TYK2 Activating Alterations in Acute Lymphoblastic Leukemia: Novel Driver Oncogenes with Potential Avenues for Precision Medicine?
Article Information
Paniz Tavakoli Shirazi1, 2*, Laura N Eadie1, 2, Susan L Heatley1, 2, Deborah L White1, 2, 3, 4
1Cancer Program, Precision Medicine Theme, South Australian Health & Medical Research Institute (SAHMRI), Adelaide, Australia
2Faculty of Health and Medical Sciences, University of Adelaide, Adelaide, Australia
3Faculty of Sciences, University of Adelaide, Adelaide, Australia
4Australian Genomics Health Alliance (AGHA)
*Corresponding Author: Paniz Tavakoli Shirazi, BSc, MBIB Biotech (Biomed), Cancer Program, Precision Medicine Theme, South Australian Health & Medical Research Institute (SAHMRI), Faculty of Health and Medical Sciences, University of Adelaide, Adelaide, Australia
Received: 19 March 2021; Accepted: 02 April 2021; Published: 12 April 2021
Citation: Paniz Tavakoli Shirazi, Laura N Eadie, Susan L Heatley, Deborah L White. TYK2 Activating Alterations in Acute Lymphoblastic Leukemia: Novel Driver Oncogenes with Potential Avenues for Precision Medicine?. Journal of Cancer Science and Clinical Therapeutics 5 (2021): 201-219.
Share at FacebookAbstract
Utilization of Next Generation Sequencing (NGS) and advances in genomic profiling have led to identification of new lesions in acute lymphoblastic leukemia (ALL) cases. TYK2 alterations are among those that warrant an in-depth characterization of the underlying mechanisms that result in leukemogenesis, targetability potential and drug response. The current literature around the functional significance and clinical importance of these alterations in driving hematological cancer (in particular, leukemia) is limited. This review focuses on recent findings demonstrating the leukemogenic potential of TYK2 alterations. Specifically, the molecular consequences of aberrant TYK2 levels are detailed and the effects of TYK2 deficiency or dysregulated activation are explored in carcinogenesis and leukemogenesis. In addition, the functional role of TYK2 in JAK/STAT signaling, possible cross talk to other cancer-related pathways and overarching avenues for pharmacological intervention in TYK2-altered ALL are also described.
Keywords
Acute Lymphoblastic; Leukemia; Oncogenes; leukemogenesis
Article Details
1. Introduction
Acute lymphoblastic leukemia (ALL) is a hematological malignancy most commonly occurring in children [1, 2]. This disease is divided into two key groups: B-cell (B-ALL) and T-cell (T-ALL) lineage, which respectively account for 75-85% and 15-25% of cases depending on the age group (childhood- adult ALL) [3-6]. Advances in the treatment of childhood ALL, by improvements in hematopoietic stem cell transplantation (SCT), CNS directed treatment and optimisation of chemotherapy regimens through risk stratification, have resulted in increases in 5-year event free survival rate from approximately 60% in the 1970s to 85% in the 1990s [5]. However, relapsed or refractory ALL still occurs in approximately 20% of childhood cases, and for these patients, outcomes are poor [5, 7, 8]. Therefore, this malignancy remains a leading cause of non-traumatic death in children. In addition, the outcome for adult patients remains extremely poor with an overall 5-year survival rate of approximately 40% and of these, nearly 7% experience a subsequent relapse [9-11]. To overcome the limitations of current chemotherapy and treatment regimens (including SCT), the ultimate approach is personalised medicine that targets specific driver lesions and pathways in individuals. Precision medicine may ultimately improve outcome for these patients and decrease the risk of treatment failure by increasing the anti-leukemic efficacy of treatment and reducing drug associated toxicities. The targeting of BCR-ABL1+ leukaemias with ABL tyrosine kinase inhibitors (ABLi) are testament to this approach, and efficacy has also been demonstrated in patients with other ABL1 and PDGFRB fusions [12-17].
ALL is a heterogenous disorder and based on the presence and functional consequence of the various lesions identified in leukemic cells, is divided into subtypes with diverse pathological and prognostic outcome [18, 19]. Technologies such as gene expression profiling, single nucleotide polymorphism (SNP) analysis and next generation sequencing (NGS) have drastically improved our understanding of the genomic basis of ALL. Genome-wide profiling studies have enabled identification of novel alterations, refinement of genomic classification and definition of genetically high risk (HR) ALL subgroups. Notably, HR ALL subtypes are characterized by alterations that activate cytokine receptor, tyrosine kinase and/or JAK/STAT signaling and are associated with poor outcome [10, 20-23]. These subtypes are of clinical importance due to potential targetability by small molecule inhibitors (SMIs) [16, 19, 22, 24-30]. TYK2 gain of function alterations, with the potential to activate the JAK/STAT pathway have only recently been described in ALL. However, the functional role of alterations involving TYK2 in leukemia development and targetability are not, to date, well understood.
2. Pathology of ALL
ALL is generally thought to be the result of deregulated transcription and maturation arrest of lymphoid lineage cells in the BM [3]. This phenomenon is caused by the acquisition of initiating lesions, including gene translocations that confer self-renewal, differentiation arrest and epigenetic reprogramming of lymphoid progenitors [1]. Accumulation of additional secondary mutations and genomic alterations, affecting multiple cellular pathways, then contribute to the clinical manifestation of the disease [1, 2]. Perturbed pathways comprise those governing lymphoid development, cell cycle regulation, tumour suppression, transcriptional regulation, epigenetic modification, Janus family of tyrosine kinases (JAK)/signal transducer and activator of transcription (STAT) signaling, phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) and RAS signaling (Figure 1). In the case of TYK2-altered disease, perturbations in JAK/STAT, PI3K/mTOR, RAS and ERK have been reported [31-35]. JAK/STAT is one of the most frequently mutated signaling pathways in cancer; recognised as one of the twelve core cancer pathways [36, 37]. In ALL, JAK activating alterations are recurrent and account for approximately 10% of HR ALL, 25% of T-ALL cases and 20% of a HR subtype of B-ALL (also known as Ph-Like ALL) [22, 23, 38, 39].
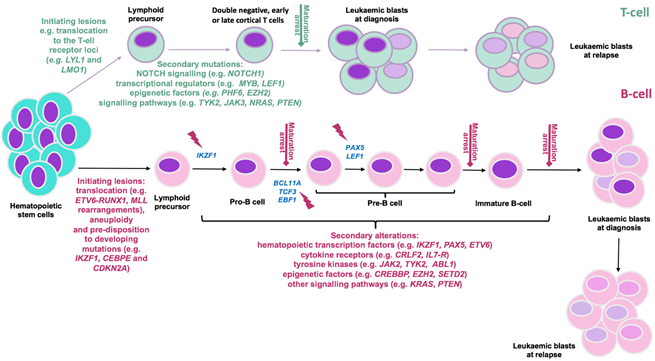
Figure 1: Predisposition and consequent development of ALL can be due to chronological acquisition of deleterious genomic alterations. The differentiation of hematopoietic cells into B- and T-lineage (represented in pink and green respectively) and their maturation is strictly regulated by transcription factors re-enforcing commitment to either fate. T-ALL initiation is mainly due to rearrangement of oncogenic transcription factors (e.g. LYL1, LMO1) into a position adjacent to T-cell receptor loci. In the B-ALL setting, changes in chromosome number (aneuploidy); acquisition of chromosomal rearrangements including translocations of genes that 1. control lymphoid development (e.g ETV6, RUNX1) 2. activate kinase signaling (e.g. ABL1) or oncogenes (e.g. MYC) 3. control epigenetic regulation (e.g. MLL(KMT2A)); and mutations in B-cell transcriptional regulator genes (e.g. IKZF1, PAX5, EBF1, CEBPE) and tumour suppressor genes (e.g, CDKN2A/2B) confer developmental arrest on lymphoid progenitors at various stages based on the altered genes (indicated by red flash). Subsequently, acquisition of additional co-operating events (as indicated) contribute to development of a genetically polyclonal disease. Selection or acquisition of further mutations can result in resistance to therapy and relapse. Adapted from [1, 2, 38].
3. TYK2 mediates cytokine signaling and activation of JAK/STAT pathway
Four human JAKs comprising JAK1, JAK2, JAK3 and TYK2 have been reported and are associated with activation of type I and II cytokines including both interleukin (IL) and interferon (IFN) receptors [40, 41]. TYK2 was the first member of the JAK family of tyrosine kinases to be described and linked to cytokine signaling and the downstream JAK/STAT pathway (Figure 2) [42, 43]. Cytokine signaling is associated with cross-specificity in activation of overlapping JAKs and STATs (44). TYK2 mediated cytokine signaling has been demonstrated, including type I, II and III IFNs (e.g. IFNa, b and ?) and ILs (e.g IL-6, IL-10, IL-12, IL-22 and IL-23) [45, 46], but importantly the capability of TYK2 to activate all STAT proteins has also been defined (46). Apart from homo-dimerization of JAK family proteins, hetero-dimerization of these proteins can also lead to activation of the JAK/STAT pathway [45, 47]. Various studies have reported TYK2 signaling upon dimerization with JAK1 and JAK2 in response to cytokines (Figure 2) [43, 45, 47-50] and the association of TYK2 with JAK1 and/or JAK2 has also been reported in hematological malignancies [32, 35, 51, 52]. Here, constitutive TYK2 auto- and trans-phosphorylation due to activating TYK2 genomic alterations predominantly results in activation of STAT1, 3 and 5 [31-34, 53]. Therefore, dependent on the specific cell types, cytokines present and also the disease, multiple JAK and STAT family proteins can be activated in response to TYK2 perturbation.
4. TYK2 mediates crosstalk with other oncogenic signaling pathways
JAK signaling and STAT family protein activation can interact with, and induce activation of, several other signaling pathways such as PI3K/mTOR and RAS [46, 54]. RAS (family of small GTPases) and PI3K/mTOR signaling pathways are responsible for signal transmission from cytokine, B-cell receptors and tyrosine kinases [55]. These signaling pathways are crucial for lineage commitment and development of B-cells in the bone marrow [55, 56]. Crosstalk with other oncogenic pathways, has been demonstrated in the setting of B- and T-cell ALL. For example, the aberrant expression of the ETV6-JAK2 fusion gene results in constitutive activation of RAS, PI3K and also NF-κB signaling pathways in the B-ALL setting [57]. In T-ALL patients, 2 of 6 TYK2 activating point mutations were reported to induce activation of extracellular signal-regulated kinase (ERK) signaling, in addition to JAK/STAT activation [33]. In other hematological malignancies, activating mutations in TYK2, resulted in aberrant signaling through additional pathways such as PI3K/mTOR, RAS and also PIM (proto-oncogene serine/threonine-protein kinases) [34, 35]. Taken together, these results demonstrate the capacity of the TYK2 protein to induce activation of additional oncogenic signaling pathways, depending on the specific cell type, mode of activation and disease context.
5. The interplay in regulation and stabilisation of TYK2 and STAT proteins
The activity of JAK family proteins including TYK2 is negatively regulated by multiple intrinsic and extrinsic factors. The first intrinsic inhibitory feature includes the regulatory ability of the pseudokinase domain (JH2), to control the kinase domain activity [58-60]. In addition, extrinsic negative regulation of the JAK/STAT pathway relies mainly on the SH2 domain-containing suppressors of cytokine signaling (SOCSs) proteins and proteases (e.g. protein tyrosine phosphatases PTPN1, PTPN6 and PTPN11) [45, 58]. The SOCS family proteins promote ubiquitination and degradation of JAK family proteins while proteases dephosphorylate activated JAKs and cytokine receptors; both actions lead to attenuation of JAK/STAT signaling [58]. Apart from the global role of these negative regulators, previous studies have demonstrated that deactivation of TYK2 signaling and acceleration of TYK2 protein degradation is largely due to direct protein-protein interaction of SOCS1/3, PTPN6 and PTPN1 with activated TYK2 [50, 61-64]. JAK and STAT family proteins are clients of heat shock proteins (HSP), in particular HSP90 [65]. The HSP90 chaperone protein plays an important role in maturation, stabilisation, folding and function of JAK and STAT proteins [65-67]. Interestingly, HSP90 is identified as the chaperone for various oncogenes [68, 69]. It promotes the functional stability of malignant cells that would otherwise be disrupted due to acquired alterations and increases their adaptability to environmental factors such as treatment [68, 69]. This function of HSP90 is also vital in JAK/STAT dependent hematological malignancies and CRLF2-rearranged and JAK2- and JAK1-mutated B-ALL [65, 70-72]. In these cases, inhibition of HSP90 by small molecule inhibitors (SMIs) or RNA interference resulted in attenuation of STATs phosphorylation and degradation of JAK proteins [70-72]. Proteomic analysis further confirmed the direct interaction of HSP90 with TYK2 protein, enhancing its stability [46, 73]. In addition, HSP90 inhibition resulted in TYK2 degradation and signal reduction in T-ALL cells harbouring wildtype and mutant TYK2 [74].
HSP90 functions through association with several proteins and co-chaperones and is subject to multiple regulatory mechanisms [66, 67]. One of these negative regulatory mechanisms involves acetylation of lysine residues on HSP90 that subsequently inhibits the binding of client and co-chaperone proteins leading to aggregation of JAK proteins [67, 75]. Therefore, histone deacetylases (HDACs) including HDAC1, 6 and 10 play an important role in facilitating HSP90 activity [67, 75]. Immunoprecipitation and western blot analysis, have demonstrated HDAC6 to be the main enzyme that stabilizes the HSP90 complex in multiple leukemia cell lines [76]. Inhibition of HDAC6 in these cells resulted in degradation of driver oncogenes such as BCR-ABL1, mutated FLT3 and subsequent reduction of their downstream signaling [76-78].
Moreover, STAT proteins interact with epigenetic co-factors such as histone acetyltransferases (HATs) and HDACs to modulate transcription of target genes [44]. Historically, HDACs activity was believed to regulate transcriptional repression only, however, recent studies revealed that both HDACs and HATs can act as activators and/or repressors of STAT-mediated transcription [44, 79-81]. HDAC mediated deacetylation of target gene transcriptional activation sites upon, STAT1, 2 and 5 binding to DNA, is essential to recruit the transcription machinery (e.g. RNA polymerase II) and initiate transcription [79, 82-86]. Hence, silencing of HDAC activity through SMIs or small interfering RNAs results in decreased STATs phosphorylation levels and expression of target genes [79, 82-86]. However, this is not without conjecture as activation of STAT3 mediated transcription, for instance, is reported to be associated with either acetylation in response to IFN stimulation in the normal setting [87, 88] or deacetylation in B-cell lymphomas [89]. It is important to note that the effect of acetylation/deacetylation on transcription can be cell type or condition specific. The exact mechanism by which chromatin modifiers interact with STATs in normal versus malignant cells remains unclear. However, the data highlighting a positive regulatory effect of HDAC is in agreement with the growing evidence of HDAC inhibitors (HDACi) efficacy in hematological malignancies and other cancers [80, 90].
The JAK/STAT signaling network is complex (Figure 2). The TYK2 protein can induce signaling redundancy, alternate signaling through other pathways and interact with positive or negative regulatory components. This interplay enables multiple therapeutic targets and emphasises the importance of careful analysis to find the appropriate inhibitors for each patient and disease context, discussed in further detail below.
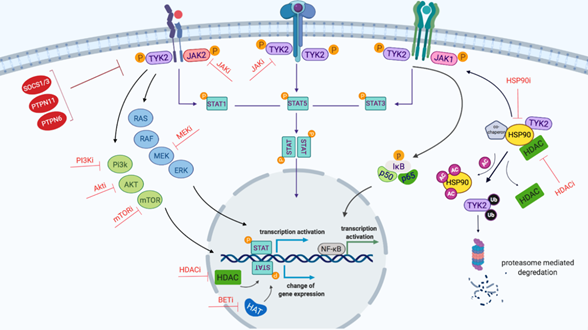
Figure 2: Schematic representation of TYK2-mediated JAK/STAT signaling network created by Biorender.com. Binding of cytokine to the cytokine receptor complex results in receptor dimerization, which consequently phosphorylates JAK proteins as the cytokine receptor itself lacks intrinsic biological activity. Activated JAKs induce the phosphorylation of STATs which, following dimerization, translocate into the nucleus and stimulate gene expression. STAT family proteins consist of seven members: STAT1-4, 5a, 5b and 6. STATs bind to the enhancer region of genes and by recruiting epigenetic modifiers (HDAC and HAT) to modulate the transcription of genes. JAKs activate other downstream signaling cascades including PI3K/mTOR, RAS and NF-κB. Furthermore, HSP90 and its co-chaperones such as HDAC proteins play an important role in facilitating signaling and JAK protein stabilisation. Red proteins (e.g SOCS1/3) are pathway regulators. Potential inhibitors of the proteins and pathways are indicated with red T-shaped lines. Abbreviations: AC, acetyl group; BET, Bromodomain and Extra-Terminal motif; HATs, histone acetyltransferases; HDAC, histone deacetylates; Ub, Ubiquitin; P, phosphorylation.
6. Can aberrant TYK2 levels cause leukemia?
The involvement of JAK1-3 in inducing cancer have been intensively studied while TYK2 has primarily been studied in the setting of auto-immune and inflammatory diseases [91, 92]. Impaired type I IFN and IL signaling due to TYK2 deficiency have been reported in several mouse models [93] but there is limited data in human cases [94, 95]. Subsequent genome wide association analyses linked TYK2 deficiency with auto-immune and inflammatory diseases [50]. Furthermore, ex vivo analysis on bone marrow cells from wild type and TYK2 deficient mice, demonstrated a reduced inhibition of B-cell lymphopoiesis upon IFNa stimulation in TYK2 deficient cells [96]. IFNa signaling can also inhibit B-cell differentiation and induce apoptosis in a normal setting [97]. In addition, reduced STAT3 signaling and response to IFNb-mediated apoptosis have been reported in TYK2 deficient pro-B cells [98]. Collectively, these findings highlighted a possible role for TYK2 in the regulation of B-cell apoptosis that may be related to B-cell leukemia. Another study suggested an increased susceptibility of TYK2 deficient mice to the development of B-cell lymphoid leukemia/lymphoma and T-ALL induced by Abelson murine leukemia virus and ETV6-JAK2 fusion gene, respectively [99]. The increased incidence of disease in TYK2 deficient mice compared to wild type controls however, may be explained by the tumour surveillance properties of TYK2, as TYK2-deficient animals also demonstrated reduced cytotoxic activity of T- and natural killer cells [50, 99]. Interestingly, the immunosurveillance properties of TYK2 are demonstrated to be independent of its canonical kinase activity. TYK2 deficient mice expressing kinase inactive TYK2 protein (harbouring TYK2 p.K923E mutation) exhibited normal development of natural killer cells in bone marrow [100]. The cytotoxic activity of these natural killer cells against a variety of tumour cells was also restored upon expression of kinase inactive TYK2 protein [107]. These results highlight the potential benefit of TYK2 inhibitors (TYK2i) to treat cancers exhibiting higher TYK2 levels and would not lead to impairment of tumour surveillance.
TYK2 overexpression and activation has been demonstrated to be associated with oncogenesis in various cancer cell lines and patient samples (breast, prostate and ovarian cancer) [97, 101-104]. For instance, a study by Ide et al. (2008) demonstrated increased invasiveness of prostate tumour cells as a result of increased TYK2 expression [102]. Another study demonstrated invasion of malignant cells into the liver upon TYK2 protein expression using in vivo transgenic mouse models of B-cell lymphoma co-expressing the c-MYC oncogene [102]. The significance of increased TYK2 expression in hematological malignancies was only recently highlighted in T-ALL (approximately 80% and 60% of lines and cases screened in the cohort, respectively) and anaplastic large cell lymphoma (ALCL) [33, 105]. TYK2 gene knock down by small interfering RNA, resulted in impaired growth and increased cell apoptosis in more than 60% of patient derived T-ALL cells and cell lines [105]. This observation was not reported in other JAK family genes and appeared specific to TYK2 [105]. In addition, TYK2 deletion in mouse models of NPM-ALK-induced ALCL and human primary ALCL cells demonstrated prolonged survival in mouse models and reduced growth/increased apoptosis in primary cells [33]. Data from both studies also demonstrated the dependency of T-ALL and ALCL cell lines and patient samples on TYK2 activation that leads to upregulation of STAT1/3 and consequently members of anti-apoptotic BCL2 family [33, 105].
7. TYK2 alterations occur in ALL patients
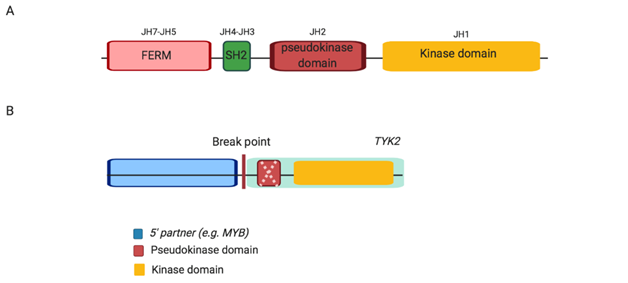
Reports on gain of function or activating TYK2 mutations and alterations in hematological malignancies have only recently emerged. In 2013, the first activating TYK2 mutations (Table 1) were reported in T-ALL cell lines and were demonstrated to have transformative ability; enabling IL-3 dependent pro-B murine Ba/F3 cells harbouring these mutations cytokine independent growth [33]. A year later, the first case of a TYK2 fusion gene was reported in HR subtype of B-ALL (also termed Ph-like ALL; MYB-TYK2) [22]. Subsequently, two more 5’ partners for TYK2 rearrangements in HR Ph-like ALL (MYB, SMARCA4 and ZNF340) were reported [22, 26]. TYK2 rearrangements have variable 5’ partners and breakpoints, yet retain an in-frame kinase domain (also known as JH1; Figure 3), potentially resulting in constitutive activation of the fusion protein. Each fusion may, or may not, contain a disrupted pseudokinase domain (termed JH2), responsible for auto-inhibition of JAK kinase domain [22, 40, 106]. In addition, the fusions lack the FERM (four-point one, erzrin, radixin, moesin) and SH-like (Src-homology) domains, which are involved in protein and cytokine receptor binding, respectively [40, 107]. Despite the diversity of the 5’ partner, 5’ genes commonly harbour a DNA binding domain and facilitate the dimerization and subsequent activation of TYK2 [22, 106].
Figure 3: Schematic structure of wild type Jak protein, JAK2 and TYK2 alterations in Ph-like ALL. (A) Wild type Jak, each Jak protein consists of four domains including FERM, SH2, pseudokinase and kinase. (B) TYK2 alterations, JAK-class fusions exhibit an intact kinase domain with either a disrupted or absent (as indicated by dashed cross) pseudokinase domain fused to variable 5’ partner genes. Abbreviation: JH, Jak homology domain.
An in vitro study demonstrated the ability of the MYB-TYK2 fusion gene to induce cytokine independent growth in pro-B murine Ba/F3 cells [26]. Only recently, additional investigations by our group demonstrated the predominant activation of JAK/STAT signaling and constitutive phosphorylation of TYK2 due to the expression of MYB-TYK2 fusion protein [108]. The leukemogenic ability of the MYB-TYK2 fusion gene was also confirmed where the resultant gene fusion induced an aggressive B-ALL in mouse models [108]. These findings provided evidence, for the first time, of the leukemogenic potential of TYK2 fusion genes. Furthermore, TYK2 alterations have been reported in other blood cancers including acute myeloid leukemia (AML), CD30-positive lymphoproliferative disorder (LPD) and ALCL [31, 32, 34, 109]. In vitro analysis of NPM1-TYK2 and NFKB2-TYK2 fusion genes, detected only in LPD and ALCL patients respectively, demonstrated constitutive auto- and trans-phosphorylation of TYK2 and downstream activation of STAT family proteins [31, 32]. However, the oncogenesis potential of these TYK2 fusion genes in in vivo models is unknown. Table 1 highlights the key TYK2 alterations and the current in vitro functional consequences in ALL.
Table 1: Key TYK2 mutations and rearrangements detected in ALL. Modified from Woss et.al (2019) (46) and St. Jude PeCan Data portal [110].
|
TYK2 alterations |
Disease |
Known functional status |
Domain |
References |
|
TYK2 p.G271S |
B-ALL |
n.d. * |
FERM |
[111] |
|
TYK2 p.W327R |
B-ALL (Ph-Like) |
n.d. * |
FERM |
[22] |
|
TYK2 p.V678L |
B-ALL |
n.d. * |
Pseudokinase (JH2) |
[111] |
|
TYK2 p.P760L |
B-ALL |
increased TYK2 autophosphorylation/ STATs activation |
Pseudokinase (JH2) |
[53] |
|
TYK2 p.G909S |
B-ALL |
n.d. |
Kinase (JH1) |
[110] |
|
TYK2 p.A1156V |
B-ALL (Ph-like) |
n.d. * |
Kinase (JH1) |
[110]. |
|
MYB-TYK2 |
B-ALL (Ph-like) |
cytokine independent growth (in vitro) +STATs activation/ increased TYK2 autophosphorylation/ induced B-ALL in mouse models |
Intact kinase (JH1) |
[22, 26] |
|
SMARCA4-TYK2 |
B-ALL (Ph-like) |
n.d. * |
Intact kinase (JH1) |
[10] |
|
ZNF340-TYK2 |
B-ALL (Ph-like) |
n.d. * |
Intact kinase (JH1) |
[10] |
|
TYK2 p.V15A |
T-ALL |
n.d. |
FERM |
[110] |
|
TYK2 p.A35V |
T-ALL |
n.d. |
FERM |
[110] |
|
TYK2 p.G36D |
T-ALL |
cytokine independent growth (in vitro) |
FERM |
[33] |
|
TYK2 p.S47N |
T-ALL |
cytokine independent growth (in vitro) |
FERM |
[33] |
|
TYK2 p.R425H |
T-ALL |
failed cytokine independent growth (in vitro) |
FERM |
[33] |
|
TYK2 p.C192Y |
T-ALL |
n.d. |
FERM |
[110] |
|
TYK2 p.R243W |
T-ALL |
n.d. |
FERM |
[110] |
|
TYK2 p.R274H |
T-ALL |
n.d. |
SH2 |
[110] |
|
TYK2 p.A375V |
T-ALL |
n.d. |
SH2 |
[110] |
|
TYK2 p.P494S |
T-ALL |
n.d. |
SH2 |
[110] |
|
TYK2 p.V678L |
T-ALL |
n.d. |
Pseudokinase (JH2) |
[110] |
|
TYK2 p.V731I |
T-ALL |
cytokine independent growth (in vitro) |
Pseudokinase (JH2) |
[33] |
|
TYK2 p.G761V |
T-ALL |
increased TYK2 autophosphorylation/ STATs activation |
Pseudokinase (JH2) |
[53] |
|
TYK2 p.G937A |
T-ALL |
n.d. |
Kinase (JH1) |
[110] |
|
TYK2 p.E957D |
T-ALL |
cytokine independent growth (in vitro) / Weak STATs activation |
Kinase (JH1) |
[33, 53] |
|
TYK2 p.M926V |
T-ALL |
no STATs activation |
Kinase (JH1) |
[53] |
|
TYK2 p.Y955H |
T-ALL |
n.d. |
Kinase (JH1) |
[110] |
|
TYK2 p.R1027H |
T-ALL |
cytokine independent growth (in vitro) /Weak STATs activation |
Kinase (JH1) |
[33] |
*These alterations are speculated to be gain of function and activating mutations and rearrangements, since no functional analyses are available. +unpublished data (Tavakoli et al, 2021, under revision). Abbreviations: n.d.=no data
8. Targeted therapeutic possibilities for TYK2-altered disease
Currently, the lack of knowledge and efficient therapeutics targeting the TYK2 oncogenic alterations, necessitate broader screening of SMIs. The subsequent JAK/STAT signaling activation due to TYK2 alterations, provide a rational avenue for the use of JAKi against this HR subtype. Ruxolitinib was the first JAK1/2i approved for treatment of patients with myeloproliferative disorders such as myeloproliferative neoplasms (MPN), polycythemia vera (PV) and essential thrombocythemia (ET), the majority of whom harboured the activating JAK2 p.V617F mutation [112-115]. The significant improvement of symptoms and reduced splenomegaly, in addition to the good tolerability of ruxolitinib treatment [116, 117], accelerated the development and clinical use of JAKi as an anti-cancer drug. The efficacy of some therapeutics against HR ALL harbouring JAK/STAT activating alterations has been demonstrated in in vitro and in vivo pre-clinical models and case studies [16, 19, 22, 24, 26-29]. The sensitivity of cell lines harbouring JAK2 fusions (i.e. PAX5-JAK2 and ATF7IP-JAK2) to ruxolitinib in vitro, plus its efficacy in reducing leukemic burden in mouse models of JAK2 rearranged ALL, has also been reported [26]. In particular, a strong in vitro and in vivo anti-leukemic effect for NDI-031301 (TYK2-specific inhibitor) has been reported against TYK2-dependent T-ALL cell lines and primary cells [118]. Similarly, our group identified the JAKi, cerdulatinib, as an efficacious therapeutic agent against cells harbouring the MYB-TYK2 fusion gene, with significantly reduced cell proliferation and decreased tumour burden in mice [108].
The protein-protein interactions and co-operation of TYK2 with HDACs and HSPs to activate JAK/STAT signaling and possible cross talk of JAK/STAT signaling to other pathways due to TYK2 alterations, provide an alternative kinase independent and even more attractive therapeutic targets [46, 73, 79]. Thus far, the sensitivity of the MYB-TYK2 fusion gene has been established to the HDACi, vorinostat and the HSP90i, tanespimycin in vitro; anti-leukemic effects of vorinostat were also demonstrated in pre-clinical in vivo models of MYB-TYK2 altered disease [119]. In addition to TYK2-altered cases, various SMIs (PI3K/mTORi, MEK1/2i, HSP90i and BETi) have demonstrated promising in vitro and in vivo efficacy against CRLF2-rearranged ALL cases exhibiting JAK/STAT hyperactivation [25, 30, 120-122]. The use of HDACi such as vorinostat alone or in combination with a chemotherapy backbone has been approved for treatment of T-cell lymphoma and demonstrated activity against other hematological malignancies such as AML [123, 124]. Interestingly, HDACi demonstrated efficacy in clinical trials for JAK mutated MPN and PV cases [125-127]. Recently, studies have also demonstrated HDACi efficacy in inducing apoptosis in cells and engraftment reduction of leukemic cells in in vitro and in vivo models of B-ALL (B-ALL cell lines and xenograft models of KMT2A-rearranged ALL and CRLF2-rearranged ALL), respectively [122, 128, 129].
Taken together, these findings highlight the potential benefit of therapies targeted to specific genomic alterations that may improve the response to treatment and subsequent outcomes in patients. Currently, there is an unmet need to widely investigate the efficacy of SMIs on other HR ALL subtypes including TYK2 alterations. The data so far, supports the further exploration of the efficacy of TYK2i, HDACi and HSP90i in larger cohort of cases with TYK2-altered disease. Furthermore, the efficacy of these SMIs in combination and/or as an addition to the chemotherapeutic backbone regimen requires future investigation. In the era of precision medicine, it is essential to understand the activated pathways of each underlying genomic alteration in individual patients. In addition, it is crucial to identify therapeutics that specifically target activated pathways, such as those in TYK-2 altered disease. This will be achieved through robust pre-clinical models to test novel therapies and facilitate more genomic alteration-specific clinical trials.
9. Future Prospects
Utilization of NGS technology has led to the identification of a growing number of TYK2 alterations in ALL cases [10, 33, 53, 111]. Given the well-established role of TYK2 in JAK/STAT signaling and its potential kinase-dependent oncogenic consequence, it is essential to inform effective targeted therapeutics for ALL cases harbouring TYK2 gain of function mutations and/or rearrangements. The prerequisite for identifying effective targeted therapies, however, relies on robust in vitro and in vivo modelling of each alteration. Aside from the recent study by our group investigating the oncogenic potential of the MYB-TYK2 fusion gene in in vivo models, the significance of other TYK2 alterations (e.g. pseudokinase or FERM mutated TYK2 cases) as a driver oncogene in leukemogenesis is unknown. It is not clear whether all TYK2 alterations are capable of inducing disease and activating similar pathways downstream of each specific alteration. Thus, future research should focus on the comprehensive characterization of the functional and prognostic consequence of these alterations and TYK2 activation in each setting as well as to their therapeutic targetability. This approach will consequently elucidate a clear picture of the leukemogenic role, clinical importance and therapeutic targetability of TYK2 alterations in ALL.
References
- Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. The Lancet 381 (2013): 1943-1955.
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. New England Journal of Medicine 373 (2015): 1541-1552.
- Jabbour EJ, Faderl S, Kantarjian HM. Adult acute lymphoblastic leukemia. InMayo Clinic Proceedings 80 (2005): 1517-1527.
- Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. The Lancet 371 (2008): 1030-1043.
- Terwilliger T, Abdul-Hay MJ. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer Journal 7 (2017): e577.
- Aifantis I, Raetz E, Buonamici S. Molecular pathogenesis of T-cell leukaemia and lymphoma. Nature Reviews Immunology 8 (2008): 380-390.
- Ko RH, Ji L, Barnette P, et al. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: a Therapeutic Advances in Childhood Leukemia Consortium study. Journal of Clinical Oncology 28 (2010): 648.
- Raetz EA, Bhatla T. Where do we stand in the treatment of relapsed acute lymphoblastic leukemia?. Hematology 2010, the American Society of Hematology Education Program Book 2012 (2012): 129-136.
- Larson S, Stock W. Progress in the treatment of adults with acute lymphoblastic leukemia. Current Opinion in Hematology 15 (2008): 400-407.
- Roberts KG, Gu Z, Payne-Turner D, et al. High frequency and poor outcome of Philadelphia chromosome–like acute lymphoblastic leukemia in adults. Journal of Clinical Oncology 35 (2017): 394.
- Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 109 (2007): 944-950.
- Daver N, Thomas D, Ravandi F, et al. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica 100 (2015): 653.
- Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome–positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood 103 (2004): 4396-4407.
- Ottmann OG, Pfeifer H. Management of Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL). ASH Education Program Book 2009 (2009): 371-381.
- Yeung DT, Moulton DJ, Heatley SL, et al. Relapse of BCR-ABL1-like ALL mediated by the ABL1 kinase domain mutation T315I following initial response to dasatinib treatment. Leukemia 29 (2015): 230-232.
- Weston BW, Hayden MA, Roberts KG, et al. Tyrosine kinase inhibitor therapy induces remission in a patient with refractory EBF1-PDGFRB–positive acute lymphoblastic leukemia. Journal of Clinical Oncology 31 (2013): e413-e416.
- Kobayashi K, Miyagawa N, Mitsui K, et al. TKI dasatinib monotherapy for a patient with Ph?like ALL bearing ATF7IP/PDGFRB translocation. Pediatric Blood & Cancer 62 (2015): 1058-1060.
- Tasian SK, Hunger SP. Genomic characterization of paediatric acute lymphoblastic leukaemia: an opportunity for precision medicine therapeutics. British Journal of Haematology 176 (2017): 867-882.
- Roberts KG, Mullighan CG. Genomics in acute lymphoblastic leukaemia: insights and treatment implications. Nature Reviews Clinical Oncology 12 (2015): 344.
- Roberts KG, Morin RD, Zhang J, et al. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell 22 (2012): 153-166.
- Reshmi SC, Harvey RC, Roberts KG, et al. Targetable kinase gene fusions in high-risk B-ALL: a study from the Children’s Oncology Group. Blood 129 (2017): 3352-3361.
- Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. New England Journal of Medicine 371 (2014): 1005-1015.
- Mullighan CG, Zhang J, Harvey RC, et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proceedings of the National Academy of Sciences 106 (2009): 9414-9418.
- Lengline E, Beldjord K, Dombret H, et al. Successful tyrosine kinase inhibitor therapy in a refractory B-cell precursor acute lymphoblastic leukemia with EBF1-PDGFRB fusion. Haematologica 98 (2013): e146.
- Maude SL, Tasian SK, Vincent T, et al. Targeting JAK1/2 and mTOR in murine xenograft models of Ph-like acute lymphoblastic leukemia. Blood, The Journal of the American Society of Hematology 120 (2012): 3510-3518.
- Roberts KG, Yang YL, Payne-Turner D, et al. Oncogenic role and therapeutic targeting of ABL-class and JAK-STAT activating kinase alterations in Ph-like ALL. Blood Advances 1 (2017): 1657-1671.
- Iacobucci I, Li Y, Roberts KG, et al. Truncating erythropoietin receptor rearrangements in acute lymphoblastic leukemia. Cancer Cell 29 (2016): 186-200.
- Maude SL, Dolai S, Delgado-Martin C, et al. Efficacy of JAK/STAT pathway inhibition in murine xenograft models of early T-cell precursor (ETP) acute lymphoblastic leukemia. Blood 125 (2015): 1759-1767.
- Treanor LM, Zhou S, Janke L, et al. Interleukin-7 receptor mutants initiate early T cell precursor leukemia in murine thymocyte progenitors with multipotent potential. Journal of Experimental Medicine 211 (2014): 701-713.
- Tasian SK, Teachey DT, Li Y, et al. Potent efficacy of combined PI3K/mTOR and JAK or ABL inhibition in murine xenograft models of Ph-like acute lymphoblastic leukemia. Blood 129 (2017): 177-187.
- Velusamy T, Kiel MJ, Sahasrabuddhe AA, et al. A novel recurrent NPM1-TYK2 gene fusion in cutaneous CD30-positive lymphoproliferative disorders. Blood 124 (2014): 3768-3771.
- Crescenzo R, Abate F, Lasorsa E, et al. Convergent mutations and kinase fusions lead to oncogenic STAT3 activation in anaplastic large cell lymphoma. Cancer Cell 27 (2015): 516-532.
- Sanda T, Tyner JW, Gutierrez A, et al. TYK2-STAT1-BCL2 pathway dependence in T-cell acute lymphoblastic leukemia. Cancer Discov. 3 (2013): 564-577.
- Tron AE, Keeton EK, Ye M, et al. Next-generation sequencing identifies a novel ELAVL1-TYK2 fusion gene in MOLM-16, an AML cell line highly sensitive to the PIM kinase inhibitor AZD1208. Leuk Lymphoma 57 (2016): 2927-2929.
- Staerk J, Kallin A, Demoulin JB, et al. JAK1 and Tyk2 activation by the homologous polycythemia vera JAK2 V617F mutation: cross-talk with IGF1 receptor. J Biol Chem 280 (2005): 41893-41899.
- Vogelstein B, Papadopoulos N, Velculescu VE, et al. Cancer genome landscapes. Science 339 (2013): 1546-1558.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 144 (2011): 646-674.
- Liu Y, Easton J, Shao Y, et al. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat Genet 49 (2017): 1211-1218.
- Tran TH, Loh ML. Ph-like acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program 2016 (2016): 561-566.
- Ihle JN, Gilliland DG. Jak2: normal function and role in hematopoietic disorders. Curr Opin Genet Dev 17 (2007): 8-14.
- Ihle JN. Cytokine receptor signalling. Nature 377 (1995): 591-594.
- Krolewski JJ, Lee R, Eddy R, et al. Identification and chromosomal mapping of new human tyrosine kinase genes. Oncogene 5 (1990): 277-282.
- Velazquez L, Fellous M, Stark GR, et al. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell 70 (1992): 313-322.
- Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem 282 (2007): 20059-20063.
- Hammaren HM, Virtanen AT, Raivola J, et al. The regulation of JAKs in cytokine signaling and its breakdown in disease. Cytokine 118 (2019): 48-63.
- Woss K, Simonovic N, Strobl B, et al. TYK2: An Upstream Kinase of STATs in Cancer. Cancers (Basel) 11 (2019).
- Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev 228 (2009): 273-287.
- Bacon CM, McVicar DW, Ortaldo JR, et al. Interleukin 12 (IL-12) induces tyrosine phosphorylation of JAK2 and TYK2: differential use of Janus family tyrosine kinases by IL-2 and IL-12. J Exp Med 181 (1995): 399-404.
- Muller M, Briscoe J, Laxton C, et al. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature 366 (1993): 129-135.
- Leitner NR, Witalisz-Siepracka A, Strobl B, et al. Tyrosine kinase 2 - Surveillant of tumours and bona fide oncogene. Cytokine 89 (2017): 209-218.
- Shide K, Shimoda K, Kamezaki K, et al. Tyk2 mutation homologous to V617F Jak2 is not found in essential thrombocythaemia, although it induces constitutive signaling and growth factor independence. Leuk Res 31 (2007): 1077-1084.
- Bousoik E, Montazeri Aliabadi H. "Do We Know Jack" About JAK? A Closer Look at JAK/STAT Signaling Pathway. Front Oncol 8 (2018): 287.
- Waanders E, Scheijen B, Jongmans MC, et al. Germline activating TYK2 mutations in pediatric patients with two primary acute lymphoblastic leukemia occurrences. Leukemia 31 (2017): 821-828.
- Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene 32 (2013): 2601-2613.
- Petkau G, Turner M. Signalling circuits that direct early B-cell development. Biochem J. 476 (2019): 769-778.
- Greaves SA, Babolin C, Torres RM, et al. Ras, Erk, and PI3K signaling pathways in the central selection of B cells 198 (2017): 202.6.
- Nguyen MH, Ho JM, Beattie BK, et al. TEL-JAK2 mediates constitutive activation of the phosphatidylinositol 3'-kinase/protein kinase B signaling pathway. J Biol Chem 276 (2001): 32704-32713.
- Babon JJ, Lucet IS, Murphy JM, et al. The molecular regulation of Janus kinase (JAK) activation. Biochem J. 462 (2014): 1-13.
- Min X, Ungureanu D, Maxwell S, et al. Structural and Functional Characterization of the JH2 Pseudokinase Domain of JAK Family Tyrosine Kinase 2 (TYK2). J Biol Chem 290 (2015): 27261-27270.
- Lupardus PJ, Ultsch M, Wallweber H, et al. Structure of the pseudokinase-kinase domains from protein kinase TYK2 reveals a mechanism for Janus kinase (JAK) autoinhibition. Proc Natl Acad Sci U S A 111 (2014): 8025-8030.
- Babon JJ, Kershaw NJ, Murphy JM, et al. Suppression of cytokine signaling by SOCS3: characterization of the mode of inhibition and the basis of its specificity. Immunity 36 (2012): 239-250.
- Piganis RA, De Weerd NA, Gould JA, et al. Suppressor of cytokine signaling (SOCS) 1 inhibits type I interferon (IFN) signaling via the interferon alpha receptor (IFNAR1)-associated tyrosine kinase Tyk2. J Biol Chem 286 (2011): 33811-33818.
- Bollu LR, Mazumdar A, Savage MI, et al. Molecular Pathways: Targeting Protein Tyrosine Phosphatases in Cancer. Clin Cancer Res 23 (2017): 2136-2142.
- Myers MP, Andersen JN, Cheng A, et al. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J Biol Chem 276 (2001): 47771-47774.
- Bocchini CE, Kasembeli MM, Roh SH, et al. Contribution of chaperones to STAT pathway signaling. JAKSTAT 3 (2014): e970459.
- Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 11 (2010): 515-528.
- Prodromou C. Mechanisms of Hsp90 regulation. Biochem J 473 (2016): 2439-2452.
- Jaeger AM, Whitesell L. HSP90: Enabler of Cancer Adaptation 3 (2019): 275-297.
- Trepel J, Mollapour M, Giaccone G, et al. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 10 (2010): 537-549.
- Weigert O, Lane AA, Bird L, et al. Genetic resistance to JAK2 enzymatic inhibitors is overcome by HSP90 inhibition. J Exp Med 209 (2012): 259-273.
- Schoof N, von Bonin F, Trumper L, et al. HSP90 is essential for Jak-STAT signaling in classical Hodgkin lymphoma cells. Cell Commun Signal 7 (2009): 17.
- Kucine N, Marubayashi S, Bhagwat N, et al. Tumor-specific HSP90 inhibition as a therapeutic approach in JAK-mutant acute lymphoblastic leukemias. Blood 126 (2015): 2479-2483.
- Taipale M, Krykbaeva I, Koeva M, et al. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell 150 (2012): 987-1001.
- Akahane K, Sanda T, Mansour MR, et al. HSP90 inhibition leads to degradation of the TYK2 kinase and apoptotic cell death in T-cell acute lymphoblastic leukemia. Leukemia 30 (2016): 219-228.
- Kramer OH, Mahboobi S, Sellmer A. Drugging the HDAC6-HSP90 interplay in malignant cells. Trends Pharmacol Sci 35 (2014): 501-509.
- Bali P, Pranpat M, Bradner J, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem 280 (2005): 26729-26734.
- Nimmanapalli R, Fuino L, Bali P, Gasparetto M, et al. Histone deacetylase inhibitor LAQ824 both lowers expression and promotes proteasomal degradation of Bcr-Abl and induces apoptosis of imatinib mesylate-sensitive or -refractory chronic myelogenous leukemia-blast crisis cells. Cancer Res 63 (2003): 5126-5135.
- Bali P, George P, Cohen P, et al. Superior activity of the combination of histone deacetylase inhibitor LAQ824 and the FLT-3 kinase inhibitor PKC412 against human acute myelogenous leukemia cells with mutant FLT-3. Clin Cancer Res 10 (2004): 4991-4997.
- Nusinzon I, Horvath CM. Unexpected roles for deacetylation in interferon- and cytokine-induced transcription. J Interferon Cytokine Res 25 (2005): 745-748.
- Buchwald M, Kramer OH, Heinzel T. HDACi--targets beyond chromatin. Cancer Lett 280 (2009): 160-167.
- Wieczorek M, Ginter T, Brand P, et al. Acetylation modulates the STAT signaling code. Cytokine Growth Factor Rev 23 (2012): 293-305.
- Rascle A, Johnston JA, Amati B. Deacetylase activity is required for recruitment of the basal transcription machinery and transactivation by STAT5. Mol Cell Biol 23 (2003): 4162-4173.
- Nusinzon I, Horvath CM. Interferon-stimulated transcription and innate antiviral immunity require deacetylase activity and histone deacetylase 1. Proc Natl Acad Sci U S A 100 (2003): 14742-14747.
- Chang HM, Paulson M, Holko M, et al. Induction of interferon-stimulated gene expression and antiviral responses require protein deacetylase activity. Proc Natl Acad Sci U S A 101 (2004): 9578-9583.
- Sakamoto S, Potla R, Larner AC. Histone deacetylase activity is required to recruit RNA polymerase II to the promoters of selected interferon-stimulated early response genes. J Biol Chem 279 (2004): 40362-40367.
- Klampfer L, Huang J, Swaby LA, et al. Requirement of histone deacetylase activity for signaling by STAT1. J Biol Chem 279 (2004): 30358-30368.
- Wang R, Cherukuri P, Luo J. Activation of Stat3 sequence-specific DNA binding and transcription by p300/CREB-binding protein-mediated acetylation. J Biol Chem 280 (2005): 11528-11534.
- Yuan ZL, Guan YJ, Chatterjee D, et al. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science 307 (2005): 269-273.
- Gupta M, Han JJ, Stenson M, et al. Regulation of STAT3 by histone deacetylase-3 in diffuse large B-cell lymphoma: implications for therapy. Leukemia 26 (2012): 1356-1364.
- Tambaro FP, Dell'aversana C, Carafa V, et al. Histone deacetylase inhibitors: clinical implications for hematological malignancies. Clin Epigenetics 1 (2010): 25-44.
- O'Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med 368 (2013): 161-170.
- Schwartz DM, Kanno Y, Villarino A, et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov 17 (2017): 78.
- Strobl B, Stoiber D, Sexl V, et al. Tyrosine kinase 2 (TYK2) in cytokine signalling and host immunity. Front Biosci (Landmark Ed) 16 (2011): 3214-3232.
- Minegishi Y, Saito M, Morio T, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity 25 (2006): 745-755.
- Kilic SS, Hacimustafaoglu M, Boisson-Dupuis S, et al. A patient with tyrosine kinase 2 deficiency without hyper-IgE syndrome. J Pediatr 160 (2012): 1055-1057.
- Shimoda K, Kamesaki K, Numata A, et al. Cutting edge: tyk2 is required for the induction and nuclear translocation of Daxx which regulates IFN-alpha-induced suppression of B lymphocyte formation. J Immunol 169 (2002): 4707-4711.
- Ubel C, Mousset S, Trufa D, et al. Establishing the role of tyrosine kinase 2 in cancer. Oncoimmunology 2 (2013): e22840.
- Gamero AM, Potla R, Wegrzyn J, et al. Activation of Tyk2 and Stat3 is required for the apoptotic actions of interferon-beta in primary pro-B cells. J Biol Chem 281 (2006): 16238-16244.
- Stoiber D, Kovacic B, Schuster C, et al. TYK2 is a key regulator of the surveillance of B lymphoid tumors. J Clin Invest 114 (2004): 1650-1658.
- Prchal-Murphy M, Witalisz-Siepracka A, Bednarik KT, et al. In vivo tumor surveillance by NK cells requires TYK2 but not TYK2 kinase activity. Oncoimmunology 4 (2015): e1047579.
- Silver DL, Naora H, Liu J, et al. Activated signal transducer and activator of transcription (STAT) 3: localization in focal adhesions and function in ovarian cancer cell motility. Cancer Res 64 (2004): 3550-3558.
- Ide H, Nakagawa T, Terado Y, et al. Tyk2 expression and its signaling enhances the invasiveness of prostate cancer cells. Biochem Biophys Res Commun 369 (2008): 292-296.
- Song XC, Fu G, Yang X, et al. Protein expression profiling of breast cancer cells by dissociable antibody microarray (DAMA) staining. Mol Cell Proteomics 7 (2008): 163-169.
- Santos J, Mesquita D, Barros-Silva JD, et al. Uncovering potential downstream targets of oncogenic GRPR overexpression in prostate carcinomas harboring ETS rearrangements. Oncoscience 2 (2015): 497-507.
- Prutsch N, Gurnhofer E, Suske T, et al. Dependency on the TYK2/STAT1/MCL1 axis in anaplastic large cell lymphoma. Leukemia 33 (2019): 696-709.
- Poitras JL, Dal Cin P, Aster JC, et al. Novel SSBP2-JAK2 fusion gene resulting from a t(5;9)(q14.1;p24.1) in pre-B acute lymphocytic leukemia. Genes Chromosomes Cancer 47 (2008): 884-889.
- Valentino L, Pierre J. JAK/STAT signal transduction: regulators and implication in hematological malignancies. Biochem Pharmacol 71 (2006): 713-721.
- Tavakoli Shirazi P EL, Heatley S, Yeung D et al. The MYB-TYK2 gene fusion induces B-cell acute lymphoblastic leukaemia in in vitro and in vivo models and can be effectively targeted by the dual SYK/JAK inhibitor, cerdulatinib. . HemaSphere 4 (2020): 136-137.
- Tomasson MH, Xiang Z, Walgren R, et al. Somatic mutations and germline sequence variants in the expressed tyrosine kinase genes of patients with de novo acute myeloid leukemia. Blood 111 (2008): 4797-4808.
- Edmonson MN, Patel AN, Hedges DJ, et al. Pediatric Cancer Variant Pathogenicity Information Exchange (PeCanPIE): a cloud-based platform for curating and classifying germline variants. Genome Res 29 (2019): 1555-1565.
- Ma X, Liu Y, Liu Y, et al. Pan-cancer genome and transcriptome analyses of 1, 699 paediatric leukaemias and solid tumours. Nature 555 (2018): 371-376.
- Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 366 (2012): 787-798.
- Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med 363 (2010): 1117-1127.
- Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 366 (2012): 799-807.
- Mascarenhas J, Hoffman R. Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res 18 (2012): 3008-3014.
- Verstovsek S, Mesa RA, Gotlib J, et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol 10 (2017): 55.
- Harrison C, Vannucchi AM. Ruxolitinib: a potent and selective Janus kinase 1 and 2 inhibitor in patients with myelofibrosis. An update for clinicians. Ther Adv Hematol 3 (2012): 341-354.
- Akahane K, Li Z, Etchin J, et al. Anti-leukaemic activity of the TYK2 selective inhibitor NDI-031301 in T-cell acute lymphoblastic leukaemia. Br J Haematol 177 (2017): 271-282.
- Tavakoli Shirazi P, Eadie LN, Heatley SL, et al. Exploring the oncogenic and therapeutic target potential of the MYB-TYK2 fusion gene in B-cell acute lymphoblastic leukemia. Oncogene (2021).
- Suryani S, Bracken LS, Harvey RC, et al. Evaluation of the in vitro and in vivo efficacy of the JAK inhibitor AZD1480 against JAK-mutated acute lymphoblastic leukemia. Mol Cancer Ther 14 (2015): 364-374.
- Ott CJ, Kopp N, Bird L, et al. BET bromodomain inhibition targets both c-Myc and IL7R in high-risk acute lymphoblastic leukemia. Blood 120 (2012): 2843-2852.
- Savino AM, Sarno J, Trentin L, et al. The histone deacetylase inhibitor givinostat (ITF2357) exhibits potent anti-tumor activity against CRLF2-rearranged BCP-ALL. Leukemia (2017).
- Zhang Q, Wang S, Chen J, et al. Histone Deacetylases (HDACs) Guided Novel Therapies for T-cell lymphomas. Int J Med Sci 16 (2019): 424-442.
- How J, Minden MD, Brian L, et al. A phase I trial of two sequence-specific schedules of decitabine and vorinostat in patients with acute myeloid leukemia. Leuk Lymphoma 56 (2015): 2793-2802.
- Finazzi G, Vannucchi AM, Martinelli V, et al. A phase II study of Givinostat in combination with hydroxycarbamide in patients with polycythaemia vera unresponsive to hydroxycarbamide monotherapy. Br J Haematol 161 (2013): 688-694.
- Rambaldi A, Dellacasa CM, Finazzi G, et al. A pilot study of the Histone-Deacetylase inhibitor Givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br J Haematol 150 (2010): 446-455.
- Mascarenhas J, Lu M, Li T, et al. A phase I study of panobinostat (LBH589) in patients with primary myelofibrosis (PMF) and post-polycythaemia vera/essential thrombocythaemia myelofibrosis (post-PV/ET MF). Br J Haematol 161 (2013): 68-75.
- Einsiedel HG, Kawan L, Eckert C, et al. Histone deacetylase inhibitors have antitumor activity in two NOD/SCID mouse models of B-cell precursor childhood acute lymphoblastic leukemia. Leukemia 20 (2006): 1435-1436.
- Stubbs MC, Kim W, Bariteau M, et al. Selective Inhibition of HDAC1 and HDAC2 as a Potential Therapeutic Option for B-ALL. Clin Cancer Res 21 (2015): 2348-2358.