 |
|||||
|
Combo |
454 |
12.7 (2.8) |
[18.8%] |
480 |
8.6 (2.4) |
[2.3%] |
**** |
|
Range |
5.7-43.8 |
3.7-20.8 |
|||||
|
1298CC |
186 |
11.6 (2.9) |
[11.8%] |
221 |
8.6 (2.5) |
[0.2%] |
*** |
|
Range |
4.0-23 |
3.5-21.3 |
|||||
|
WT |
278 |
11.5 (2.6) |
[8.6%] |
356 |
8.4 (3.0) |
[0.2%] |
*** |
|
Range |
6.4-23.3 |
3.8-25.5 |
|||||
|
1298AC |
422 |
11.6 (2.7) |
[9%] |
517 |
8.5 (2.4) |
[0.2%] |
*** |
|
Range |
4.1-25.2 |
3.5-22.4 |
|||||
|
677CT |
502 |
11.9 (2.8) |
[11.6%] |
584 |
8.5 (2.5) |
[2.4%] |
*** |
|
Range |
6.2-95.6 |
3.7-25.1 |
Table 1: Mean (SD) circulating Hcy values (µMoles per liter) in men and women. N: number of patients in each group [% < 15 µM): percentage of patients having HHcy>15 µMolar. Combo: double/combined Heterozygoty C677T/A1298C
*Comparison of Hcy levels between men and women: all the gender differences are significant whatever the MTHFR SNP combination. ****p<10-5, ***p< 10-4. All the percentage of HHCy are significantly different between men and women
*Impact in each gender group of the MTHFR SNP vs WT on HHCy (odds ratios)
Women: The only mutation increasing significantly the level of homocysteine (vs WT) is the Homozygotic T677T/A1298A form. Mean comparison (t = 4.9)
|
T677T/A1298A |
Odds ratio |
Lower 95% |
Upper 95% |
1/Odds |
|
8.97 |
3.72 |
21.64 |
0.11 |
Men: The two significant combinations increasing highly significantly the Hcy (Vs WT) are the homozygous state T677T/A1298A (Mean comparison, t= 10.9) and the combo state C677T/A1298C (mean comparison, t=8)
|
Odds ratio |
Lower 95% |
Upper 95% |
1/Odds |
|
|
C677T/A1298C |
2.5 |
1.5 |
4.0 |
0.40 |
|
T677T/A1298A |
14.1 |
8.7 |
22.9 |
0.07 |
4. Discussion
During reproductive life, methylation is a universal ubiquitous regulatory process, affecting DNA, histones and RNAs during gametogenesis and then as early as fertilization and the first stages of embryo development. It involves two integrated cycles: the folates and the one carbon cycles. The function of the one-carbon cycle differs between men and women during their reproductive life. Firstly, the baseline level for a “normal/safe” Hcy can be estimated as 8.5µMoles/L (µM) in women vs 11.5µMoles/L in men (based on the values for WT). The 677TT isoform status presents the greatest hazard, especially for genomic DNA methylation [40]. followed by combined heterozygosity; this confirms that determining the 1298AC isoform is also of benefit, especially in consideration of the fact that ultimately, the genetic background of the embryo is an important feature [14]. Irrespective of the threshold, women appear to have greater protection against HHcy and Hcy-related oxidative stress [41]. In some respects, this favors oocyte and ovarian protection, as overall scientific literature links elevated follicular fluid Hcy with poor oocyte quality. It is evident that MTHFR SNPs create a source of abnormal regulation in local Hcy recycling. This feature has also been confirmed in the testis [21,23,24]. Mitochondria, of maternal origin in the embryo, need to have protection against HHcy, especially as cellular Hcy transport is shared by transporters for most of the classical amino acids. Notably, Hcy competes with methionine for transport in the embryo [1], albeit with a 4.5 times lower affinity. Hcy decreases the cellular methylation index.
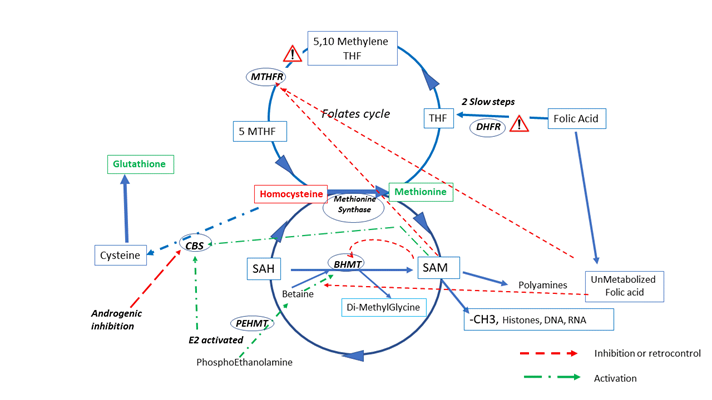
The mechanism of Hcy regulation in women can be explained via two major pathways (Figure 1):
- Estrogens stimulate BHMT (Betaine Homocysteine Methyl Transferase) activity [42,44], increasing betaine
availability by upregulation of Phosphoethanolamine Methyl Transferase (PEMT); this pathway removes Hcy and allows regeneration of methionine.
- Estrogens also stimulate activity of the CBS (Cystathionine Beta Synthase) pathway: “the homocysteine
concentration varies inversely with estradiol concentration” [43] leading to the formation of cysteine and then glutathione from Hcy. This facilitates better protection against oxidative stress for epigenetic marks, but can impair/affect epigenetic/methyl setting during a critical period of maturation.
Estrogens increase the activity of the CBS (cystathionine beta synthase) and the BHMT (Betaine Homocysteine Methyl Transferase) pathways, decreasing the Hcy. In contrary this pathway is inhibited by androgen [45] SAM synthesis is strongly regulated: it increases also the CBS pathway, allowing an equilibrium between the MS (methionine synthase) and the CBS pathways. But it also regulates negatively the BHMT and the MTHFR activity. Elevated activity of CBS reduces Hcy but may reduce the methylation process as Methionine then SAM synthesis is reduced.
FA (folic acid) has a low capacity to enter the folate cycle: DHFR activity is poorly efficient in human [47] to form THF (tetrahydrofolate). In MTHFR SNP carriers, the capacity to metabolize FA is low: these two bottle necks lead to UMFA (Un metabolized Folic acid) elevation. These 2 metabolic steps are then strongly inhibited by a Michaelis and Menten effect. SAH and Hcy are both strong inhibitors or methylation processes, not depending of gender
Two secondary systems of regulation are also active: SAM normally exerts negative feedback (retro control) on two enzymatic reactions, MTHFR and BHMT [44]. Low concentrations of SAM, associated with high estrogen levels result in high BHMT and CBS activity, allowing removal of Hcy mainly towards the formation of Cysteine. However, this upheaval has certain limits: the CBS pathway is usually poorly expressed in the oocyte/ early embryo before the stage of zygote genomic activation. There is clearly a risk that the early embryo will suffer a shortage of methionine and then SAM, especially in carriers of the MTHFR 6777SNP. This isoform associates with lower steroids concentration both androgens and estrogens [46] in follicular fluid. Then a shortage of SAM, will deregulate the folate cycle. Moreover, one has to consider that estrogens are strong inducers/regulators of epigenetically related gene expression [47].
Dosage of Hcy, at the age over 2 (age 2 to 7) in 20 of the children “at risk”, based on the MTHFR SNP background of the parents and their circulating Hcy, shows a mean value of 6.2 (SD: 1.7) micromolar with no difference between boys (9) and girls (11). This level is low and similar to the one described in literature [48]. Before puberty circulating Hcy remains low, but may fluctuate according to MTHFR SNPs; our data are in line with an OCC dependence of the sexual hormones.
In men, the combination of Hcy/MTHFR 677TT has a noticeable impact on sperm quality, through a significant effect of this mutation on sperm DNA methylation [21,22]. It is also clear that Hcy is involved in sperm DNA degradation, as cause and a consequence of oxidative stress [41]. Oxidative stress impairs methylation and affects the “methylation” setting via formation of 5-OH Cytosine; 5,6-diOH Cytosine; Cytosine glycol and uracil (deamination). Hcy also induces thrombophilia and circulatory system problems, known to affect spermatogenesis.
From a therapeutic point of view, the metabolism of folic acid (FA), pteroyl glutamic acid is crucial, especially with regard to its capacity to enter the folates cycle. It is usually prescribed at more or less high doses at the beginning of pregnancy and even before (preparation to IVF treatments in women). The first two steps of the cycle are driven by dihydrofolate reductase (DHFR), and these steps are very slow and poorly efficient [49]. This leads to the accumulation of unmetabolized folic acid, UMFA. UMFA prevents entry of natural folate, probably by saturating the receptors FolRα and SLC 19A1 [50]. The situation is more severe in carriers of the MTHFR SNPs, for whom the formation of 5-Methyl tetrahydrofolate (5-MTHF), the active form that is required for recycling Hcy to Methionine by the methionine synthase enzyme. An excess of unmetabolized substrate provokes a succession of inhibitory Michaelis and Menten reactions. El Aarabi et al. [23,24] observed that high doses of folic acid administered to men carriers of the 677TT mutation clearly have a damaging effect on sperm methylation profiles. The use of 5-MTHF instead of FA is preferable, and thus far has yielded promising results [31-33, ,51]. Based on the consequence of these features on embryo development [14,52,53], when both partners of a couple are affected by the MTHFR mutation; 5-MTHF treatment is mandatory for both.
In conclusion, investigating carrier status for the two main MTHFR SNPs (677TT and 677CT/1298AC), together with evaluation of circulating Hcy is a relevant strategy for patients suffering long-duration/idiopathic infertility. The paternal effect, which is often neglected, should not be overlooked [51,53] especially since Hcy levels are higher in men and can reach easily pathological values. Our data are in line with a major role of Estrogens and androgens on the impact of circulating homocysteine. They highlight the interference with the MTHFR SNPS, as it has been demonstrated in menopausal women [54-57]. Concerns regarding elevated circulating Hcy and its negative impact in conceptus quality and fertility have recently led the “Rotterdam periconception cohort” to recommend the “routine analysis of homocysteine levels in preconceptional and pregnant women and their partners” [58]. Patients must be informed of these pleiotropic medical implications for their own health, as well as for the health of future children, especially boys [59], Hcy can be high in MTHFR carrier infants. The potential impact of elevated homocysteine on psychiatric diseases, and multiple other diseases must also be considered [59]. Information about the parental genetic background and the risk of transmitting 677TT and 677CT/1298AC to male children may have important implications [59-60]. However, our preliminary observations do not show any noticeable Hcy increase in the young children, in agreement with the marked impact of sexual hormones on the CBS pathway. Since treatment with 5-MTHF decreases Hcy down to baseline levels, with no UMFA accumulation, this therapeutic option should be considered especially for male carriers of the MTHFR SNPs at puberty and before, in order to fulfill the ubiquitous needs for methylation [31-33,59,60] as this compound has proven its safety in infant and children [61].
Conflict of interest:
None to declare
Funding:
No funding
References
- Menezo Y, Khatchadourian C, Gharib A, et al. Regulation of S-adenosyl methionine synthesis in the mouse embryo. Life Sci 44 (1989):1601-9.
- Zhang H P, Wang YH, Ma SC, et al. Homocysteine inhibits endothelial progenitor cells proliferation via DNMT1-mediated hypomethylation of Cyclin A. Exp Cell Res 362 (2018): 217-226.
- Yi P, Melnyk S, Pogribna M, et al. Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. J. Biol. Chem 275 (2000): 29318–29323.
- James SJ, Melnyk S, Pogribna M, et al. Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology. J Nutr 132 (2002): 2361S-2366.
- Menezo YJ, Silvestris E, Dale B, et al. Oxidative stress and alterations in DNA methylation: Two sides of the same coin in reproduction. Reprod. Biomed. Online 33 (2016): 668–683.
- Boxmeer JC, Macklon NS, Lindemans J, et al. IVF outcomes are associated with biomarkers of the homocysteine pathway in monofollicular fluid. Hum. Reprod 24 (2009): 1059–1066.
- Szyma?ski W, Kazdepka?Ziemi?ska A. Effect of homocysteine concentration in follicular fluid on a degree of oocyte maturity. Ginekol. Pol 74 (2003): 1392–1396.
- Berker B, Kaya C, Aytac R, et al. Homocysteine concentrations in follicular fluid are associated with poor oocyte and embryo qualities in polycystic ovary syndrome patients undergoing assisted reproduction. Hum. Reprod 24 (2009): 2293–2302.
- Razi Y, Eftekhar M, Fesahat, F, et al. Concentrations of homocysteine in follicular fluid and embryo quality and oocyte maturity in infertile women: A prospective cohort. J. Obstet. Gynaecol 41 (2021):588–593.
- Kaplan P, Tatarkova Z, Sivonova MK, et al. Homocysteine and Mitochondria in Cardiovascular and Cerebrovascular Systems. J.Int J Mol Sci 21 (2020):7698
- Chen L, Xu T, Qiu Y, et al. Homocysteine induced a calcium?mediated disruption of mitochondrial function and dynamics in endothelial cells. J. Biochem. Mol. Toxicol 35 (2021): e22737.
- Zhang T, Huang D, Hou J, et al. High concentration homocysteine inhibits mitochondrial respiration function and production of reactive oxygen species in neuron cells. Cerebrovasc Dis 29 (2020):105109.
- Lu S, Hoestje SM, Choo E, et al. Induction of caspase-dependent and -independent apoptosis in response to methionine restriction. Int J Oncol 22 (2003): 415-20.
- Enciso M, Sarasa J, Xanthopoulou L, et al. Polymorphisms in the MTHFR gene influence embryo viability and the incidence of aneuploidy. Hum. Genet 135 (2016): 555–568.
- Zeng H, Liu Z Zhang L, et al. MTHFR 677TT is associated with decreased number of embryos and cumulative live birth rate in patients undergoing GnRHa short protocol: a retrospective study. BMC Pregnancy Childbirth 22 (2022): 170.
- D'Elia PQ, dos Santos AA, Bianco B, et al. MTHFR polymorphisms C677T and A1298C and associations with IVF outcomes in Brazilian women. Reprod BioMed Online 28 (2014):733–8.
- Sun H, Kang J, Su J, et al. Methionine adenosyltransferase 2A regulates mouse zygotic genome activation and morula to blastocyst transition. Biol. Reprod 100 (2019): 601–617.
- Ishitani H, Ikeda S, Egashira K, et al. Embryonic MTHFR contributes to blastocyst development. J Assist Reprod Genet 37 (2020): 1807-1814.
- Mu H, Zhang, T, Yang Y, et al. METTL3-mediated mRNA N6-methyladenosine is required for oocyte and follicle development in mice. Cell Death Dis 12 (2021): 989.
- Sui X, Hu, Y Ren C, et al. METTL3-mediated m6A is required for murine oocyte maturation and maternal-to-zygotic transition. Cell Cycle 19 (2020): 391–404.
- Aitken RJ, Flanagan HM, Connaughton H, et al. Involvement of homocysteine, homocysteine thiolactone, and paraoxonase type 1 (PON-1) in the aetiology of defective human sperm function. Andrology 4 (2016): 345–60.
- Gong M, Dong W, He T, et al. MTHFR 677C>T polymorphism increases the male infertility risk: a meta-analysis involving 26 studies. PLoS One 10 (2015): e0121147.
- Aarabi M, San Gabriel M C, Chan D, et al. . High-dose folic acid supplementation alters the human sperm methylome and is influenced by the MTHFR C677T polymorphism. J. Hum. Mol. Genet 24 (2022): 6301–6313.
- Aarabi M, Christensen KE, Chan D, et al. Testicular MTHFR deficiency may ex-plain sperm DNA hypomethylation associated with high dose folic acid supplementation. J. Hum. Mol. Genet 27 (2018): 1123–1135.
- Duthie S, Narayanan S, Brand G, et al. Impact of folate deficiency on DNA stability. J. Nutr 132 (2002): 2444S–2449S.
- Håberg SE, Page CM, Lee Y, et al. DNA methylation in newborns conceived by assisted reproductive technology. Nat. Commun 13 (2022): 1896.
- Hattori H, Hiura H, Kitamura A, et al. Association of four imprinting disorders and ART. Clin. Epigenetics 11 (2009): 21.
- Hiura H, Okae H, Miyauchi N, et al. Characterization of DNA methylation errors in patients with disorders conceived by assisted reproduction technologies. Hum. Reprod 27 (2012): 2541–2548.
- Choux C, Binquet C, Carmignac V, et al. The epigenetic control of transposable elements and imprinted genes in newborns is affected by the mode of conception: ART versus spontaneous conception without underlying infertility. Hum. Reprod 33 (2018): 331–340.
- Song S, Ghosh J, Mainigi, M, et al. . DNA methylation differences between in vitro and in vivo conceived children are associated with ART procedures rather than infertility. Clin. Epigenetics 7 (2015): 41–51.
- Servy EJ, Jacquesson-Fournols L, Cohen M, et al. MTHFR isoform carriers. 5-MTHF (5-methyl tetrahydrofolate) vs folic acid: a key to pregnancy outcome: a case series. J Assist Reprod Genet 35 (2018): 1431-1435
- Servy E & Menezo Y. The Methylene Tetrahydrofolate Reductase (MTHFR) isoform challenge. High doses of folic acid are not a suitable option compared to 5 Methyltetrahydrofolate treatment Clin Obstet Gynecol Reprod Med 3(2017): 1-5
- Goyco Ortiz LE, Servy EJ, Menezo YJR. A successful treatment with 5 methyltetrahydrofolate of a 677 TT MTHFR woman suffering premature ovarian insufficiency post a NHL (non-Hodgkin's lymphoma) and RPL (repeat pregnancy losses). J Assist Reprod Genet 36 (2019): 65-67.
- Alnabbat KI, Fardous A M, Cabelof DC, et al. Excessive Folic Acid Mimics Folate Deficiency in Human Lymphocytes. Curr Issues Mol Biol 44 (2022): 1452-1462.
- Page R, Wong A, Arbuckle TE, et al. The MTHFR 677C>T polymorphism is associated with unmetabolized folic acid in breast milk in a cohort of Canadian women. Am J Clin Nutr 110 (2019): 401-409
- Clément A, Menezo Y, Cohen M, et al. 5- Methyltetrahydrofolate reduces blood homocysteine level significantly in C677T methyltetrahydrofolate reductase single nucleotide polymorphism carriers consulting for infertility. Gynecol Obstet Hum Reprod 49 (2020): 101622.
- Clément A, Chouteau J, Clément P, et al. Importance of the determination of MTHFR SNPs (Methylene Tetrahydrofolate Reductase Single Nucleotide Polymorphisms) in couple infertility. Gynecol Obstet Fertil Senol 48 (2020): 422-27.
- Škovierová H, Vidomanová E, Mahmood S, et al. The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int J Mol Sci 17 (2016): E1733.
- Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med 346 (2002): 476–83.
- Friso S, Choi SW, Girelli D, et al. Common mutation in the 5,10 methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci U S A 99 (2002): 5606-11.
- Hoffman M. Hypothesis: hyperhomocysteinemia is an indicator of oxidant stress. Med Hypotheses 77 (2011): 1088-93.
- Estienne A, Portela VM, Choi Y, et al. The endogenous hydrogen sulfide generating system regulates ovulation. Free Radic Biol Med 138 (2019): 43-52.
- Kim R, Nijhout HF, Reed MC. One-carbon metabolism during the menstrual cycle and pregnancy. PLoS Comput Biol 17 (2021): e1009708.
- Pey AL, Majtan T, Sanchez-Ruiz JM, et al. Human cystathionine beta-synthase (CBS) contains two classes of binding sites for S-adenosylmethionine (SAM): complex regulation of CBS activity and stability by SAM. Biochem J 449 (2013):109-21.
- Giltay EJ, Hoogeveen EK, Elbers JM, et al. Effects of sex steroids on plasma total homocysteine levels: a study in transsexual males and females. J Clin Endocrinol Metab 83 (1998): 550-3.
- Pavlik R, Hecht S, Noss U, et al. Reduced Steroid Synthesis in the Follicular Fluid of MTHFR 677TT Mutation Carriers: Effects of Increased Folic Acid Administration. Geburtshilfe Frauenheilkd 82 (2022): 1074-1081.
- Kovács T, Szabó-Meleg E, Ábrahám IM. Oestradiol-Induced Epigenetically Mediated Mechanisms and Regulation of Gene Expression. Int J Mol Sci 2 (2022): 3177.
- Caldeira-Araújo H, Ramos R, Florindo C, et al. Homocysteine Metabolism in Children and Adolescents: Influence of Age on Plasma Biomarkers and Correspondent Genotype Interactions. . Nutrients 11 (2019): 646.
- Bailey SW & Ayling JE. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc Natl Acad Sci U S A 106 (2009):15424-9.
- Menezo Y, Elder K, Clement P, et al. Biochemical Hazards during Three Phases of Assisted Reproductive Technology: Repercussions Associated with Epigenesis and Imprinting. Int J Mol Sci 23 (2022): 8916.
- Jacquesson-Fournols L, Alvarez S, Cohen M, et al. A paternal effect of MTHFR SNPs on gametes and embryos should not be overlooked: case reports. J Assist Reprod Genet 36 (2019): 1351-1353.
- Williamson JM, Arthurs AL; Smith MD, et al. Perturbed One-Carbon Metabolism and Gestational Diabetes Mellitus. Nutrients 14 (2022): 3930.
- Tara SS, Ghaemimanesh F, Zarei S, et al. Methylenetetra hydrofolate Reductase C677T and A1298C Polymorphisms in Male Partners of Recurrent Miscarriage Couples. J Reprod Infertil 16 (2015): 193-8.
- Lambrinoudaki I, Papadimitriou D, Kaparos G, et al. MTHFR C677T polymorphism modifies the effect of HRT on metabolic parameters in postmenopausal women. Climacteric 16 (2013): 568-75.
- Somekawa Y, Kobayashi K, Tomura S, et al. Effects of hormone replacement therapy and methylenetetrahydrofolate reductase polymorphism on plasma folate and homocysteine levels in postmenopausal Japanese women. Fertil Steril 77 (2002): 481-6.
- Mijatovic V, van der Mooren MJ. Homocysteine in postmenopausal women and the importance of hormone replacement therapy. Clin Chem Lab Med 39 (2001): 764-7.
- Mijatovic V, Kenemans P, Jakobs C, et al. A randomized controlled study of the effects of 17beta-estradiol-dydrogesterone on plasma homocysteine in postmenopausal women. Obstet Gynecol 91 (1998): 432-436.
- Rubini E, Snoek KM, Schoenmakers S, et al. First Trimester Maternal Homocysteine and Embryonic and Fetal Growth: The Rotterdam Periconception Cohort. Nutrients 14 (2022): 1129.
- Yverneau M, Leroux S, Imbard A, et al. Influence of early identification and therapy on long-term outcomes in early-onset MTHFR deficiency. J Inherit Metab Dis 45 (2022): 848-861.
- Koklesova L, Mazurakova A, Samec M, et al. Homocysteine metabolism as the target for predictive medical approach, disease prevention, prognosis, and treatments tailored to the person. EPMA J 12 (2021): 477-505
- Troesch B, Demmelmair J, Gimpfl M, et al. MEFOLIN Study Group. Suitability and safety of L-5- methyltetrahydrofolate as a folate source in infant formula: A randomized-controlled trial. PLoS ONE 14 (2019): e0216790.