Treatment of Systemic Inflammation with Itolizumab: A Single Center Experience
Article Information
Lazaro M Filgueira1, Armando Caballero2, Julio Betancourt1, Carlos Hidalgo1, Naivy Sanchez1, Ariam Senrra1, Delvis Gonzalez1, Yaumara Aguilera1, Geidy Lorenzo3, Meylan Cepeda3, Danay Saavedra3, Ana Laura Ane4, Irinia Valdivia5, Carmen Valenzuela3, Patricia Lorenzo Luaces3, Zaima Mazorra3, Mayra Ramos3, Kalet Leon3, Tania Crombet3*
1Manuel “Piti” Fajardo Rivero Hospital”, Santa Clara, Cuba
2Arnaldo Milián Castro University Hospital, Santa Clara, Cuba
3Center of Molecular Immunology (CIM), Havana, Cuba
4Institute of Basic and Preclinical Science Victoria de Giron, Havana, Cuba
5Center of Immunoassay, Havana, Cuba
*Corresponding Author: Tania Crombet Ramos, Center of Immunoassay, Havana, Cuba
Received: 03 February 2021; Accepted: 19 March 2021; Published: 20 May 2022
Citation: Lazaro M Filgueira, Armando Caballero, Julio Betancourt, Carlos Hidalgo, Naivy Sanchez, Ariam Senrra, Delvis González, Yaumara Aguilera, Geidy Lorenzo; Meylán Cepeda, Danay Saavedra, Ana Laura Ane, Irinia Valdivia, Carmen Valenzuela, Patricia Lorenzo Luaces; Zaima Mazorra; Mayra Ramos, Kalet Leon, Tania Crombet. Treatment of Systemic Inflammation with Itolizumab: A Single Center Experience. Archives of Clinical and Medical Case Reports 6 (2022): 442-451.
Share at FacebookAbstract
A hypercytokinemia ending in pulmonary and systemic illness occurs in severe COVID-19 patients. Mature T lymphocytes express CD6, a molecule that regulates antigen specific responses of T cells. Itolizumab is an anti-CD6 antibody, which inhibits the proliferation of naïve T-cells and reduces the secretion of pro-inflammatory cytokines. The objective of this study was to preliminary assess the safety and impact of itolizumab on interleukin 6, seroconversion and clinical outcome of 40 COVID-19 patients treated at the Manuel Fajardo Hospital in Santa Clara, Cuba. Itolizumab was used in combination with other drugs as part of an expanded access trial. The protocol was registered in the Cuban Registry for Trials (http://rpcec.sld.cu/trials/RPCEC00000311-En) in April 2020. Median age was 78.5 and almost all subjects had at least one condition predisposing to higher mortality. Thirteen patients received a single infusion, 24 received 2 doses and 3 patients needed 3 itolizumab infusions. Time from hospital admission to trial entry was 13, 5 and 1 day for critical, severe and moderate patients. The antibody was safe and did not increase lymphopenia. Post-itolizumab secondary infections were only seen in patients with lengthy ICU stay. Interleukin-6 decreased in critical patients with high concentration and did not augment in moderate and severe individuals. IgG seropositivity against the SARS-CoV-2 nucleocapside peptides increased on time. In spite of their poor prognosis, 29 out of 40 patients (72.5%) successfully recovered. Although this was not a randomized study, preliminary data suggest that itolizumab reduced the death probability in comparison to observed matched controls.
Keywords
COVID-19; Itolizumab; Cytokine release syndrome; Monoclonal antibody; SARS-CoV2
COVID-19 articles; Itolizumab articles; Cytokine release syndrome articles; Monoclonal antibody articles; SARS-CoV2 articles
Article Details
1. Introduction
Cytokine storm is a complication of SARS-CoV-2 infection that occurs in 10-20% of patients. It is associated with significant lethality [1]. Monocytes and macrophages but also dysregulated T cell responses may contribute to the cytokine release syndrome [2]. Several authors have reported lymphoid infiltrate at the necropsies of COVID-19 [3] while others have found CD4-T cells able to secrete large ex-vivo pro-inflammatory cytokines in critically ill patients [2]. CD6 is a surface glycoprotein expressed on mature T lymphocytes, medullary thymocytes and B1 cells among other hematopoietic cells [4]. Interactions between CD6 and CD6 ligands regulate antigen specific and autoreactive responses of human T lymphocytes [4]. CD6 activation triggers the release of inflammatory cytokines like tumor necrosis factor alpha (TNFα), Interleukin (IL) 1 and IL-6 [5]. Itolizumab is a humanized anti-CD6 Monoclonal Antibody (MAb) binding domain 1 of CD6 with high affinity [6]. It blocks an important co-stimulatory pathway, leading to inhibition of proliferation of naïve T-cells and to a marked reduction of pro-inflammatory cytokines [7]. Itolizumab does not induce in vitro apoptosis, complement or antibody dependent cell cytotoxicity in peripheral blood mononuclear cells from normal donors [8]. The humanized molecule was not immunogenic in monkeys or humans [8]. The antibody has been previously used for the treatment of several autoimmune diseases including psoriasis, rheumatoid arthritis and type 1 diabetes [7]. The safety and efficacy of itolizumab in psoriasis was evaluated in several trials in Cuba and India. In Cuba, a compassionate study was done in 80 subjects with moderate-to-severe disease. After 1 year, the Psoriasis Area and Severity index (PASI)-75 was 60% when using itolizumab at a dose of 1.6 mg/kg for 6 months. In India, at least 2 randomized studies were carried out in the same indication. In the phase II and III trials, the week-12 PASI-50 were 72.5% and 67%, respectively, in subjects with baseline PASI ≥10 [9, 10]. Overall, itolizumab was safe and the main related adverse events were infusion reactions including chills, fever, nausea and headache [9, 10]. No opportunistic infections were observed in Cuba [7] while, in India, there was no activation of latent tuberculosis and patients did not experience severe infections during or after treatment [9]. We speculate that itolizumab can impact on key aspects beneath COVID-19 pathogenesis by inhibiting the overactivation of effector T cells, the secretion of multiple cytokines, by hindering migration of effector T cells into the lung and other infected tissues and by skewing the repertoire toward a regulatory phenotype [11]. Considering this rational, an expanded access trial with itolizumab was approved by the National Regulatory Agency. This manuscript describes the results of treating 40 patients with itolizumab at the leading investigational site.
2. Material and Methods
This was a single-arm expanded access trial, where patients of any gender or skin color, older than 18 years, diagnosed with SARS-CoV2 by RT-PCR were recruited. In addition, subjects should have at least one of the following conditions: confirmed multifocal interstitial pneumonia, need for oxygen support to keep saturation of oxygen (SaO2) >93%, worsening of lung involvement, dyspnea, increased respiratory frequency, partial arterial oxygen pressure <65 mm Hg, worsening of the radiological image, persistent fever, reduction of initial values of hemoglobin, platelets or neutrophils, increase of the initial values of triglycerides or triglycerides greater than 3 mmol/L, increase of the baseline ferritin or absolute ferritin ≥ 2000 ng/ml, amino aspartate transferase ≥30 IU/L, increase in dimer D, fibrinogen < 2.5 g/L or onset of neurological manifestations. Severely-ill subjects had clinical signs of pneumonia plus one of the following: respiratory rate > 30, respiratory distress or SaO2 < 93% on room air, while critically-ill individuals had shock, multiple organ failure or required mechanical ventilation. Moderate patients were those with disease symptoms but no signs of severe pneumonia and SaO2 ≥ 93% on room air. Only moderately ill patients with high-risk of aggravation were included in the trial. Risk factors for worsening were age ≥ 65 and comorbidities associated with COVID-19 mortality: hypertension, cardiovascular disease, diabetes mellitus, chronic kidney disease, cancer, chronic obstructive pulmonary disease (COPD), obesity and nutrition deficit. Itolizumab was presented in sterile and pyrogen-free solution for intravenous infusion and formulated at 5 mg/mL (25 mg/vial). The administered dose was 200 mg diluted in 200 mL of saline solution at 0.9%. Treatment was administered by intravenous route during 2 hours, under hospital conditions. Adverse reactions were defined as those unfavorable medical signs or symptoms or unintended responses probably, possibly or definitively related to the antibody. Reactions were classified by the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 published on November 27, 2017. The oxygenation index (PO2/FiO2 ratio), the quick Sequential Organ Failure Assessment qSOFA) score [12], and the Acute Physiology and Chronic Health Evaluation II (APACHE II) scale [12] were used to evaluate all critical and severe patients at baseline and at 72 and 168 hrs post-itolizumab.
Interleukin 6 (IL-6) was measured at baseline and 48 hrs after itolizumab by using a Human IL-6 Quantikine ELISA Kit from R&D Systems (Cat# S6050, Minneapolis, USA). IgG antibodies to SARS-CoV-2 were evaluated by using a qualitative, indirect ultra-micro ELISA (UMELISA SARS-CoV-2) developed by the Center of Immunoassay. The assay uses synthetic peptides of the immunodominant regions of the virus's nucleocapsid protein as capture antigen. The UMELISA SARS-CoV-2 sensitivity and specificity was 71.6% and 89.3%, respectively, in serum samples taken after the 7th day of symptoms. Seroconversion was evaluated at baseline and after 5 to 7 days of itolizumab infusion. The trial was approved by the Institutional Review Board (IRB) of the Pedro Kouri Institute, and also by the IRB of the Manuel Fajardo Hospital, the main inclusion site. The study was accepted by the National Regulatory Agency, the State Center for Drug Quality Control (CECMED). Subjects with intact mental consciousness gave informed consent prior to itolizumab treatment. Patients sedated at trial entry provided consent upon recovery. A legal representative granted the approval for patients with cognitive problems. The protocol was registered in the Cuban Registry for Clinical Trials (http://rpcec.sld.cu/trials/RPCEC00000311-En). In addition, to preliminary assess the impact of itolizumab on survival, a control group was selected among the Cuban COVID-19 patients, who received the rest of the drugs included in the national protocol, but not immunomodulatory agents, according the national database closed on May 15, 2020. All patients treated at the ICU without biologics were selected (n=57). In addition, all patients older than 65, hospitalized at the conventional ward were chosen (n=32). Control and treated patients were matched regarding sex, age and comorbidities associated with higher COVID-19 mortality [13]. Odds ratio (OR) for death was estimated for control vs itolizumab. The number of patients needed to treat (NNT) to prevent an additional poor outcome (death event) was also calculated. Descriptive statistics was used to analyze the data set. The analyses were made with the SPSS version 25.0.
3. Results
Forty patients (16 male and 24 female) were treated with itolizumab at the Manuel Fajardo Hospital, in the Villa Clara province. Sixteen patients (9 critical and 7 severe) were treated at the intensive care unit (ICU) while 24 patients with moderate illness and poor prognosis received therapy at the regular ward. Median age was 78.5 (28 to 100) and 87.5% had at least one associated primary condition. Most common comorbidities were hypertension, cardiovascular disease, diabetes mellitus, nutrition deficit, Chronic Obstructive Pulmonary Disease (COPD) or bronchial asthma, chronic kidney disease and hypothyroidism (Table 1). Six patients (15%) have toxic habits (smoking or alcoholism). Nineteen of the 24 moderate patients (~ 80%) were enrolled after a local transmission event. Median age of the moderate patients was 76.5 years.
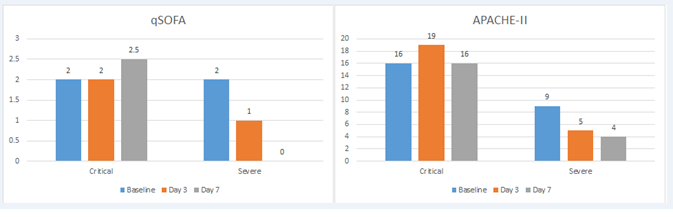
All patients at the ICU required oxygen support including 8 critically-ill who were on mechanical ventilation. Nine moderately ill subjects (43%) also needed oxygen supplement at the general ward. Most frequent symptoms at hospital admission were fever, dyspnea and dry cough. Patients were treated by the national protocol for COVID-19 including lopinavir/ritonavir, chloroquine and interferon α2b. Time from hospital admission to trial entry was 13, 5 and 1 day for critical, severe and moderate patients. For critical and severe cases, time from ICU admission and trial enrolment was 5 days and 1 day, respectively. Thirteen patients received a single infusion of the antibody, 24 subjects received 2 doses and 3 patients needed 3 itolizumab doses, at the discretion of the treating physicians. qSOFA, APACHE and oxygenation index (PO2/FiO2) were evaluated for patients treated at the ICU. Three days after itolizumab, the oxygenation index improved in 60% of the critical patients and in all severe subjects. Only 3 additional patients (one critical and 2 severe at baseline) required new mechanical ventilation after itolizumab. As expected, qSOFA and APACHE-II were different at baseline between severe and critical patients. After itolizumab, median qSOFA and APACHE-II scores decreased for severe but not for critical subjects. Patient’s outcome at 3 and 7 days according qSOFA and APACHE-II is shown in figure 1. Nine patients (6 critical, 2 severe and 1 moderate) of the 40, died by day 14 of trial entry. By day 28, 11 patients died (7 critical, 2 severe and 2 moderate). There was a direct correlation between disease severity at baseline and lethality. The 14-day lethality rate was 22.5% while the 28-day lethality rate was 27.5%.
The mortality risk at day 28 of observed control patients, not receiving immunomodulatory drugs, was compared with the itolizumab series. Both groups were homogeneous in terms of demographics and significant comorbidities, except for nutrition deficit that was more frequent in itolizumab individuals (Table 1). The 28-day lethality rate in the control group was 43.8%. Controls had higher mortality risk (OR 2.05, 95% CI 0.914, 4.625) than itolizumab patients. Every 6 patients treated with itolizumab, one death was prevented (p=0.05, test χ2, NNT: 6.18). An additional analysis was done for treated and control patients with 2 or more comorbidities. The 28-day lethality rate was 56.4 and 28.6% for control and itolizumab subjects, respectively. Controls had greater death probability than patients treated with itolizumab: OR 3.23 (95% CI 1.215, 8.586). Every 4 patients treated with itolizumab, one death was prevented (p=0.009, test χ2, NNT: 3.59). Interleukin 6 was evaluated before and after 48 hrs of itolizumab. Median IL-6 was significantly higher in critical patients as compared to severe and moderate. Two days after the antibody, IL-6 decreased in critical while it did not significantly vary in severe and moderate patients. The baseline median values for critical, severe and moderate patients corresponded to 334, 31.6 and 19.1 pg/ml, respectively. Two days after the antibody, IL-6 concentration was 214, 22.45 and 18.27 pg/ml for the corresponding severity groups. The antibody response against the SARS-CoV2 nucleocapsid peptides was evaluated before and 5 to 7 days after itolizumab. Within a week, antibody positivity increased from 40.6% to 68%, following antibody administration. The anti-virus antibody titles increased 4 folds in the critically ill vs. 2 folds in severe and moderately ill patients. Only 2 patients had related adverse events consisting on mild chills and fever. No serious adverse events were reported. Grade 2 lymphopenia (lymphocyte count < 0.5x109L) was detected in only 2 patients, 7 days after itolizumab. An extensive characterization was done on the secondary infections in patients treated at the ICU with itolizumab. Among the 16 patients, 7 patients had bacterial or fungal infections before itolizumab while only 3 individuals developed sepsis after the antibody. Before the antibody, the most frequent microorganisms were klebsiella pneumoniae, escherichia coli and pseudmona aeruginosa. After itolizumab the most common germs were acinetobacter, pseudomona aeruginosa, escherichia coli and enterobacter aerogenes. Secondary infections were associated with prolonged hospitalization at the ICU.
|
Itolizumab |
Control |
χ2 |
||||
|
N=40 |
N=89 |
P-value |
||||
|
Freq |
Freq |
Freq |
% |
|||
|
Sex |
F |
24 |
60% |
42 |
47.50% |
0.309 |
|
M |
16 |
40% |
47 |
52.80% |
||
|
Age group |
19-39 |
1 |
2.50% |
1 |
1.1%% |
0.803 |
|
40-64 |
8 |
20% |
16 |
18% |
||
|
65 or more |
31 |
77.50% |
72 |
80.90% |
||
|
Number of comorbidities |
0-1 |
12 |
30% |
34 |
38.20% |
0.368 |
|
2 or more |
28 |
70% |
55 |
61.80% |
||
|
Hypertension |
30 |
75% |
77 |
77% |
0.552 |
|
|
Diabetes mellitus |
16 |
40% |
38 |
42.70% |
0.464 |
|
|
Cardiovascular diseases |
19 |
47.50% |
30 |
33.70% |
0.098 |
|
|
COPD |
6 |
15% |
13 |
14.60% |
0.574 |
|
|
Obesity |
2 |
5% |
3 |
3.40% |
0.494 |
|
|
Chronic kidney disease |
6 |
15% |
13 |
14.60% |
0.574 |
|
|
Nutrition deficit |
12 |
30 |
1 |
1.10% |
0 |
|
Table 1: Baseline characteristics of patients treated with itolizumab vs. controls not receiving immunomodulatory drugs.
4. Discussion
The majority of COVID-19 patients display minor symptoms and have a good prognosis. However, 15 to 20% of the cases progress to severe disease, particularly older individuals with underlying comorbidities [1]. The role of activated of T cells in COVID-19 pathogeny is not fully understood [2]. Zhou et al, found larger subsets of IL-6 and GM-CSF secreting CD4 T cells in severe as compared to mild patients [14]. Monocytes and macrophages release IL-6 and other cytokines to invigorate the innate immune response. Then, cytokines recruit T cells which further stimulate a pro-inflammatory response, amplifying the cascade [2]. The detection of high levels of other inflammatory cytokines like TNFα, IFN-γ and IL-2 also suggests a possible pathogenic role of CD4 T cells [2]. In this scenario, the use of itolizumab, a humanized antibody targeting a key checkpoint for regulating effector T-cells, is very appealing. This manuscript describes the results of using itolizumab in 40 patients in a single hospital. The antibody was safe and did not increase lymphopenia. Post-itolizumab secondary infections were only seen in patients with lengthy ICU stay. These results are compatible with previous findings in psoriasis and rheumatoid arthritis, and represent a large advantage as compared to other biologics [7]. Anti-inflammatory drugs, including steroids and anti-IL-6 receptor (IL-6R) or TNFα antibodies can reduce tissue harm but augment the risk of sepsis [15]. In COVID-19, IL-6 can have either deleterious or positive effects, depending on the infection phase and host response [16]. IL-6 is the main mediator of the hyperinflammatory response but also modulates the B cell differentiation and IgG production [16]. B cell response is crucial for controlling viral replication [17]. IL-6 decreased in critical patients with very high concentration and did not augment in moderate and severe individuals, which showed lower basal levels, albeit high. One possible interpretation is that the anti-CD6 antibody prevented the exacerbation of the cytokine release syndrome.
In our series, IgG seropositivity against the SARS-CoV-2 nucleocapside peptides increased on time. According Keam et al, the level of antibodies against the nucleocapside or the receptor binding domain, correlated with their neutralizing capacity [17]. So, despite reducing or stabilizing IL-6 level, itolizumab did not delay the antibody response, and all surviving subjects had a negative viral load upon clinical recovery. Immune-senescence and chronic inflammation predispose to cytokine storm and death due to COVID-19 [16]. In our series, roughly 50% of the patients were older than 80 and have important associated conditions. In spite of their poor prognosis, 29 of 40 patients (72.5%) successfully recovered after using itolizumab. The antibody enhanced the oxygenation index of severe and critical patients. However, q-SOFA and APACHE-II deteriorated in critical patients. Lethality was also high in this patient subset. Overall, critical patients were treated after 5 days on mechanical ventilation and 13 days of hospital admission, presumably at a moment when hipercytokinemia might have permanently damaged the respiratory, cardiovascular, urinary or gastrointestinal tract. Although this was not a randomized study, preliminary data suggest that itolizumab reduced the death probability in comparison to observed matched controls. Control individuals were treated at the same time, with the rest of the drugs included in the national protocol, but without immunomodulatory biologics. Itolizumab administration in patients with 2 or more comorbidities, further decreased mortality.
Other drugs have been evaluated to control the hyperinflammatory response. In a Phase II randomized trial done in a similar population in Brazil, methylprednisolone did not reduce the mortality of patients with moderate, severe or critical disease as compared to a placebo [18]. Overall 28-day mortality was 38.2% in the placebo group vs 37.1% in the experimental arm. In the largest controlled study in 6,000 patients who received the best supportive therapy with or without dexamethasone, the mortality rate was 41.4% for control patients who required mechanical ventilation [19]. In another controlled trial in 3,924 critical individuals, 40.6% of patients who did not receive therapy with the anti-IL-6R MAb tocilizumab died [20]. In the referred studies, the mortality rate was reduced by 10% after the use of dexamethasone or tocilizumab, respectively [19,20]. In summary, this 40 patients study showed that itolizumab was well tolerated and did not seem to increase the risk of opportunistic infections. In addition, the antibody reduced or prevented IL-6 increase and did not hamper the humoral response against COVID-19. We conclude that the opportune use of drugs like itolizumab that does not fully inhibit the immune system, but its exaggerated activation, combined with an antivirals and anti-coagulants, could be a good treatment strategy. These preliminary findings will be confirmed in larger clinical trials.
Declarations
Funding
This research was funded by the Center of Molecular Immunology and the Cuban Ministry of Health.
Conflicts of Interest/Competing Interests
Nine authors currently work for the Center of Molecular Immunology, the institution that generated and originally patented itolizumab. The rest of the authors do not have any commercial or financial relationships that could be taken as a potential conflict of interest.
Authors' Contributions
TC and MR designed the clinical trial, informed consent and CRFs of the expanded access trial; LF, AC, JB, CH, AS and DG administered the experimental drug plus SOC and followed the 19 COVID patients at the hospital ICU and general ward; GL and MC did the primary data collection, monitoring and experimental drug control; DZ, ALA, ZM and NS evaluated IL-6 concentration; YA did the microbiology evaluation, IV evaluated patient’s seroconversion against COVID-19; PL and CV did statistical analysis. LF, AC, JB, CH, MR, DS, ZM, KL and TC analyzed and interpreted the clinical and laboratory data. All authors reviewed and approved the final manuscript.
Ethics Approval
The trial was approved by the institutional review board (IRB) of the IRB of the Manuel Fajardo Hospital.
Consent to Participate
Subjects with intact mental consciousness gave informed consent prior to itolizumab treatment. Patients sedated at trial entry provided consent upon recovery. A legal representative granted the approval for patients with cognitive problems.
Consent for Publication
All authors gave consent for publication
Acknowledgments
The authors appreciate the contribution of all medical staff that supported this clinical investigation and treated COVID-19 patients.
References
- Mayor. Intensive immunosuppression reduces deaths in covid-19-associated cytokine storm syndrome. study finds BMJ 370 (2020).
- N Vabret, GJ Britton, C Gruber, et al. Sinai Immunology Review, Immunology of COVID-19: Current State of the Science, Immunity 52 (2020): 910-941.
- M Ackermann, SE Verleden, M Kuehnel, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19, N Engl J Med 383 (2020): 120-128.
- NG Singer, BC Richardson, D Powers, et al. Fox, Role of the CD6 glycoprotein in antigen-specific and autoreactive responses of cloned human T lymphocytes, Immunology 88 (1996): 537-543.
- S Dogra, S Uprety, SH Suresh, et al. a novel anti-CD6 monoclonal antibody: a safe and efficacious biologic agent for management of psoriasis, Expert Opin Biol Ther 17 (2017): 395-402.
- LI Garner, A Hartland, J Breuning, et al CD6 monoclonal antibodies differ in epitope, kinetics and mechanism of action, Immunology 155 (2018): 273-282.
- Hernandez, E Moreno, LE Aira, et al Therapeutic Targeting of CD6 in Autoimmune Diseases: A Review of Cuban Clinical Studies with the Antibodies IOR-T1 and Itolizumab, Curr Drug Targets 17 (2016): 666-677.
- L Roque-Navarro, C Mateo, J Lombardero, et al. Humanization of predicted T-cell epitopes reduces the immunogenicity of chimeric antibodies: new evidence supporting a simple method, Hybrid Hybridomics 22 (2003): 245-257.
- L Budamakuntla, HV Shree-Lakshmi, A Bansal, et al Spotlight on itolizumab in the treatment of psoriasis - current perspectives from India, Psoriasis (Auckl) 9 (2019): 19-27.
- DS Krupashankar, S Dogra, M Kura, et al. Efficacy and safety of itolizumab, a novel anti-CD6 monoclonal antibody, in patients with moderate to severe chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, phase-III study, J Am Acad Dermatol 71 (2014): 484-492.
- S Loganathan, SN Athalye, SR Joshi, et al. an anti-CD6 monoclonal antibody, as a potential treatment for COVID-19 complications, Expert Opin Biol Ther (2020): 1-7.
- P Balasubramanian, N Sharma, M Biswal, et al. Kumar, Critical Illness Scoring Systems: Sequential Organ Failure Assessment, Acute Physiology and Chronic Health Evaluation II, and Quick Sequential Organ Failure Assessment to Predict the Clinical Outcomes in Scrub Typhus Patients with Organ Dysfunctions, Indian J Crit Care Med 22 (2018): 706-710.
- S Richardson, JS Hirsch, M Narasimhan, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, JAMA 323 (2020): 2052-2059.
- Y Zhou, B Fu, X Zheng, et al. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+ CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus (2020).
- A Stallmach, A Kortgen, F Gonnert, et al. Infliximab against severe COVID-19-induced cytokine storm syndrome with organ failure-a cautionary case series, Crit Care 24 (2020): 444.
- EO Gubernatorova, EA Gorshkova, AI Polinova, et al. IL-6: Relevance for immunopathology of SARS-CoV-2, Cytokine Growth Factor Rev 53 (2020): 13-24.
- S Keam, D Megawati, SK Patel, et al. Harapan, Immunopathology and immunotherapeutic strategies in severe acute respiratory syndrome coronavirus 2 infection, Rev Med Virol (2020).
- CMP Jeronimo, MEL Farias, FFA Val, et al. Methylprednisolone as Adjunctive Therapy for Patients Hospitalized With COVID-19 (Metcovid): A Randomised, Double-Blind, Phase IIb, Placebo-Controlled Trial, Clin Infect Dis (2020).
- RC Group, P Horby, WS Lim, et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report, N Engl J Med (2020).
- S Gupta, W Wang, SS Hayek, et al. Investigators, Association Between Early Treatment With Tocilizumab and Mortality Among Critically Ill Patients With COVID-19, JAMA Intern Med (2020).