Two Cases of Pneumocystis In Patients Treated with Osimertinib
Article Information
Foch Emilie*, Masse Laurie, Allou Nathalie, Moreau Diane, Andre Michel
Pneumologie, Centre Hospitalier Universitaire Felix Guyon, Allée des Topazes, Saint Denis, France
*Corresponding Author: Foch Emilie, Pneumologie, Centre Hospitalier Universitaire Felix Guyon, Allée des Topazes, Saint Denis, France
Received: 21 January 2020; Accepted: 14 April 2020; Published: 24 May 2021
Citation: Foch Emilie, Masse Laurie, Allou Nathalie, Moreau Diane, Andre Michel. Two Cases of Pneumocystis In Patients Treated with Osimertinib. Archives of Clinical and Medical Case Reports 5 (2021): 479-481.
Share at FacebookKeywords
Lung adenocarcinoma; Osimertinib; Pneumocystis
Article Details
1. Background
The first-line treatment in patients with advanced lung adenocarcinoma and EGFR-activating mutation is osimertinib 80 mg/day [1]. In patients with advanced adenocarcinoma of the lung and EGFR exon 20 mutation, osimertinib 160 mg/day can be given off-label after at least one line of treatment [2]. Here we report two cases of pneumocystis in patients treated with osimertinib.
2. Case Report
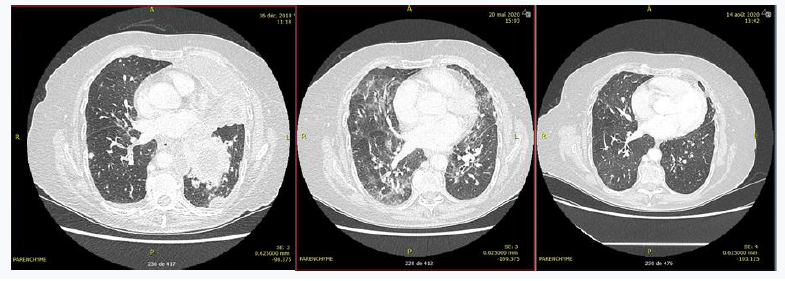
Patient 1 was a 78-year-old patient treated for stage IVB adenocarcinoma of the lung with EGFR-activating mutation. First-line treatment with osimertinib 80mg/day was initiated on day 10 (12/26/2019), followed by osimertinib 40 mg/day on day 52 (renal and cutaneous side effects were observed). The patient was hospitalized on day 162 for dyspnea. A thoracic CT scan showed ground-glass interstitial lung disease (Figure 1). Biological results confirmed the presence of grade 2 lymphopenia (with a nadir of 0.7 G/L), which had appeared at day 40. Bronchoalveolar lavage was positive by PCR for Pneumocystis carinii. HIV serology was negative. The patient was treated with corticosteroids 1mg/kg with gradual decrease and cotrimoxazole, but developed digestive and cutaneous side effects. She was then treated with wellvone, which led to clinical and radiological improvement (Figure 1).
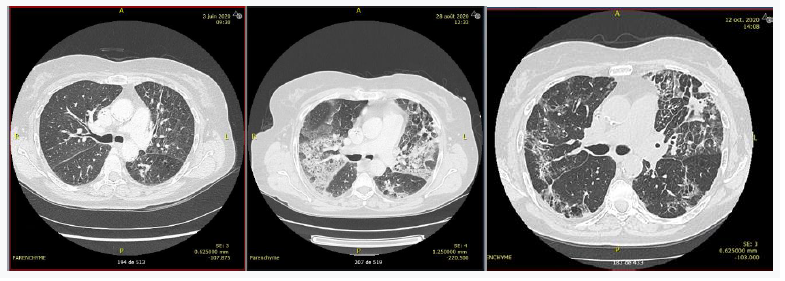
Patient 2 was a 76-year-old patient treated for stage IVA adenocarcinoma of the lung with EGFR exon 20 mutation. Third-line treatment with osimertinib 160 mg/day was initiated on day 53. The patient was hospitalized on day 84 for dyspnea. A thoracic CT scan showed ground-glass interstitial lung disease (Figure 2). Biological results showed grade 0 lymphopenia (with a nadir of 1.09 G/L), which was attributed to the use of osimertinib. Induced sputum was positive by PCR for Pneumocystis carinii. HIV serology was negative. The patient was treated with corticosteroids 1 mg/kg with gradual decrease and cotrimoxazole, but developed neurological side effects. She was then treated with atovaquone, which led to clinical and radiological improvement (Figure 2).
3. Discussion
In the FLAURA and AURA studies (AURA3, AURAex, AURA2, and AURA1), lymphopenia as a side effect of osimertinib was found in 67% of included patients, of whom 7.2% had grade 3 lymphopenia [3]. No other cases of pneumocystis in patients treated with osimertinib were found in the literature or in public databases [4-5]. These two cases raise the question of prophylaxis for pneumocystis in patients with lymphopenia due to osimertinib.
References
- Soria J-C, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 378 (2018): 113-125.
- Piotrowska Z, et al. - ASCO® 2020 – Abs.#9513.
- Summary of product characteristics – Osimertinib.
- Public drug database. available at http://basedonnees-publique.medicaments.gouv.fr/
- Hematox®. available at http://www.biourtox.com/Mediquick7/Abstract.cfm?id_dci=1268