Ultrasound-Faciliated Endovascular Fibrinolysis for Acute Bilateral Pulmonary Embolism in a Patient with SARS-CoV-2 Infection: Case Report
Article Information
Hani Al-Terki1*, Tobias Paulus2, Michael Gotzmann1, Andreas Mügge1
1University Hospital St. Josef-Hospital Bochum, Cardiology and Rhythmology, Ruhr University Bochum, Bochum, Germany
2University Hospital St. Josef-Hospital Bochum, Department of Interventional radiology, Ruhr University Bochum, Bochum, Germany
*Corresponding author: Hani Al-Terki, 1University Hospital St. Josef-Hospital Bochum, Cardiology and Rhythmology, Ruhr University Bochum, Bochum, Germany
Received: 17 Febraury 2023; Accepted: 27 Febraury 2023; Published: 17 March 2023
Citation: Hani Al-Terki, Tobias Paulus, Michael Gotzmann, Andreas Mügge. The Safety and Efficacy of Ultrasound-Accelerated-Catheter- Directed-thrombolysis with Urokinase in Patients with Intermediate-High Risk Pulmonary Embolism. Cardiology and Cardiovascular Medicine. 7 (2023): 86-89.
Share at FacebookAbstract
A 73-year-old man was admitted with dyspnoea (NYHA III) and hypoxemia, making oxygen treatment necessary. The polymerase chain reaction (PCR) test was positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Echocardiography revealed a right heart dysfunction, a CT scan confirmed massive pulmonary embolism. Additionally, troponin and NTproBNP levels were elevated. According to the clinical findings and the elevated sPESI score, the patient was classified as intermediate–high risk. He was treated with heparin therapy as well as ultrasound-assisted, catheter-directed thrombolysis (USAT) over 6 hours. With this therapy, his vital parameters rapidly improved, oxygen therapy could be suspended, and the patient was transferred to the general ward the next day. Four days after admission, the patient was discharged under oral anticoagulation without any complaints.
Keywords
Dyspnoea; Echocardiography; Polymerase Chain Reaction; Ultrasound-Assisted, Catheter-Directed Thrombolysis (USAT)
Dyspnoea articles; Echocardiography articles; Polymerase Chain Reaction articles; Ultrasound-Assisted articles, Catheter-Directed Thrombolysis (USAT) articles
Article Details
1. Introduction
Acute pulmonary embolism (PE) is one of the most common acute cardiovascular diseases, with an annual incidence of up to 115 per 100,000 population [1] and a mortality of approximately 10% [2]. An RV/LV ratio of > 0.9, indicating right heart decompensation, has been shown to be a predictor of poor clinical outcome [2]. According to current ESC guidelines, PE patients with hemodynamical instability, as defined by a drop in systolic blood pressure (SBP) of < 90 mmHg accompanied by an increased heart rate to > 110 beats/min, have the indication for intravenous fibrinolysis therapy. Since this therapy is associated with an increased risk of bleeding, it should be reserved for those patients with an increased risk of death in the first few hours or days (referred to as a high-risk group in the guidelines) [1]. Patients with severe PE who are not hypotensive but who have right heart dysfunction (RV/LV ≥ 0,9) with signs of myocardial damage (troponin elevation) have a 90-day mortality rate between 21% and 29%. The therapeutic options in this so-called intermediate–to-high-risk groups are less well defined. To overcome the bleeding risk of systemic fibrinolysis, local applications of fibrinolytic drugs, using pulmonary artery catheters, have been developed. In order to keep the blood concentration of fibrinolytic drugs as low as possible, an ultrasound-assisted endovascular lysis therapy has been introduced and FDA approved in 2014 (EKOS system; Boston Scientific, Marlborough, MA). Ultrasound energy is used to loosen the fibrin structure and to achieve a more effective and accelerated lysis effect. According to current ESC guidelines, EKOS is indicated in intermediate–high-risk PE patients and when systemic fibrinolysis is contraindicated or failed [1]. Certain accompanying diseases or circumstances in PE patients may alter the coagulation system. In patients with a SARS CoV-2 infection, an obstructive thromboinflammatory syndrome has been reported which may increase the risk thrombosis even despite adequate anticoagulation [4]. In this subset of patients the optimal therapy for accompanying PE is challenging, and the experience is low. We report on a patient with a SARS CoV-2-associated intermediate–high-risk PE who was treated successfully and uneventful with an ultrasound-assisted fibrinolysis system. To the best of our knowledge, this case is the second patient reported to the literature.
2. Case Presentation
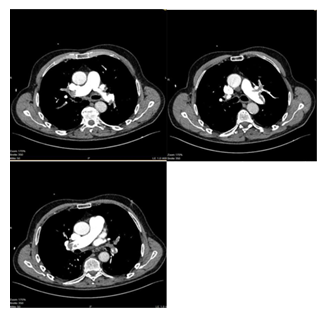
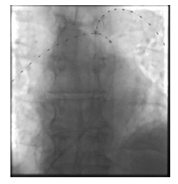
A 73-year-old male patient (BMI of 25 kg/m2) presented to our emergency department with progressive dyspnoea (NYHA III–IV) over the previous two weeks, deteriorating during the last 24 hours. A history for relevant cardiac or pulmonary diseases was negative. The antigen and PCR tests for SARS-CoV-2 were positive. At admission, the general condition was reduced with a peripheral oxygen saturation of 82% under ambient air, respiration rate of 26/min, a blood pressure of 105/83 mmHg and a heart rate of 116 bpm. Echocardiography showed a normal left ventricular ejection fraction but acute signs of right heart dysfunction, with an RV/LV ratio of 1.7, tricuspid regurgitation and a peak pressure gradient across the tricuspid valve of 44 mmHg. Laboratory tests revealed increased troponin T (0.34 ng/ml, norm < 0.01 ng/ml) and D-dimer values (> 20 μg/ml, norm < 0.5 μg/ml) as well as NT-proBNP value (432 pg/ml, norm < 125 pg/ml). CT angiography of the pulmonary arteries confirmed a massive bilateral LAE involving the main branches of the pulmonary artery tree (Figure 1). Pulmonary infiltrates typical for a SARS CoV-2 pneumonia were not detected. Duplex sonography showed no deep vein thrombosis. The patient was admitted to the intensive care unit. With 6 L/min of oxygen via oxygen mask, blood saturation improved to 94%. Intravenous anticoagulation with unfractionated heparin was started with a target PTT of 60–80 seconds. The sPESI score revealed 2 points. The patient was classified as an intermediate-to-high risk patient, and a decision was made in favour of USAT. An alternative therapeutic approach using systemic lysis was discarded because the clinical course regarding the SARS CoV-2 infection seemed unclear. Two EKOS catheters were positioned into the right and left pulmonary artery (Figure 2) and, in addition to on-going heparinisation, a reperfusion therapy was carried out for a total of 6 hours with a dosage of 1 mg/catheter/hour rt-plasminogen activator (Alteplase®, Boehringer Ingelheim). This regimen was followed by switching to an oral therapy with 10 mg of apixaban twice daily. The clinical situation improved rapidly, and the supply with oxygen could be suspended. The patient could be transferred to the general ward the next day. Echocardiography 48 hours later revealed no signs of right heart stress. The patient was discharged at home in a stable condition four days after admission, with the recommendation to continue the isolation for further 6 days, to reduce the dosage of apixaban to 5 mg twice daily and to continue the therapy with apixaban for a total of 6 months.
3. Discussion
PE is a common condition, particularly among hospitalized patients. Its incidence has increased significantly over the past two years due to the COVID-19 pandemic. The number of deaths related to SARS-Cov-2 in Germany up to February 2022 was 121,474 [9]. The main cause of death besides acute, therapy-refractory respiratory failure (ARDS), was circulatory failure due to septic venous and arterial embolism. The diagnosis of PE in patients with a SARS CoV-2 infection can be challenging, since dyspnoea and elevated troponin and D-dimer laboratory values are common symptoms or finding in both entities. The incidence of PE in patients with a SARS CoV-2 infection is not precisely known. However, some smaller studies have reported a high co-incidence of up to 23% [10]. Even in the presence of prophylactic anticoagulation, the incidence of PE may be higher in patients with a SARS CoV-2 infection than in hospitalized non-COVID-19 patients [11]. No randomized trial has evaluated the efficacy and safety of different anticoagulants for the treatment of acute venous thromboembolism in hospitalized or critically ill patients with a SARS CoV-2 infection [12]. There is also no evidence so far for the effectiveness and safety of systemic fibrinolysis therapy in these patients. The ESC guidelines for the treatment of pulmonary artery embolism recommend a risk stratification of patients into a low, intermediate–low, intermediate–high-, and high-risk group, based on the pulmonary embolism severity index (PESI or sPESI score) [1]. The effectiveness and safety of systemic fibrinolysis in intermediate PE patients were investigated by the PEITHO study [12]. The study compared anticoagulation plus systemic fibrinolysis vs. anticoagulation alone in 1005 patients. Systemic fibrinolysis reduced significantly death and hemodynamic decompensation by 56%, but at the expense of a 4-fold increase in the rate of severe extracranial bleeding (6.3% vs. 1.2%) and a 12-fold increase in the rate of intracranial bleeding haemorrhagic strokes (2.4% vs. 0.2%). Alternatively, to systemic fibrinolysis, a catheter-based therapy can be considered [1]. The pulmonary embolism response to fragmentation, embolectomy, and catheter thrombolysis (PERFECT) study included 101 patients with either massive or submassive pulmonary embolism who underwent EKOS therapy [13]. The study showed a significant symptomatic and clinical improvement in right ventricular pump function and a decrease in pulmonary artery pressure, with minimal procedural complications [13]. Similar results were achieved in the SEATTLE II study, which included 150 patients who showed improved right ventricular pump function and a decrease in RV/LV ratio in pulmonary arterial pressure after treatment with EKOS [14]. The ultrasound accelerated thrombolysis of pulmonary embolism (ULTIMA) study conducted 59 patients and showed significant improvement of right ventricular dysfunction in patients receiving combination therapy with EKOS and anticoagulation (heparin) compared to patients who received anticoagulation (heparin) alone. No increase in complication rates was found [15]. Khan et al. [16] published the first case with COVID-19-associated pulmonary artery embolism, which was successfully treated with the EKOS technique. The 31-year-old patient, diagnosed with massive bilateral PE and a sPESI score of 1, received local lysis by EKOS over 12 hours. The result was an improvement in symptoms and an increase in oxygen saturation, and the patient was discharged 48 hours later. The 73-year-old male patient in this case-report with a SARS CoV-2 infection was also diagnosed with a pronounced bilateral pulmonary artery embolism and signs of right heart strain. Due to the reduced oxygen saturation of < 90% and tachycardia—and thus a sPESI score of 2 points—in combination with the increased troponin, the decision was made to use EKOS therapy based on these indications. The therapy was uneventful, and the catheters were removed 6 hours later. The patient improved clinically. The previously initiated oxygen therapy could be terminated so that transfer to the general ward could take place the next day. Ultrasound-assisted local fibrinolysis may be an alternative approach in PE patients with imminent right heart failure. In particular in PE patients in the connection with an accompanying SARS CoV-2 infection and its uncertain course, this alternative approach appears to be hopeful therapy. Further studies are necessary to verify its effectiveness and to evaluate possible limitations.
4. Take Home Messages
- Ultrasound-assisted local fibrinolysis appears to be a promising therapeutic option in patients with acute pulmonary embolism and the risk of imminent right heart failure.
- This technique has been uneventfully used in an elder patient with a SARS CoV-2 associated massive pulmonary embolism. Our patient is to the best of our knowledge the second case reported in the literature. Further studies are necessary to verify its effectiveness and to evaluate possible limitations in this subset of patients.
References
- Konstantinides S, Meyer G, Becattini C, et.al. ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). European Heart Journal 41 (2019).
- Frémont B, Pacouret G, Jacobi D, et al. Prognostic value of echocardiographic right/left ventricular end-diastolic diameter ratio in patients with acute pulmonary embolism: results from a monocenter registry of 1,416 patients. Chest 133 (2008): 358-362.
- Anderson FA, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 151 (1991): 933-938.
- Ciceri F, Beretta L, Scandroglio AM, et.al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis, Crit Care Resusc (2020).
- Bouchlarhem A, Haddar L, Nasri S, et. al. Brainstem stroke: a fatal thromboembolic event after new onset atrial fibrillation during covid-19 infection: a case report and literature review, Radiol Case Rep 16 (2021): 3244-3249.
- Chen T, Wu T, Chen H, et.al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study, BMJ 368 (2020): m1091.
- Boudihi A, Derar C, Mazouzi M, et.al. Association of pulmonary embolism and acute coronary syndrome during COVID-19 infection: Case report and a brief review. Annals of Medicine and Surgery 73 (2022): 103152.
- https://de.statista.com/statistik/daten/studie/1102667/umfrage/erkrankungs-und-todesfaelle-aufgrund-des-coronavirus-in-deutschland/#professional
- Grillet F, Behr J, Calame P, et.al. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology (2020): 201544.
- Wichmann D, Sperhake JP, Lutgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study [published online ahead of print May 6, 2020]. Ann Intern Med 173 (2020): 268-277.
- Moores LK, Tritschler T, et.al. Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019 CHEST Guideline and Expert Panel Report. CHEST Journal 158 (2020): 1143-1163.
- Meyer G, Vicaut E, Konstantinides SV. Fibrinolysis for intermediate- risk pulmonary embolism. N Engl J Med 371 (2014): 581-582.
- Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest 148 (2015): 667-673.
- Piazza G, Hohlfelder B, Jaff MR, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv 8 (2015): 1382-1392.
- Gupta JD, Marek J, Rana MA, et.al. Same-Day ICU Discharge in Selected Patients With Severe Submassive Pulmonary Embolism Treated With Catheter-Directed Thrombolysis. Vasc Endovascular Surg 54 (2020): 58-64.
- Khan Z, Gupta A, Pabani UK, et.al. EkoSonicTM Endovascular System-Directed Thrombolysis in a Patient With COVID-19 Infection Presenting With Bilateral Large Pulmonary Embolism Causing Right Ventricular Strain: A Case Report. Cureus (2022).