Use of Selective Pulmonary Vasodilators to Treat Pulmonary Arterial Hypertension Prior to Successful Surgical Closure of Atrial-Level Shunts
Article Information
Yuli Y. Kim MD1, Stephanie Fuller MD2, Benjamin D’Souza MD3, Yoshiya Toyoda MD4, Paul Forfia MD5*
1Division of Cardiovascular Medicine, Hospital of the University of Pennsylvania, Philadelphia, PA, USA and Division of Cardiology, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA
2Division of Cardiac Surgery, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA
3Division of Cardiovascular Medicine, Penn Presbyterian Medical Center, Philadelphia PA, USA
4Divison of Cardiac Surgery, Temple University Hospital, Philadelphia, PA, USA
5Division of Cardiology, Temple University Hospital, Philadelphia, PA, USA
*Corresponding Author: Paul Forfia MD, Professor of Medicine, Director, Pulmonary Hypertension, Right Heart Failure and CTEPH/PTE Program, Temple University Hospital, 3401 N. Broad St, 9th Floor Parkinson Pavilion, Suite 945, Philadelphia, PA 19140, United States
Received: 20 January 2020; Accepted: 31 January 2020; Published: 06 February 2020
Citation: Yuli Y Kim, Stephanie Fuller, Benjamin D’Souza, Yoshiya Toyoda, Paul Forfia. Use of Selective Pulmonary Vasodilators to Treat Pulmonary Arterial Hypertension Prior to Successful Surgical Closure of Atrial-Level Shunts. Archives of Clinical and Medical Case Reports 4 (2020): 226-234.
Share at FacebookAbstract
Background: Significant pulmonary hypertension [PH] is often considered a contraindication for closure of atrial septal defects [ASD]. Treatment of pulmonary arterial hypertension [PAH] with selective pulmonary vasodilators can allow for surgical repair of ASD otherwise considered prohibitive. We describe our experience in the assessment and treatment of adults with ASD and PAH with pulmonary vasodilator therapy who underwent successful surgical repair.
Methods: Retrospective case series of patients with atrial level shunts and significant PAH who met clinical criteria for repair. Clinical and demographic data were collected through review of the electronic medical records. Echocardiographic data were analyzed by a single cardiologist.
Results: Four patients with ASD and PAH were identified. All four patients experienced a decrease in pulmonary vascular resistance and increase in shunt fraction with institution of PH-directed therapy. Each underwent successful surgical repair with improvements in six-minute walk distance, right ventricular systolic function, and natriuretic peptide levels. All patients remained improved and stable on PH medications at last follow-up.
Conclusion: With appropriate selection and application of therapy, patients with atrial level shunts and severe PAH can successfully undergo surgical closure following medical treatment of their pulmonary arterial hypertension.
Keywords
Atrial septal defect; Pulmonary hypertension; Congenital heart surgery
Atrial septal defect articles, Pulmonary hypertension articles, Congenital heart surgery articles
Article Details
1. Introduction
The clinical spectrum of pulmonary hypertension [PH] associated with atrial septal defects [ASD] varies from elevated pulmonary pressures due to increased pulmonary blood flow, to elevated pulmonary vascular resistance [PVR] (pulmonary arterial hypertension or PAH), to Eisenmenger syndrome with resultant bidirectional or right-to-left flow across the defect. For patients with PAH and ASD who do not have Eisenmenger syndrome, pulmonary vasodilator therapy raises the possibility of modifying the underlying physiology such that ASD closure can be performed in patients previously deemed too high risk for closure. This “treat and close” strategy has been described in the literature with differing approaches and medical therapy[1, 2]. We aim to add to current knowledge by describing our experience and approach to patients with ASD-level shunts who undergo operative repair after medical treatment for severe PAH.
2. Methods
This was a dual-center retrospective case series of patients ≥ 18 years with PAH and atrial-level shunts who were medically treated prior to surgical repair. The study was approved by the Institutional Review Board at the Hospital of the University of Pennsylvania and Temple University of Hospital and waiver of informed consent was granted.
Data were abstracted from electronic medical records. Echocardiographic data were collected and measured by a single cardiologist (P.F.). PH was defined as mean pulmonary arterial pressure greater than 25 mm Hg , while PAH was hemodynamically defined as PH with PVR ≥ 3 WU and left atrial pressure < 15 mmHg [3].
3. Results
3.1 Case 1
A 48-year-old male was referred after a screening ECG showed evidence of right ventricular hypertrophy and subsequently diagnosed with sinus venosus ASD associated with anomalous drainage of a right superior pulmonary vein into the superior vena cava. Right heart catheterization showed a PVR of 5.1 WU and Qp/Qs of 1.7 at baseline, improving to a PVR of 3.6 and Qp/Qs of 2.1 following acute administration of inhaled epoprostenol [EPOi]. He was then initiated on phosphodiesterase-5 [PDE5] inhibitor, tadalafil 40 mg daily (Table 1).
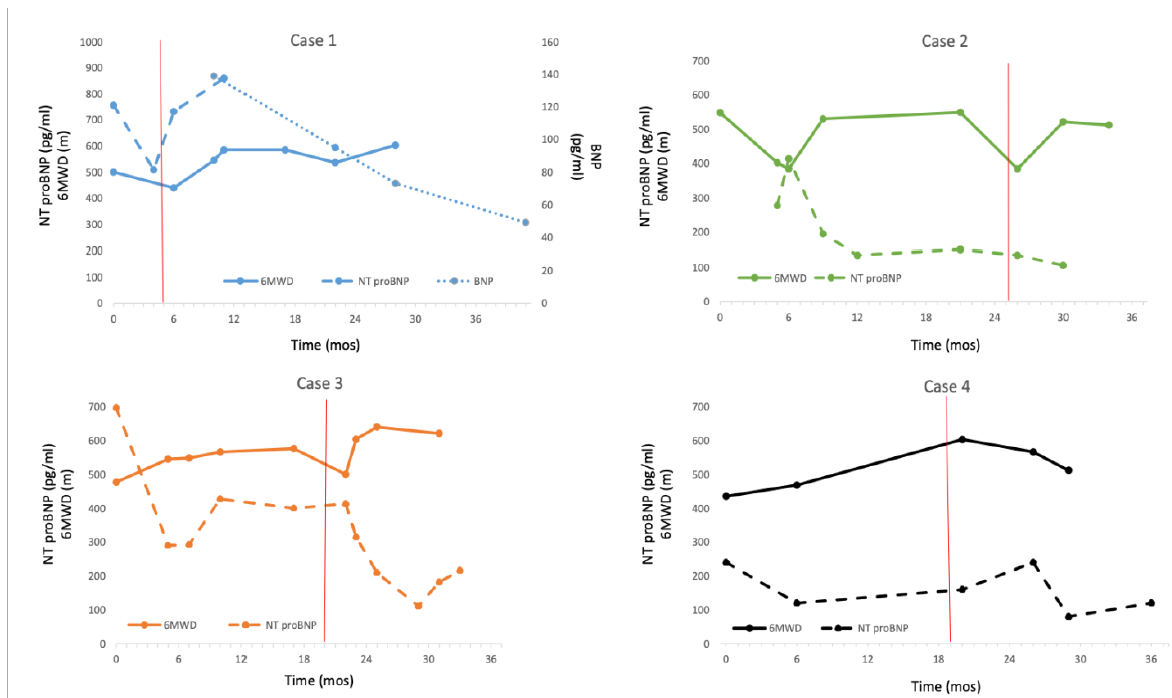
After three months of treatment, he underwent double pericardial patch repair of the sinus venous ASD and discharged on post-operative day 10 on his prior dose of tadalafil. Serial six-minute walk distance and B-type natriuretic peptide measurements improved (Figure 1). He is asymptomatic, maintained on low dose of furosemide and tadalafil with no hospital admissions 7 years post-operatively at last follow-up.
3.2 Case 2
A 33-year-old female presented with dyspnea and hypoxemia two days after vaginal delivery of her second pregnancy. Work-up by cardiac MRI showed a large sinus venosus ASD with anomalous drainage of a right upper pulmonary vein into the superior vena cava-right atrial junction and Qp/Qs 1.4. Subsequent right heart catheterization revealed pulmonary artery pressure 125/25 mmHg and PVR 5.3. She was initiated on PDE5 inhibitor sildenafil (20 mg three times daily) followed by endothelin receptor antagonist [ERA] bosentan (125 mg twice daily). On therapy, PVR fell to 2.6 WU and Qp/Qs rose to 2.5 (Table 1). Eighteen months after therapy, she underwent double patch repair of the sinus venous ASD without complications. She was discharged on post-op day 11 on her prior PDE5 inhibitor and ERA therapies. She was maintained on her PH medications and followed closely for 22 months post-surgery without issue (Figure 1).
3.3 Case 3
A 27-year-old female was diagnosed with a large secundum ASD and PH in India approximately 5 years prior to presentation. She underwent fenestrated ASD patch closure but developed worsening dyspnea on exertion. She was started on sildenafil (25 mg twice daily) and bosentan (32.5 mg twice daily) and subsequently moved to the United States. Echo demonstrated a 1.2 cm residual ASD with left-to-right flow and right ventricular systolic pressure estimate of 70 mmHg. Right heart catheterization demonstrated PVR of 5.5 WU, decreasing to 1.9 WU with EPOi and a simultaneous increase in Qp/Qs from 1.5 to 3.6. Her sildenafil was gradually increased to 80 mg three times daily, bosentan increased to 125 mg twice daily and inhaled iloprost was added (5 microgram/breath 6 times daily) with marked clinical improvement (Table 1).
Twenty-six months after PH treatment, she had successful redo ASD patch closure. She was discharged on post-operative day 9 on her previous PH medications. Ten months after her surgery she was successfully weaned off inhaled iloprost (Figure 1). One year after surgical closure, the patient was weaned off bosentan in order to consider possible pregnancy. Off the bosentan, her PH remained mild and highly responsive to sildenafil and inhaled treprostinil (6 micrograms/breath, 9 breaths 4 times daily). She subsequently became pregnant and had an uneventful, healthy full- term pregnancy.
3.4 Case 4
A 36-year-old female was diagnosed with a secundum ASD seven years prior to surgery. Baseline hemodynamics showed PA pressure 97/29 mmHg and PVR 7.9 WU in the presence of normal pulmonary capillary wedge pressure [PCWP]. Qp/Qs was 1.7. She was treated with tadalafil (40 mg daily) and bosentan (125 mg twice daily). Inhaled treprostinil (6 micrograms/breath, 9 breaths 4 times daily) was initiated with sporadic adherence and she was lost to follow-up. Six years after her initial diagnosis, she re-initiated care (on tadalafil and ERA which changed to macitentan 10 mg per day) at which time repeat hemodynamics demonstrated vasoreactivity to EPOi with a drop in her PA pressure from 75/24 to 69/18 and PVR from 6 WU to 4.5 WU and an unchanged PCWP. Baseline Qp/Qs increased from 2.0 to 2.6 (Table 1). She underwent surgical ASD patch closure and discharged on post-operative day 9 on sildenafil 40 mg three times daily and macitentan 10 mg per day. She is now 18 months post-ASD closure and asymptomatic (Figure 1).
4. Discussion
The goal of ASD closure is to mitigate worsening PH, right ventricular failure, exercise intolerance, arrhythmias, and stroke. [9, 10] However, PAH and ASD can coexist- prevalence of PAH associated with either repaired or unrepaired ASDs is 6-8% [4-6]. Natural history and epidemiology studies suggest that the development of PAH in ASDs is more complex than simply a flow-mediated process that eventually progresses to PAH. Most patients with unrepaired ASDs do not develop PAH and likewise a small proportion of patients undergoing ASD closure in the setting of normal PVR develop PAH years, even decades, later [7, 8].
Guidelines on the management of ASD and PH recommend ASD closure for those of hemodynamic significance in the absence of significantly elevated PVR [3, 9-11]. The 2015 European Society of Cardiology/European Respiratory Society Pulmonary Hypertension Guidelines consider PVR > 4.6 WU a contraindication [10]. Similarly, the 2018 American Heart Association/American College of Cardiology Adult Congenital Heart Disease Guidelines recommend closure of secundum ASD provided systolic pulmonary artery pressure <50% systemic and PVR < 1/3 systemic vascular resistance [11]. For those with PAH, the decision-making process in determining who can or cannot safely undergo ASD closure is complex. Outcomes of patients with ASD and PAH who do not initially meet indications for ASD closure but do so after treatment with selective pulmonary vasodilator therapy are not well-known [12-14].
With all four cases, treatment with PH-specific medications were intended to transition the shunt physiology from a high pressure/high resistance state to a higher flow/lower resistance state, marked by right heart catheterization showing a fall in PVR with simultaneous rise in Qp/Qs. On PH therapy, we target a PVR < 4 WU and/or a PVR < 1/3 systemic vascular resistance with a corresponding rise in Qp/Qs (≥2:1), and improved right ventricular systolic function (i.e. tricuspid annular plane systolic excursion [TAPSE] ≥2.0 cm) before surgical referral. Time to referral ranged widely, underscoring the variability in time until hemodynamic criteria are met as well as patient-related factors such as adherence to follow-up.
After surgery, clinical improvement was validated by increases in 6-minute walk distance and decreases in natriuretic peptide levels. Post-operative right heart catheterization in the two patients for whom invasive hemodynamic data was available demonstrated persistent, however, dramatically improved PH after surgical closure of the shunt. In all four cases, global right ventricular function as measured by right ventricular fractional area change also improved. TAPSE typically fell after surgical ASD closure, in keeping with prior observations that longitudinal right ventricular function measures such as TAPSE fall following pericardiotomy even amidst improvements in global right ventricular function [15]. All four patients continued to require PAH treatment at last follow-up despite improvements in functional and imaging parameters.
Our case series demonstrates feasibility and success in the “treat and close” strategy of managing adults with concomitant ASD and PAH. Safe surgical closure of ASD in patients with PAH can be accomplished through proper patient selection and the use of PH-directed therapy to lower PVR and alter the shunt physiology toward a higher flow, lower resistance pulmonary vascular bed. Pulmonary vasoreactivity testing has been utilized to risk stratify patients for surgery [16] but may be used to identify those in whom medical treatment could be of benefit prior to closure [2]. This approach requires validation in a broader construct as well as continued follow-up for long-term consequences of surgical closure.
|
CASE 1 |
|||||||||||
|
ECHO |
RVSP (mm Hg) |
RV dysfunction |
RVEDD/LVEDD ratio |
TAPSE |
RV FAC (%) |
||||||
|
Baseline |
94 |
Moderate-severe |
1.3 |
2.9 |
32 |
||||||
|
Post PAH therapy (4 months) |
85 |
Moderate-severe |
1.4 |
2.8 |
27 |
||||||
|
Post-op (1 month) |
71 |
Moderate-severe |
1.3 |
2.0 |
28 |
||||||
|
Post-op (6 months) |
53 |
Moderate |
1.1 |
1.9 |
39 |
||||||
|
Post-op (12 months) |
40 |
Mild-moderate |
0.7 |
1.6 |
43 |
||||||
|
Post-op (18 months) |
52 |
Mild |
0.9 |
1.8 |
46 |
||||||
|
Post-op (30 months) |
52 |
Mild |
1.0 |
1.8 |
49 |
||||||
|
CATH |
|||||||||||
|
Baseline |
86/29/48 |
12 |
5.1 |
15.3 |
0.33 |
1.7 |
|||||
|
Pulmonary vasodilator challenge |
85/30/48 |
12 |
3.6 |
10.9 |
0.33 |
2.1 |
|||||
|
Post-op (8 months) |
56/25/35 |
19 |
2.9 |
16.5 |
0.18 |
1 |
|||||
|
CASE 2 |
||||||||||
|
ECHO |
RVSP (mm Hg) |
RV dysfunction |
RVEDD/LVEDD ratio |
TAPSE |
RV FAC (%) |
|||||
|
Baseline |
101 |
Severe |
1.5 |
2.2 |
29 |
|||||
|
Post PAH therapy (5 months) |
93 |
Moderate |
1.4 |
1.9 |
32 |
|||||
|
Post PAH therapy (17 months) |
55 |
Moderate |
1.2 |
2.1 |
42 |
|||||
|
Post-op (1 month) |
60 |
Moderate |
1.3 |
1.8 |
41 |
|||||
|
Post-op (9 months) |
26 |
Mild |
0.9 |
2.1 |
39 |
|||||
|
CATH |
||||||||||
|
Baseline |
125/25/58 |
8 |
5.3 |
13.4 |
0.40 |
1.6 |
||||
|
Post PAH therapy (12 months) |
99/30/53 |
7 |
4.3 |
13 |
0.33 |
1.8 |
||||
|
Post PAH therapy (18 months) |
89/23/45 |
12 |
2.6 |
15.1 |
0.17 |
2.5 |
||||
|
CASE 3 |
|||||||||
|
ECHO |
RVSP (mm Hg) |
RV dysfunction |
RVEDD/LVEDD ratio |
TAPSE |
RV FAC (%) |
||||
|
Baseline* |
64 |
Moderate |
1.2 |
2.2 |
29 |
||||
|
Post PAH therapy (6 months) |
58 |
Moderate |
1.2 |
2.4 |
27 |
||||
|
Post PAH therapy (22 months |
47 |
Moderate |
1.1 |
2.0 |
34 |
||||
|
Post-op (1 month) |
43 |
Mild-moderate |
1.1 |
1.8 |
43 |
||||
|
Post-op (4 months) |
24 |
Moderate |
1.2 |
1.8 |
39 |
||||
|
Post-op (8 months) |
22 |
Moderate |
0.9 |
1.6 |
44 |
||||
|
CATH |
|||||||||
|
Baseline |
82/29/47 |
9 |
5.5 |
17 |
0.32 |
1.5 |
|||
|
Pulmonary vasodilator challenge |
58/24/35 |
8 |
1.9 |
19.5 |
0.10 |
3.6 |
|||
|
Post PAH therapy (23 months) |
70/30/43 |
13 |
3.7 |
18 |
0.21 |
2.1 |
|||
|
Post-op (9 months) |
44/18/27 |
6 |
3.8 |
15.9 |
0.24 |
1.2 |
|||
|
CASE 4 |
||||||||||
|
ECHO |
RVSP (mm Hg) |
RV dysfunction |
RVEDD/LVEDD ratio |
TAPSE |
RV FAC (%) |
|||||
|
Baseline* |
100 |
Moderate-severe |
1.5 |
1.5 |
25 |
|||||
|
Post PAH therapy (7 years) |
60 |
Mild |
1.3 |
2.2 |
35 |
|||||
|
Post-op (1 month) |
50 |
None |
1.0 |
1.5 |
40 |
|||||
|
CATH |
||||||||||
|
Baseline |
97/29/52 |
8 |
7.9 |
20 |
0.4 |
1.4 |
||||
|
Post PAH therapy (7 years) Pulmonary vasodilator challenge |
75/24/41 69/18/35 |
8 7 |
6.0 4.5 |
25 24 |
0.24 0.19 |
2.0 2.6 |
||||
* Baseline is bosentan and sildenafil and PAH therapy is inhaled prostaclin FAC, fractional area change; LVEDD, left ventricular end-diastolic dimension; Qp/Qs, pulmonary flow to systemic flow shunt fraction; PA, pulmonary artery; PAH, pulmonary arterial hypertension; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RV, right ventricle; RVEDD, right ventricular end-diastolic dimension; SVR, systemic vascular resistance; TAPSE, tricuspid annular plane systolic excursion; WU, Wood Units
Table 1: Imaging and hemodynamic data at baseline, after medical therapy, and after surgical closure.
Figure 1: Six-minute walk distance and brain natriuretic peptide levels over time. Time 0 is the start of pulmonary hypertension therapy. The vertical line denotes time of surgical repair. 6MWD, six-minute walk distance; NT-proBNP, N-terminal pro-brain natriuretic peptide; BNP, brain natriuretic peptide.
Acknowledgements
The authors wish to acknowledge Katherine Awh for editing assistance. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.
References
- Kijima Y, Akagi T, Takaya Y, et al. Treat and Repair Strategy in Patients With Atrial Septal Defect and Significant Pulmonary Arterial Hypertension. Circ J 80 (2016): 227-234.
- Bradley EA, Chakinala M, Billadello JJ. Usefulness of medical therapy for pulmonary hypertension and delayed atrial septal defect closure. Am J Cardiol 112 (2013): 1471-1476.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62 (2013): D34-D41.
- Steele PM, Fuster V, Cohen M, et al. Isolated atrial septal defect with pulmonary vascular obstructive disease--long-term follow-up and prediction of outcome after surgical correction. Circulation 76 (1987): 1037-1042.
- Duffels MG, Engelfriet PM, Berger RM, et al. Pulmonary arterial hypertension in congenital heart disease: an epidemiologic perspective from a Dutch registry. Int J Cardiol 120 (2007): 198-204.
- Lowe BS, Therrien J, Ionescu-Ittu R, et al. Diagnosis of pulmonary hypertension in the congenital heart disease adult population impact on outcomes. J Am Coll Cardiol 58 (2011): 538-546.
- Gabriels C, De Meester P, Pasquet A, et al. A different view on predictors of pulmonary hypertension in secundum atrial septal defect. Int J Cardiol 176 (2014): 833-840.
- van Riel AC, Blok IM, Zwinderman AH, et al. Lifetime Risk of Pulmonary Hypertension for All Patients After Shunt Closure. J Am Coll Cardiol 66 (2015): 1084-1086.
- Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 31 (2010): 2915-2957.
- Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37 (2016): 67-119.
- Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol (2018).
- Beghetti M, Galie N, Bonnet D. Can "inoperable" congenital heart defects become operable in patients with pulmonary arterial hypertension? Dream or reality? Congenit Heart Dis 7 (2012): 3-11.
- Dimopoulos K, Peset A, Gatzoulis MA. Evaluating operability in adults with congenital heart disease and the role of pretreatment with targeted pulmonary arterial hypertension therapy. Int J Cardiol 129 (2008): 163-171.
- Myers PO, Tissot C, Beghetti M. Assessment of operability of patients with pulmonary arterial hypertension associated with congenital heart disease. Circ J 78 (2014): 4-11.
- Raina A, Vaidya A, Gertz ZM, et al. Marked changes in right ventricular contractile pattern after cardiothoracic surgery: implications for post-surgical assessment of right ventricular function. J Heart Lung Transplant 32 (2013): 777-783.
- Balzer DT, Kort HW, Day RW, et al. Inhaled Nitric Oxide as a Preoperative Test (INOP Test I): the INOP Test Study Group. Circulation 106 (2002): I76-181.