Update on Female Fertility Preservation
Article Information
Elsa Labrune1, 2, 3*, Bruno Salle1, 2, 4, Jacqueline Lornage1, 2, 4*
1Hospices Civils de Lyon, Hôpital Mère Enfant, Service de Médecine de la Reproduction, Bron, France
2Université Claude Bernard, Faculté de Médecine Laennec, Lyon cedex, France
3INSERM Unité 1208, Bron cedex, France
4Université Claude Bernard, Faculté de Médecine Lyon Sud, Oullins cedex, France
*Corresponding Author: Elsa Labrune, Hospices Civils de Lyon, Hôpital Mère Enfant, Service de Médecine de la Reproduction, 59 boulevard Pinel, 69677 Bron, France
Jacqueline Lornage, Hospices Civils de Lyon, Hôpital Mère Enfant, Service de Médecine de la Reproduction, 59 boulevard Pinel, 69677 Bron, France
Received: 24 November 2020; Accepted: 03 December 2020; Published: 10 May 2021
Citation: Elsa Labrune, Bruno Salle, Jacqueline Lornage. Update on Female Fertility Preservation. Journal of Cancer Science and Clinical Therapeutics 5 (2021): 221-234.
Share at FacebookAbstract
Cancer is the second leading cause of death for women under 40. Survival rates are increasing due to earlier diagnosis and advanced treatment. One of the side effects of treatment is premature ovarian failure. This is why fertility preservation is part of the initial care of patients, allowing for a better quality of life after the disease. The main causes of premature ovarian failure are iatrogenic (mainly chemotherapy and radiotherapy) in more than a third of cases, idiopathic, genetic and autoimmune. This preservation of fertility should be offered as soon as possible, ideally before gonadotoxic treatments. The same is true when the cause is non-iatrogenic because the age of the woman has a determining role on the quality of the gametes obtained and therefore on the results. The different techniques are embryo vitrification, oocyte vitrification and freezing of ovarian tissue, to be combined when possible with ovarian blockage and/or ovarian transposition. The most common technique is oocyte vitrification, which allows oocytes to be conserved, although this is not possible in pre-pubescent girls and in cases of urgent treatment. In these cases the freezing of the ovarian tissue is the solution.
Keywords
Fertility preservation; Cancer; Chemotherapy; Oocyte vitrification; Ovarian tissue; Embryo vitrification; Premature ovarian failure
Fertility preservation articles; Cancer articles; Chemotherapy articles; Oocyte vitrification articles; Ovarian tissue articles; Embryo vitrification articles; Premature ovarian failure articles
Fertility preservation articles Fertility preservation Research articles Fertility preservation review articles Fertility preservation PubMed articles Fertility preservation PubMed Central articles Fertility preservation 2023 articles Fertility preservation 2024 articles Fertility preservation Scopus articles Fertility preservation impact factor journals Fertility preservation Scopus journals Fertility preservation PubMed journals Fertility preservation medical journals Fertility preservation free journals Fertility preservation best journals Fertility preservation top journals Fertility preservation free medical journals Fertility preservation famous journals Fertility preservation Google Scholar indexed journals Cancer articles Cancer Research articles Cancer review articles Cancer PubMed articles Cancer PubMed Central articles Cancer 2023 articles Cancer 2024 articles Cancer Scopus articles Cancer impact factor journals Cancer Scopus journals Cancer PubMed journals Cancer medical journals Cancer free journals Cancer best journals Cancer top journals Cancer free medical journals Cancer famous journals Cancer Google Scholar indexed journals Chemotherapy articles Chemotherapy Research articles Chemotherapy review articles Chemotherapy PubMed articles Chemotherapy PubMed Central articles Chemotherapy 2023 articles Chemotherapy 2024 articles Chemotherapy Scopus articles Chemotherapy impact factor journals Chemotherapy Scopus journals Chemotherapy PubMed journals Chemotherapy medical journals Chemotherapy free journals Chemotherapy best journals Chemotherapy top journals Chemotherapy free medical journals Chemotherapy famous journals Chemotherapy Google Scholar indexed journals Oocyte vitrification articles Oocyte vitrification Research articles Oocyte vitrification review articles Oocyte vitrification PubMed articles Oocyte vitrification PubMed Central articles Oocyte vitrification 2023 articles Oocyte vitrification 2024 articles Oocyte vitrification Scopus articles Oocyte vitrification impact factor journals Oocyte vitrification Scopus journals Oocyte vitrification PubMed journals Oocyte vitrification medical journals Oocyte vitrification free journals Oocyte vitrification best journals Oocyte vitrification top journals Oocyte vitrification free medical journals Oocyte vitrification famous journals Oocyte vitrification Google Scholar indexed journals Ovarian tissue articles Ovarian tissue Research articles Ovarian tissue review articles Ovarian tissue PubMed articles Ovarian tissue PubMed Central articles Ovarian tissue 2023 articles Ovarian tissue 2024 articles Ovarian tissue Scopus articles Ovarian tissue impact factor journals Ovarian tissue Scopus journals Ovarian tissue PubMed journals Ovarian tissue medical journals Ovarian tissue free journals Ovarian tissue best journals Ovarian tissue top journals Ovarian tissue free medical journals Ovarian tissue famous journals Ovarian tissue Google Scholar indexed journals Embryo vitrification articles Embryo vitrification Research articles Embryo vitrification review articles Embryo vitrification PubMed articles Embryo vitrification PubMed Central articles Embryo vitrification 2023 articles Embryo vitrification 2024 articles Embryo vitrification Scopus articles Embryo vitrification impact factor journals Embryo vitrification Scopus journals Embryo vitrification PubMed journals Embryo vitrification medical journals Embryo vitrification free journals Embryo vitrification best journals Embryo vitrification top journals Embryo vitrification free medical journals Embryo vitrification famous journals Embryo vitrification Google Scholar indexed journals Premature ovarian failure articles Premature ovarian failure Research articles Premature ovarian failure review articles Premature ovarian failure PubMed articles Premature ovarian failure PubMed Central articles Premature ovarian failure 2023 articles Premature ovarian failure 2024 articles Premature ovarian failure Scopus articles Premature ovarian failure impact factor journals Premature ovarian failure Scopus journals Premature ovarian failure PubMed journals Premature ovarian failure medical journals Premature ovarian failure free journals Premature ovarian failure best journals Premature ovarian failure top journals Premature ovarian failure free medical journals Premature ovarian failure famous journals Premature ovarian failure Google Scholar indexed journals autoimmune articles autoimmune Research articles autoimmune review articles autoimmune PubMed articles autoimmune PubMed Central articles autoimmune 2023 articles autoimmune 2024 articles autoimmune Scopus articles autoimmune impact factor journals autoimmune Scopus journals autoimmune PubMed journals autoimmune medical journals autoimmune free journals autoimmune best journals autoimmune top journals autoimmune free medical journals autoimmune famous journals autoimmune Google Scholar indexed journals embryo vitrification articles embryo vitrification Research articles embryo vitrification review articles embryo vitrification PubMed articles embryo vitrification PubMed Central articles embryo vitrification 2023 articles embryo vitrification 2024 articles embryo vitrification Scopus articles embryo vitrification impact factor journals embryo vitrification Scopus journals embryo vitrification PubMed journals embryo vitrification medical journals embryo vitrification free journals embryo vitrification best journals embryo vitrification top journals embryo vitrification free medical journals embryo vitrification famous journals embryo vitrification Google Scholar indexed journals gonadotoxic treatments articles gonadotoxic treatments Research articles gonadotoxic treatments review articles gonadotoxic treatments PubMed articles gonadotoxic treatments PubMed Central articles gonadotoxic treatments 2023 articles gonadotoxic treatments 2024 articles gonadotoxic treatments Scopus articles gonadotoxic treatments impact factor journals gonadotoxic treatments Scopus journals gonadotoxic treatments PubMed journals gonadotoxic treatments medical journals gonadotoxic treatments free journals gonadotoxic treatments best journals gonadotoxic treatments top journals gonadotoxic treatments free medical journals gonadotoxic treatments famous journals gonadotoxic treatments Google Scholar indexed journals
Article Details
1. Introduction
Cancer is the second leading cause of death for women under 40 years of age. Survival rates are increasing due to earlier diagnosis and therapeutic advances. An improvement in age-standardized net survival at 5 years is observed for most solid tumors diagnosed between 1989 and 2010 [1]. In France, the average number of new pediatric cancers diagnosed between 2000 and 2004 was 8,473 per year. It is estimated that one child in 440 will be diagnosed with cancer before the age of 15. The most frequent cancers are leukemia (29%), central nervous system tumors (23%) and lymphomas (12%). The overall survival rate is 81.6% at 5 years, over the period from 2000 to 2008 [2]. The Agence de Biomédecine carried out a retrospective study based on the Cancer Cohort database created since 2010. This study estimated that each year, approximately 10,700 women of childbearing age (under 40 years old) with cancer should benefit from information on fertility preservation; due to the gonadotoxic risk of the various treatments received or to come [3]. Chemotherapy as well as radiotherapy can be gonadotoxic [4]. The preservation of female fertility is also of interest for certain non-tumoral pathologies such as non-malignant hemopathies (severe sickle cell disease, thalassemia major), or autoimmune pathologies (severe systemic lupus erythematosus) that require recourse to these gonadotoxic treatments. There are different fertility preservation strategies whose choice depends on several parameters: patient age, type of treatment, marital status, urgency of treatment. The aim of this review is to present who is interested in female fertility preservation, when to propose it and how to carry it out.
2. For Whom?
Premature ovarian failure (POF) accounts for 1% of women under 40 years of age, 1 per 1000 women under 30 years of age and 1 per 10,000 women under 20 years of age [5]. The main causes are iatrogenic, idiopathic, genetic and autoimmune.
2.1 Iatrogenic treatments
2.1.1Chemotherapy: The type and dose of chemotherapy as well as the age of the patients at the time of treatment are predictive of the risk of premature ovarian failure. In a study of 460 women treated for Hodgkin's lymphoma, a significant risk of POF during alkylating treatment and during dose escalation treatment with alkylating agents was demonstrated. It also showed a linear increase in the risk of POF according to age at the time of treatment. The cut-off would be at 32 years of age [6]. The prospective study by Fisher et al. reported the incidence of POF in women treated for breast cancer with cyclophosphamide, busulfan and melphalan: 22% of women under 40 years of age had POF versus 73% of women over 40 years of age [7]. The risk of POF depends on the type of molecule, which is why chemotherapies are classified into 3 categories: low risk of POF, intermediate risk and high risk of POF [8] (Table 1). Alkylating agents are the most gonadotoxic drugs, all the more so if the treatment takes place at the time of puberty, which would be a critical period. Indeed, there would be 9 times more risk of developing POF if chemotherapy is given during puberty [9]. Another method of classifying treatments has been carried out by the American Society of Oncology (Table 2). It classified the different protocols commonly used in oncology according to the risk of developing POF. This classification makes it possible to apprehend the effect of a protocol that often combines several molecules used at variable doses. The protocols most at risk are the pre-graft protocols using cyclophosphamide associated with busulfan or total body irradiation and the adjuvant treatment of breast cancer in women over 40 years of age. It is important to note that the ABVD protocol, which is used for the treatment of Hodgkin's disease as first-line therapy, presents a low risk of gonadotoxicity despite the presence of an alkylating agent, dacarbazine [10].
|
High risk |
Intermediate risk |
Low risk |
|
Cyclophosphamide |
Cisplatine |
Vincristine |
|
Ifosfamide |
Carboplatine |
Méthotrexate |
|
Chlorméthine |
Doxorubicine |
Dactinomycine |
|
Busulfan |
Bléomycine |
|
|
Melphalan |
Mercaptopurine |
|
|
Procarbazine |
Vinblastine |
|
|
Chlorambucil |
Table 1: Classification of chemotherapies according to their risk of causing POFs [8].
|
Degree of Risk |
Cancer Treatment |
|
High risk (>80%) |
Hematopoietic stem cell transplantation with cyclophosphamide/total body irradiation or cyclophosphamide/busulfan External Beem Radiation to a field that includes the Ovaries CMF, CEF, CAF×6 cycles in women age 40 and older (adjuvant breast cancer therapy with combinations of cyclophosphamide, methotrexate, fluorouracil, doxorubicin, epirubicin) |
|
Intermediate risk |
CMF, CEF, CAF×6 cycles in women age 30-39 (adjuvant breast cancer therapy with combinations of cyclophosphamide, methotrexate, fluorouracil, doxorubicin, epirubicin) AC×4 in women age 40 and older (adjuvant breast cancer therapy with doxorubicin/cyclophosphamide) |
|
Lower risk (<20%) |
ABVD (doxorubicin/bleomycin/vinblastine/dacarbazine) CHOP×4-6 cycles (cyclophosphamide/doxorubicin/vincristine/prednisone) CVP (cyclophosphamide/vincristine/prednisone) AML therapy (anthracycline/cytarabine) All therapy (multi-agent) CMF, CEF, CAF×6 cycles in women less than 30 (adjuvant breast cancer therapy with combinations of cyclophosphamide, methotrexate, fluorouracil, doxorubicin, epirubicin) AC×4 in women less than 40 (adjuvant breast cancer therapy with doxorubicin/ cyclophosphamide) |
|
Very low or no risk |
Vincristine Methotrexate Fluorouracil |
|
Unknown risk (examples) |
Taxanes Oxaliplatin Irinotecan Monoclonal antibodies (trastuzumba, bevacizumab, cetuximab) Tyrosine kinase inhibitors (erlotinib, imatinib) |
Table 2: Risk of POF according to chemotherapy protocols, ASCO recommendations [10].
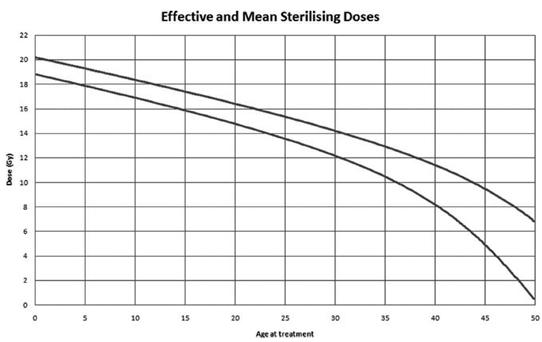
Figure 1: Doses of radiotherapy leading to infertility according to the patient's age at the time of treatment [11].
|
Technique |
Benefits |
Disadvantages |
|
Embryo cryopreservation |
· No invasive surgery · No risk of reintroduction of the malignant pathology |
· Puberty patient · Patient in couple and embryos belonging to the couple · Need for ovarian stimulation (contraindicated in case of therapeutic emergency, hypo- response, hormone-dependent cancer) · Ovarian puncture |
|
Oocyte cryopreservation |
· No invasive surgery · Oocytes belonging to the patient · No risk of reintroduction of the malignant pathology |
· Puberty patient · Need for ovarian stimulation (contraindicated in case of therapeutic emergency, hypo-response, hormone-dependent cancer) · Ovarian puncture |
|
Cryopreservation of ovarian tissue |
· Regardless of age · Lack of ovarian stimulation · Can be carried out in an emergency · Possible if hormone-dependent cancer |
· Invasive surgery · Risk of reintroduction of the malignant pathology |
Table 2: Synthetic presentation of the advantages and disadvantages of fertility preservation techniques used routinely.
The mechanism of action of chemotherapies is not identical for each family of drugs and remains unknown for some. As for the growing follicles, they would be destroyed by most chemotherapies leading to transient amenorrhea. The prospective study by Sukumvanich et al. showed that 84% of women treated with doxorubicin and cyclophosphamide had amenorrhea at the end of their treatment. Almost half of them return to menstrual cycles within 9 months after treatment [12]. The follicles that make up the reserve (the primordial follicles) are not all affected by these treatments, so POF is not systematic. The chemotherapeutic agents that can act on these follicles are mainly alkylating agents [13]. According to Morgan et al., this depletion of primordial follicles could be directly due to chemotherapeutic agents or it could be indirectly secondary: the destroyed growing follicles would lead to the recruitment of primordial follicles, thus diminishing the reserve of primordial follicles [14]. In order to better understand the action of chemotherapies on the follicles in reserve, some authors have put ovarian follicles in contact with a chemotherapy molecule and studied its impact. In 2002, Raz et al. demonstrated a nuclear alteration of the granulosa cells of human ovarian follicles cultured in vitro and brought into contact with cyclophosphamide in high concentration, a concentration much higher than that used in human therapy [15]. Another study conducted by Soleimani et al. investigated the effect of doxorubicin by incubating human ovarian follicles in vitro with doxorubicin. It reported an increase in double-stranded DNA breaks as well as an increase in dose-dependent apoptosis of primordial follicles [16]. A third study, conducted by Morgan et al., reported the effects of cisplatin and doxorubicin. Cisplatin appeared to induce apoptosis of primary follicles through direct action on germ cells by altering DNA. Growing follicles were not affected. Doxorubicin, on the other hand, seemed to affect the granulosa cells. It would lead to apoptosis of the growing follicles and not of the primary follicles, contrary to the results of the work of Soleimani et al. [14]. Follicular loss would be the result of apoptosis activation [17], this is still discussed by other authors who evoke a mechanism of necrosis with atresia [18] or autophagy [19]. The mechanism of apoptosis induction was unclear: studies described a direct effect of chemotherapy on the oocyte [14], others suggested that oocyte apoptosis was the result of follicular cell damage [15]. Finally, the study by Soleimani et al. reported an impact on the oocyte and on the follicular cells [16] The ovarian reserve would not be the only target of chemotherapy. Chemotherapy could also alter the ovarian stroma. Meirow et al. reported abnormalities of the vessel, anarchic neovascularization, and the presence of cortical fibrosis [20].
2.1.2 Radiotherapy: The risk of POF depends on the dose received, its fractionation and the radiation field. Age at the time of treatment was not a predictor of POF in the study of 706 women by Thomas-Teinturier et al. [9]. On the other hand, Wallace et al. showed that age had an impact on the dose responsible for the development of POF [11]. As the woman's age increased and consequently the follicular reserve decreased, the lower the dose of radiotherapy to be received to induce POF was (Figure 1). Radiotherapy did not only alter the ovarian reserve. When irradiation in childhood was pelvic, it could cause uterine radiation damage in adulthood resulting in a decrease in the volume of the uterus. This lesion was probably irreversible. This leads to problems with embryo implantation and pregnancy development [21]. A deleterious fibrosis for the uterus appeared as of 14 Gray and was marked starting from 30 Gray.
2.1.3 Other treatments at risk of POF: All ovarian surgery must be considered in order to evaluate the risk of premature ovarian failure and therefore the preservation of female fertility.
2.2 Etiologies known to cause POF [5, 22]
2.2.1 Genetics: Genetic pathologies affecting X are at risk of premature ovarian failure. The most frequent etiology is Turner syndrome, which is a 45.X monosomy in 50% of cases and the other forms are essentially mosaic forms (45,X/46,XX, etc.) and more rarely structural abnormalities of the X chromosome [23]. It affects 1 female newborn out of 2500. Other genetic pathologies are Fragile X syndrome and X translocations. Autosomal damage is variable, with alterations in NOBOX, GFD9, BMP15 and estrogen receptors.
2.2.2 Auto immune: In polyglandular autoimmune syndromes, premature ovarian failure is a diagnostic criterion. It may be the first symptom [23].
2.2.3 Societal reason: Another reason for preserving female fertility is societal. Women who are single or who do not have a desire for an immediate child at an advanced age use these fertility preservation techniques to postpone their parenting plans. Societal cryopreservation is not permitted in all countries, for example France.
3. When?
The management of patients to preserve their fertility will depend on the time available before the start of gonadotoxic treatments. If the treatment is not urgent, depending on the patient's age and pathology, it is preferable not to expose the gametes to gonadotoxic treatments. If the treatment is urgent, the question then arises as to whether to preserve the gametes as an emergency before treatment or to wait for the first chemotherapy treatments. The impact of potentially gonadotoxic treatments on the ovarian reserve has been the subject of numerous studies since the 1990s. Himelstein et al. in 1978 and Waxman et al. in 1983 described an alteration of ovarian tissue following chemotherapy treatments. Himelstein et al. after a macroscopic study of 31 ovaries of girls with acute leukaemia showed a decrease in the number of growing follicles [24]. In a meta-analysis, Waxman reported the presence of ovarian tissue fibrosis [25]. Marcello et al. in 1990 studied the effect of chemotherapy on follicular reserve by light microscopy in 10 girls aged 4 to 14 years with acute leukemia. They showed a tendency to decrease the total number of follicles in the biopsies performed. They also observed in the same study the ultrastructure of 4 biopsies of treated patients. They highlighted capillary abnormalities. On the other hand, the follicles did not present any abnormalities [26]. In contrast, Familiari et al. observed electron microscopy ovarian biopsies of 4 patients treated with chemotherapy (ABVD and/or MOPP) in the context of Hodgkin's disease. They found the presence of intraocytic vacuoles and vacuoles in the granulosa cells, the significance of which is not described [27].
However, the boom in publications really took place in the 2000s, at the beginning of ovarian freezing as part of the preservation of female fertility, when the question of the indication for self-preservation for patients who had received treatment arose. The results of these studies are divergent. In fact, several authors have found no differences between patients who have received chemotherapy cures and patients who were free of treatment prior to ovarian cryopreservation. Poirot et al. in 2002 studied the follicular density of 31 fragments of ovarian cortex from 31 patients, 22 of whom had received chemotherapy. The mean age was 17.9 years (3-34 years). They concluded that there was an association between patient age and follicular density, and that there was no impact of prior chemotherapy [28]. Similarly Seshadri et al. found no correlation between follicular density and chemotherapy in their cohort of 26 patients with Hodgkin's disease. The average age was 22 years (13-29 years). Seven patients had been treated with ABVD. They found no evidence of a link between age and follicular density [29], contrary to the work of Poirot et al. of 2002. The same team that in 2005 confirmed the results obtained in 2002 on a larger cohort of pre-pubescent patients. 45 patients, treated before ovarian self-conservation, were included. The median age was 5 years (1-15 years). Follicular density was studied: it would depend on the age of the patients and not on the treatments received [30]. On the other hand, Abir et al. in 2008 and Fabbri et al. in 2012 reported more nuanced results. In their study of 41 patients, Abir et al. performed histological analysis of the ovarian cortex using light and electron microscopy. Twenty-three patients had received prior chemotherapy. The mean age of the 18 patients without chemotherapy was 23 years (8-39 years) and the mean age of the 23 patients with chemotherapy was 17 years (5-39 years). The authors described an absence of correlation of follicular density with the presence or absence of treatment, but they described an increase in ultra-structural abnormalities in patients who received chemotherapy before ovarian cryoconservation: presence of intra-ovocyte vacuoles, and nuclear abnormalities of granulosa cells [31]. The study by Fabbri et al. was conducted on 45 pre-pubescent or recently pubescent patients. Twenty-two were treated with chemotherapy. The mean age was 13.4 years (1.5-17.9 years). They did not find a link between follicular density and treatment, but they described a tendency to oocyte abnormalities after chemotherapy [32]. The abnormalities described by Abir et al. and Fabbri et al. could be an apoptotic phenomenon present in the oocyte and in the granulosa cells. However, this hypothesis has not been demonstrated.
Finally, some authors have described a dependence of follicular density and chemotherapies that included alkylating agents in both studies. Oktem and Oktay conducted a study of ovarian fragments from 26 patients with a mean age of 27 years (4-44 years). Ten had been treated with chemotherapy prior to fertility preservation surgery. Follicular density was inversely correlated with age and the presence of chemotherapy treatment [33]. Meirow et al. reported in their study of 18 patients without treatment and 17 patients with treatment, several abnormalities found in patients previously treated with chemotherapy: vessel wall abnormalities, uncontrolled neovascularization and the presence of cortical fibrosis. However, these abnormalities were also found in the control group (patients without treatment) in the oldest women. The mean age of the patients was high: 30.5 years for the unexposed group and 27.4 years for the chemotherapy exposed group [20]. It should be taken into account that all of these studies have a small number of participants.
In view of these results it would be logical to carry out the cryopreservation of the ovarian tissue before any treatment. However, one or more chemotherapy treatments prior to the ovary removal was intended to reduce the risk of malignant cells being present in the tissue, and therefore the risk of reintroducing the initial pathology at the time of transplantation. This question arises essentially for malignant hematological pathologies including acute leukemia and malignant lymphomas. In fact, a retrospective study showed that in 2027 women under 40 years of age who were autopsied following leukemia, 8.4% presented an invasion of the ovarian tissue by malignant cells. Regarding malignant lymphomas, in 736 autopsied women under 40 years of age, 13.3% had ovarian metastases [34]. This result should be interpreted cautiously, as the autopsied patients had a more advanced stage of malignant pathology than the patients with ovarian self-preservation. The largest cohort of patients with preserved ovarian tissue described to date was that of Dolmans et al. and included 582 patients. The team reported 1.3% of patients whose biopsies were invaded after reading by light microscopy. These patients had hematological malignancies (2 non-Hodgkin's malignant lymphomas and 3 acute leukemias) [35]. Fertility preservation should be carried out as early as possible, ideally before any potentially sterilizing treatment. It can be carried out after chemotherapy when the treatment is extremely urgent and/or when the risk of invasion of the ovarian tissue is important.
4. How?
4.1 Ovarian suppression
Ovarian suppression consists of putting the ovaries at rest to preserve the follicular reserve from gonadotoxic treatments. This blockage was obtained with GnRH agonists (monthly or quarterly injections). This method could be used immediately, without the need for ovarian stimulation treatment or surgery, and also had the advantage of a contraceptive effect and induced amenorrhea (allowing a reduction in the hemorrhagic effects during chemotherapy). The side effects described were hot flashes, vaginal dryness and bone loss related to induce hypoestrogenesis. However, the effectiveness of ovarian blockade remains controversial since the 2000s [36]. About 50 studies have been reported to date. Most are in favor of GnRH agonists but these studies are often non-randomized, retrospective and conducted on a small number of subjects [37-39]. Ovarian suppression is therefore currently proposed, although the level of evidence is low, due to the absence of significant side effects. It must always be combined with a fertility preservation technique [40].
4.2 Ovarian transplantation
Radiotherapy is used for the treatment of many pediatric tumors and has a special place in the treatment of gynecological cancers in adults. Ovarian irradiation leads to a depletion of the primordial follicle stock resulting in premature ovarian failure. The severity of the lesions depends on the dose received, the fractionation pattern and the age of the patient at the time of irradiation [41]. Schematically, from 2 Gray, more than 50% of the oocytes are destroyed and 20 Gray corresponds to a sterilizing dose [42]. In case of radiotherapy or brachytherapy of more than 6 Gray, AFSOS recommends ovarian transposition in women under 40 years old. This technique allows one or both ovaries to be surgically moved out of the radiation field to decrease the gonadal dose received. The ovary is most often placed in the homolateral parietocolic gutter. The protection is only partial, the lateral transposition dividing approximately by 10 the dose of radiation received by the ovaries. The ovarian transposition must be discussed with the radiotherapist according to the dose that will be received by the ovaries. The systematic review of the literature by Hoekman et al concluded that ovarian function is preserved in 20-100% of cases. Pregnancies have been described after ovarian transposition: spontaneous or by assisted reproduction technology (ART), with or without repositioning [43]. This technique was associated with an increased risk of functional ovarian cysts [44]. Ovarian suppression as well as ovarian transposition are not sufficient to guarantee fertility preservation. These are techniques that must be combined with another preservation technique whose effectiveness has been established: embryo vitrification, oocyte vitrification or ovarian tissue freezing (Table 1).
4.3 Embryo cryopreservation
Embryo cryopreservation is the oldest fertility preservation technique. It is intended for pubescent patients and couples. After ovarian stimulation lasting 2 to 3 weeks, the mature oocytes recovered by egg retrieval are fertilized in vitro. The resulting embryos are vitrified. They belong to the couple. This technique raises a major ethical problem of the fate of embryos in the event of separation of the couple or the death of one of the partners. This technique offers the best chance of pregnancy among fertility preservation techniques, but is not the most used because of its disadvantages: contraindication in case of therapeutic emergency, hypo-response, hormone-dependent cancer, prepubescent patient, single, or in couples without parental plans. (Figure 2). Comparison of ART data from patients with and without cancer showed no difference in the number of oocytes recovered, embryos obtained and live births, although cancer patients have fewer good quality embryos [45]. In the context of fertility preservation, there are few data in the literature on pregnancy rates due to the small number of patients who undergo an embryo transfer after recovery. After cryopreservation for cancer, Barcroft et al. and Courbière et al. estimate the percentage of deliveries by embryo transfer in their centers to be 22% and 27% [46, 47]. These results in terms of pregnancy rates would therefore fall between those of infertile couples (19.2% of deliveries by frozen embryo transfer in 2017 in France) and those of fertile couples using PGD (30.4% of deliveries by fresh or frozen embryo transfer in 2017 in France, [3].
4.4 Oocyte cryopreservation
Oocyte cryopreservation is intended for pubescent patients who are single or in couples without parental plans. This technique requires one to several cycles of ovarian stimulation to obtain a sufficient number of oocytes. Griveau et al estimate the number of mature oocytes required for a live birth at 22.53 between 23 and 37 years of age and 55.50 between 38 and 43 years of age [48]. The length of time required for ovarian stimulation means that chemotherapy should not be initiated as a matter of urgency. The mature oocytes obtained by egg retrieval are vitrified. Vitrification has supplanted the slow freezing technique, allowing for a better oocyte survival rate of more than 90% [49, 50]. In the case of parental project after healing, the oocytes will be warmed up and then fertilized by microinjection. De Munck et al. in their review of the literature in 2017 find fertilization and pregnancy rates by transfer at least equal to those obtained with fresh oocytes [50]. The use of these cryopreserved oocytes remains low. Balkenende et al. reported data from their center between 2009 and 2015. 31% of the women who have vitrified their oocytes had a parental project. Of these women with a parental project, 76% achieved pregnancy spontaneously, 17% by ART without oocyte warming and only 7% by ICSI after oocyte warming [51].
4.5 Cryopreservation of ovarian tissue
It is the only technique available for prepubertal patients or in case of chemotherapy to be started urgently, as it does not require ovarian stimulation treatment. It involves freezing the ovarian cortex containing the primordial follicles. The removal of the entire ovary or a fragment of ovary is done by laparoscopy under general anesthesia. The ovary is then prepared in the laboratory into ovarian cortex slices which will be cryopreserved. After recovery, the patient will be able to benefit from an autograft, which in more than 90% of the cases will restore endocrine ovarian activity within 4 to 6 months after the transplant, with a graft survival of up to 5 to 7 years (ONCO AURA; Réseau Régional de Cancérologie OncoPaca-Corse). Today, more than 150 births have been described after ovarian autograft in the world, with a live birth rate of about 30% [52, 53]. However, the risk of reintroduction of malignant cells is a major disadvantage of this technique, especially in case of preservation for hemopathy (leukemia), Ewing's sarcoma or neuroblastoma [45]. For the time being, the preservation of fertility for these pathologies is a therapeutic impasse that may be resolved by in vitro folliculogenesis or artificial ovary.
5. Conclusion
Preservation of female fertility is an important part of patient management before gonadotoxic treatments or when a non-iatrogenic cause of premature ovarian failure is discovered. This improves the quality of life of patients by offering solutions to enable a live birth. Oocyte vitrification is the most widely used technique in the world. It offers results in birth rates close to those described with fresh oocytes [50]. When vitrification is not feasible, due to the young age of the patient or the urgency of treatment, cryopreservation of ovarian tissue is the only solution. The results are satisfactory with a recovery of endocrine function 4 to 6 months after transplantation and live birth rates of approximately 30% [52, 54]. There is still a therapeutic impasse for patients with a pathology with a high risk of dissemination of malignant cells in the ovarian tissue and whose treatment is urgent. The solutions may be in vitro folliculogenesis or artificial ovary, which are currently avenues of research. The current alternatives are oocyte donation and adoption.
References
- INCA - Les cancers en France’
- Lacour B, Guyot-Goubin A, Guissou S, et al. Incidence of childhood cancer in France: National Children Cancer Registries, 2000–2004: European Journal of Cancer Prevention 19 (2010): 173-181.
- Agence de la biomédecine. Evaluation des résultats des centres d’assistance (2020).
- Demeestere I, Basso O, Moffa F, et al. Fertility Preservation in Female Cancer Patients. Obstetrics and Gynecology International (2012).
- Fenton AJ. Premature ovarian insufficiency: Pathogenesis and management. Journal of Mid-Life Health 6 (2015): 147-153.
- van der Kaaij MAE, Heutte N, Meijnders P, et al. Premature ovarian failure and fertility in long-term survivors of Hodgkin’s lymphoma: a European Organisation for Research and Treatment of Cancer Lymphoma Group and Groupe d’Etude des Lymphomes de l’Adulte Cohort Study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 30 (2012): 291-299.
- Fisher B, Sherman B, Rockette H, et al. 1-phenylalanine mustard (L-PAM) in the management of premenopausal patients with primary breast cancer: lack of association of disease-free survival with depression of ovarian function. National Surgical Adjuvant Project for Breast and Bowel Cancers. Cancer 44 (1979): 847-857.
- Bricaire L, Laroche E, Bourcigaux N, et al. Premature ovarian failures. Presse Medicale (Paris, France: 1983) 42 (2013): 1500-1507.
- Thomas-Teinturier C, Allodji RS, Svetlova E, et al. Ovarian reserve after treatment with alkylating agents during childhood. Human Reproduction (Oxford, England) 30 (2015): 1437-1446.
- Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 24 (2006): 2917-2931.
- Wallace WHB, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. International Journal of Radiation Oncology, Biology, Physics 62 (2005): 738-744.
- Sukumvanich P, Case LD, Van Zee K, et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: a prospective study. Cancer 116 (2010): 3102-3111.
- Meirow D, Biederman H, Anderson RA, et al. Toxicity of chemotherapy and radiation on female reproduction. Clinical Obstetrics and Gynecology 53 (2010): 727-739.
- Morgan S, Lopes F, Gourley C, et al. Cisplatin and doxorubicin induce distinct mechanisms of ovarian follicle loss; imatinib provides selective protection only against cisplatin. PloS One 8 (2013): e70117.
- Raz A, Fisch B, Okon E, et al. Possible direct
- cytoxicity effects of cyclophosphamide on cultured human follicles: an electron microscopy study. Journal of Assisted Reproduction and Genetics 19 (2002): 500-506.
- Soleimani R, Heytens E, Darzynkiewicz Z, et al. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging 3 (2011): 782-793.
- Hussein MR, Bedaiwy MA, Falcone T. Analysis of apoptotic cell death, Bcl-2, and p53 protein expression in freshly fixed and cryopreserved ovarian tissue after exposure to warm ischemia. Fertility and Sterility 85 (2006): 1082-1092.
- de Bruin JP, Dorland M, Spek ER, et al. Ultrastructure of the resting ovarian follicle pool in healthy young women. Biology of Reproduction 66 (2002): 1151-1160.
- Choi JY, Jo MW, Lee EY, et al. The role of autophagy in follicular development and atresia in rat granulosa cells. Fertility and Sterility 93 (2010): 2532-2537.
- Meirow D, Dor J, Kaufman B, et al. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Human Reproduction (Oxford, England) 22 (2007): 1626-1633.
- Larsen EC, Schmiegelow K, Rechnitzer C, et al. Radiotherapy at a young age reduces uterine volume of childhood cancer survivors. Acta Obstetricia Et Gynecologica Scandinavica 83 (2004): 96-102.
- Fraison E, Crawford G, Casper G, et al. Pregnancy following diagnosis of premature ovarian insufficiency: a systematic review. Reproductive BioMedicine Online 39 (2019): 467-476.
- Christin-Maitre S, Pasquier M, Donadille B, et al. L’insuffisance ovarienne prématurée. Annales d’Endocrinologie 67 (2006): 557-566.
- Himelstein-Braw R, Peters H, Faber M. Morphological study of the ovaries of leukaemic children. British Journal of Cancer 38 (1978): 82-87.
- Waxman J. Chemotherapy and the adult gonad: a review. Journal of the Royal Society of Medicine 76 (1983): 144-148.
- Marcello MF, Nuciforo G, Romeo R, et al. Structural and ultrastructural study of the ovary in childhood leukemia after successful treatment. Cancer 66 (1990): 2099-2104.
- Familiari G, Caggiati A, Nottola SA, et al. Ultrastructure of human ovarian primordial follicles after combination chemotherapy for Hodgkin’s disease. Human Reproduction (Oxford, England) 8 (1993): 2080-2087.
- Poirot C, Vacher-Lavenu M-C, Helardot P, et al. Human ovarian tissue cryopreservation: indications and feasibility. Human Reproduction (Oxford, England) 17 (2002): 1447-1452.
- Seshadri T, Gook D, Lade S, et al. Lack of evidence of disease contamination in ovarian tissue harvested for cryopreservation from patients with Hodgkin lymphoma and analysis of factors predictive of oocyte yield. British Journal of Cancer 94 (2006): 1007-1010.
- Poirot C, Brugières L, Genestie C, et al. Ovarian tissue cryopreservation for prepubertal girls: indications and feasibility]. Gynecologie, Obstetrique & Fertilite 33 (2005): 799-803.
- Abir R, Ben-Haroush A, Felz C, et al. Selection of patients before and after anticancer treatment for ovarian cryopreservation. Human Reproduction (Oxford, England) 23 (2008): 869-877.
- Fabbri R, Vicenti R, Macciocca M, et al. Cryopreservation of ovarian tissue in pediatric patients. Obstetrics and Gynecology International 2012 (2012): 910698.
- Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 110 (2007): 2222-2229.
- Kyono K, Doshida M, Toya M, et al. Potential indications for ovarian autotransplantation based on the analysis of 5,571 autopsy findings of females under the age of 40 in Japan. Fertility and Sterility 93 (2010): 2429-2430.
- Dolmans M-M, Jadoul P, Gilliaux S, et al. A review of 15 years of ovarian tissue bank activities. Journal of Assisted Reproduction and Genetics 30 (2013): 305-314.
- Thomin A, Torre A, Daraï É, et al. Role of GnRH agonists in preserving female fertility. Journal De Gynecologie, Obstetrique Et Biologie De La Reproduction 43 (2014): 267-274.
- Recchia F, Saggio G, Amiconi G, et al. Gonadotropin-releasing hormone analogues added to adjuvant chemotherapy protect ovarian function and improve clinical outcomes in young women with early breast carcinoma. Cancer 106 (2006): 514-523.
- Potolog-Nahari C, Fishman A, Cohen I. Protection of ovarian function and fertility using a combination of gonadotropin-releasing hormone (GnRH) agonist and GnRH antagonist during cancer treatment in young females. Gynecological Endocrinology: The Official Journal of the International Society of Gynecological Endocrinology 23 (2007): 290-294.
- Blumenfeld Z. Fertility Preservation Using GnRH Agonists: Rationale, Possible Mechanisms, and Explanation of Controversy. Clinical Medicine Insights. Reproductive Health 13 (2019): 1179558119870163.
- Oktay K, Harvey BE, Loren AW. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update Summary. Journal of Oncology Practice 14 (2018): 381-385.
- Chaput L, Grémeau A-S, Vorilhon S, et al. Préservation de la fertilité en cancérologie. Bulletin du Cancer 105 (2018): 99-110.
- Hoekman EJ, Broeders EABJ, Louwe LA, et al. Ovarian function after ovarian transposition and additional pelvic radiotherapy: A systematic review. European Journal of Surgical Oncology: The Journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 45 (2019): 1328-1340.
- Phelippeau J, Cazalis CG, Koskas M. Ovarian protection and fertility preservation in women with cancer: a French national registry analysis between 2005 and 2014. Journal of Gynecology Obstetrics and Human Reproduction 48 (2019): 705-710.
- Martin JR, Kodaman P, Oktay K, et al. Ovarian cryopreservation with transposition of a contralateral ovary: a combined approach for fertility preservation in women receiving pelvic radiation. Fertility and Sterility 87 (2007): 189.e5-189.e7.
- Fisch B, Abir R. Female fertility preservation: past, present and future. Reproduction (Cambridge, England) 156 (2018): F11-F27.
- Barcroft J, Dayoub N, Thong KJ. Fifteen year follow-up of embryos cryopreserved in cancer patients for fertility preservation. Journal of Assisted Reproduction and Genetics 30 (2013): 14071413.
- Courbiere B, Decanter C, Bringer-Deutsch S, et al. Emergency IVF for embryo freezing to preserve female fertility: a French multicentre cohort study. Human Reproduction (Oxford, England) 28 (2013): 2381-2388.
- Griveau JF, Lopes M, Jouve G, et al. Vitrification: Principles and results. Journal De Gynecologie, Obstetrique Et Biologie De La Reproduction 44 (2015): 485-495.
- Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Human Reproduction Update 22 (2016): 440-449.
- De Munck N, Vajta G. Safety and efficiency of oocyte vitrification. Cryobiology 78 (2017): 119-127.
- Balkenende EM, Dahhan T, van der Veen F, et al. Reproductive outcomes after oocyte banking for fertility preservation. Reproductive Biomedicine Online 37 (2018): 425-433.
- Donnez J, Dolmans M-M. Fertility Preservation in Women. The New England Journal of Medicine 378 (2018): 400-401.
- Lotz L, Dittrich R, Hoffmann I, et al. Ovarian Tissue Transplantation: Experience From Germany and Worldwide Efficacy. Clinical Medicine Insights. Reproductive Health 13 (2019): 1179558119867357.
- Marin L, Bedoschi G, Kawahara T, et al. History, Evolution and Current State of Ovarian Tissue Auto-Transplantation with Cryopreserved Tissue: a Successful Translational Research Journey from 1999 to 2020. Reproductive Sciences (Thousand Oaks, Calif.) 27 (2020): 955-962.
- ONCO AURA R régional de cancérologie A-R-A Référentiel - Préservation de la fertilité. In ONCO AURA.
- Réseau Régional de Cancérologie OncoPaca-Corse La Préservation de la fertilité.
