Transcatheter Closure of a Ragged Post-Myocardial Infarction Ventricular Septal Defect Using an Off-Label ASD Occluder
Article Information
Christine Quast1, Shazia Afzal1, Pia Leuders1, Maryna Masyuk1, Kathrin Klein1, Malte Kelm1,2, Amin Polzin1, Tobias Zeus1*
1Heinrich-Heine-University Düsseldorf, Medical Faculty, Division of Cardiology, Pulmonology and Vascular Medicine, Düsseldorf 40225, Germany
2CARID (Cardiovascular Research Institute Düsseldorf), Moorenstrasse 5, Düsseldorf 40225, Germany
*Corresponding Author: Tobias Zeus, Department of Cardiology, Pulmonology and Vascular Medicine, Heinrich Heine University, Medical Faculty, Moorenstraße 5, D-40225 Düsseldorf, Germany
Received: 13 January 2022; Accepted: 21 January 2022; Published: 10 February 2022
Citation: Christine Quast, Shazia Afzal, Pia Leuders, Maryna Masyuk, Kathrin Klein, Malte Kelm, Amin Polzin, Tobias Zeus. Transcatheter Closure of a Ragged Post-Myocardial Infarction Ventricular Septal Defect Using an Off-Label ASD Occluder. Archives of Clinical and Medical Case Reports 6 (2022): 66-71.
Share at FacebookAbstract
Post-myocardial infarction ventricular septal defect has become a rare event following ST Elevation Myocardial Infarction (STEMI) but is still a life-threatening complication with high mortality. Transcatheter closure of post-myocardial infarction ventricular septal defects has emerged as an alternative therapeutic approach but often requires individual strategies due to complex morphology. We report a case of a 62-year-old man who was admitted to our hospital after STEMI of the inferior wall with pulmonary congestion due to a post-myocardial infarction VSD for interdisciplinary evaluation and therapy. The patient was characterized by estimated high perioperative risk and underwent interventional closure of the VSD according to heart team decision. Because of ragged nature of the VSD closure with a conventional Occlutech™ VSD occluder was not possible. Therefore, we decided to close the defect using an off-lable device for ASD closure which was successfully implanted without relevant residual shunt.
Keywords
ASD occluder; Occluder; Post-myocardial infarction ventricular septal defect; VSD; Transcatheter closure
ASD occluder articles; Occluder articles; Post-myocardial infarction ventricular septal defect articles; VSD articles; Transcatheter closure articles
Article Details
Abbreviations:
ASD: Atrial septal defect; LAO: Left anterior oblique; CRAN: Cranial; LDH: Lactat dehydrogenase; MI: Myocardial infarction; PIVSD: Post-infarction ventricular septum defect; RCA: Right coronary artery; STEMI: ST Elevation myocardial infarction; VSD: Ventricular septal defect
1. Introduction
Although post-myocardial infarction ventricular septal defect (VSD) has become a rare event following ST elevation myocardial infarction (STEMI) it is still a life-threatening complication with high mortality. With conservative management mortality of post-myocardial infarction VSD ranges over 90% [1] and therefore closure should be attempted where possible to improve survival [2]. After the first transcatheter closure of ventricular septal defects in 1988 new vascular occlusion devices were developed and lead to reduced complication rates [3]. Transcatheter closure of post-myocardial infarction ventricular septal defects has emerged as a feasible alternative therapeutic approach [4-7]. Due to complex morphology of post-myocardial infarction VSD [8] interventional closure often requires individual strategies for successful therapy.
2. Case Presentation
A 62-year-old male patient was initially diagnosed with a ST segment elevation myocardial infarction of the inferior wall. He presented with typical and progressive angina pectoris symptoms, dyspnoea at rest and asthenia since previous day. Coronary angiography revealed a proximal occlusion of the right coronary artery which was successfully treated by percutaneous coronary intervention with a coronary stent (2.5x24mm, drug eluting stent). Initial cardiac biomarkers were elevated and indicated an elapsed smouldering myocardial infarction with high-sensitive cardiac Troponin T 547.7 pg/ml (cut-off < 14 pg/ml), Creatine Kinase 605 U/l (< 190 U/l) and LDH 316 U/l (cut-off < 150 U/l). Four days after successful coronary recanalisation the patient developed symptoms of acute cardiac decompensation with incipient pulmonary congestion. A hemodynamically relevant post-myocardial infarction ventricular septal defect was observed by echocardiography. The patient was devoid of need for catecholamines and assigned for interdisciplinary evaluation and therapy. Echocardiographic analysis displayed a ragged and frayed VSD located in the basal to midventricular interventricular septum with relevant left-to-right shunt (Qp:Qs ratio 2.8:1). Due to estimated high perioperative risk precluding for surgical repair interdisciplinary heart team decision yielded a transcatheter closure of the hemodynamically relevant post-myocardial infarction VSD. Transcatheter VSD closure was performed eleven days after coronary angioplasty to allow for early myocardial remodeling following myocardial infarction and prevent hemodynamic deterioration of the patient at the same time. After detailed informed consent the patient was put under mild conscious sedation. Angiography with simultaneous and continous transoesophageal echocardiography imaging was performed. After VSD sizing (13mm x 13mm) a 9 French venous sheath over an arterio-venous-circuit-wire was placed and an Occlutech™ Muscular VSD Occluder 18mm (Occlutech International AB, Helsingborg, Sweden) was applied. Due to a ragged and fissured margin, stable positioning of the VSD occluder was not accomplished. Therefore, the interventional approach was revised, adjusted and an Occlutech™ Figulla® Flex II ASD Occluder 19.5mm was successfully implated sealing the VSD reducing the shunt immediately from 2.8 to 1.3. The patient recovered fast and was discharged at home after complete recompensation five days after transcatheter VSD closure without heart block or any other adverse events.
3. Discussion
Transcatheter closure of post-myocardial infarction ventricular septal defects has emerged as an accepted and feasible alternative therapeutic approach [4-7]. Though, individual therapeutic strategies are necessary for sufficient closure adressing time of intervention and technique [9]. Timepoint for closure is controversially discussed. We observed an increasing congestive hepatopathy as a sign of therapy-refractory right heart failure with a need for early improvement. In our case we decided to close the VSD eleven days after STEMI to benefit from initiated myocardial remodeling processes and early hemodynamic improvement of the patient to avoid clinical impairment and decrease mortality. Closure in the chronic phase after myocardial infarction (>14 days after MI) seems to be characterized by lower morbidity and mortality and a higher rate of complete closure [5]. Scarring at the edges of the defect has to be taking into account as beneficial feature for successful closure [10]. Post-myocardial infarction ventricular septal defects are highly variable between patients and also vary in size and shape during the cardiac cycle [8] which determines suitability of the device. As in our case, RCA post-myocardial infarction VSDs tend to be more complex potentially because of more interdigitating fibers in the posterior interventricular groove [11] or the orientation of myocardial fibers to direct intramyocardial dissection along the inferior heart [8] and require individual decision-making in therapy. There are reported successful VSD closures using a VSD occluder device [8, 9, 12]. In our case, stable positioning of an Occlutech™ VSD Occluder was not accomplished due to anatomical requirements. We therefore applied an Occlutech™ ASD Occluder 19.5 mm with sufficient closure. There are only a few reports on individual cases with off-lable use of ASD occluders in VSD position after unsuccessful surgical closure [13] or very large post-infarction VSD [14]. In line with this, our case report highlights the necessity for individual decision-making depending on morphology in a ragged post-myocardial infarction VSD.
4. Conclusion
This case report highlights the necessity for individual decision-making in therapy depending on morphology of the post-myocardial infarction VSD. After careful risk-benefit assessment off-lable use and implantation of an ASD occluder may lead to therapeutic success in a ragged post-myocardial infarction VSD with high morphological complexity.
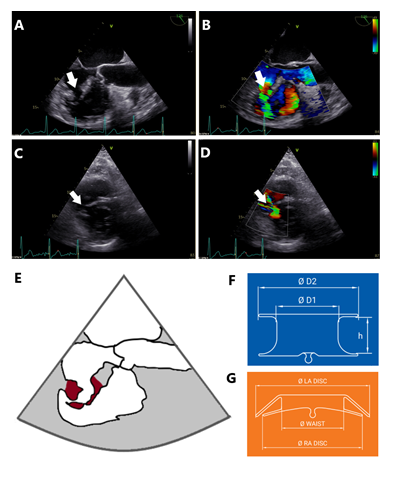
Figure 1: Preprocedural VSD evaluation. (A) Transesophageal echocardiography, angle of sounding 126°, reveals a ragged VSD (arrow) with local intramyocardial dissection. (B) Colour doppler indicates left-to-right shunt across the ventricular septal defect (arrow). (C) Transthoracic echocardiography, parasternal short axis view at midventricular level shows the VSD with finger-shaped ragged margin of the VSD (arrow). (D) Short axis view with colour doppler, left-to-right shunt across VSD is characterised by two jets due to intramyocardial dissection (arrow). (E) Schematic drawing corresponding to (A), dark red indicates tissue of the ventricular septum being involved in ragged VSD. (F) Schematic drawing of Occlutech™ VSD Occluder (15), D1=diameter of the waist, D2=diameter of the discs, h=height of the waist. (G) Schematic drawing of Occlutech™ Figulla® Flex II ASD Occluder (16), LA=left atrium, RA=right atrium. In contrast to the VSD occluder in F, the ASD occluder was able to grasp and stabilize ragged and dissected parts of the VSD by its tilted disc.
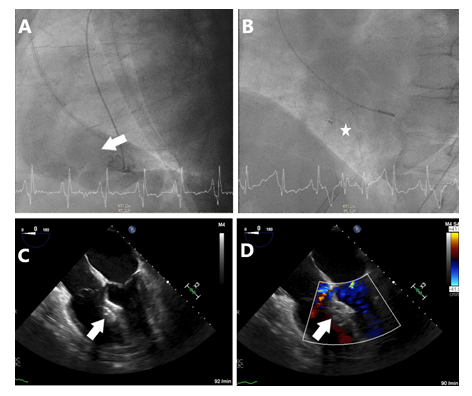
Figure 2: Periprocedural fluoroscopy and transesophageal echocardiography. (A) LAO 87.9°, CRAN 0.2°, Ventriculogram with pigtail catheter in left ventricle and hemodynamically relevant left-to-right shunt across VSD (arrow). (B) LAO 52.4°, CRAN 1.3° fluoroscopy depicting the position of the Occlutech™ ASD occluder. (C) Transesophageal echocardiography, 4-chamber view at 0° after successful implantation of ASD occluder (arrow). (D) 4-chamber view with colour doppler, ASD occluder without relevant residual shunt.
Authors’ Contributions
Made substantial contributions to conception and drafting: C. Quast, P. Leuders, A. Polzin, M. Kelm, T. Zeus. Made substantial contributions to data acquisition, analysis and supervision: S. Afzal, M. Masyuk, K. Klein
Disclosures
All authors have nothing to disclose.
Funding
No funding.
Conflicts of Interest
All authors declared that there are no conflicts of interest.
References
- Omar S, Morgan GL, Panchal HB, et al. Management of post-myocardial infarction ventricular septal defects: A critical assessment. Journal of interventional cardiology 31 (2018): 939-948.
- Trivedi KR, Aldebert P, Riberi A, et al. Sequential management of post-myocardial infarction ventricular septal defects. Archives of cardiovascular diseases 108 (2015): 321-330.
- Morray BH. Ventricular Septal Defect Closure Devices, Techniques, and Outcomes. Interventional cardiology clinics 8 (2019): 1-10.
- Ferraioli D, Santoro G, Bellino M, et al. Ventricular Septal Defect Complicating Inferior Acute Myocardial Infarction: A Case of Percutaneous Closure. Journal of cardiovascular echography 29 (2019):17-19.
- Sabiniewicz R, Huczek Z, Zbronski K, et al. Percutaneous Closure of Post-Infarction Ventricular Septal Defects-An Over Decade-long Experience. Journal of interventional cardiology 30 (2017): 63-71.
- Schlotter F, de Waha S, Eitel I, et al. Interventional post-myocardial infarction ventricular septal defect closure: a systematic review of current evidence. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 12 (2016): 94-102.
- Evans MC, Steinberg DH, Rhodes JF, Tedford RJ. Ventricular septal defect complicating delayed presentation of acute myocardial infarction during COVID-19 lockdown: a case report. European heart journal Case reports 5 (2021): 27.
- Hamilton MCK, Rodrigues JCL, Martin RP, et al. The In Vivo Morphology of Post-Infarct Ventricular Septal Defect and the Implications for Closure. JACC Cardiovasc Interv 10 (2017): 1233-1243.
- Turner MS, Hamilton M, Morgan GJ, et al. Percutaneous Closure of Post-Myocardial Infarction Ventricular Septal Defect: Patient Selection and Management. Interventional cardiology clinics 2 (2013): 173-180.
- Shafiei I, Jannati F, Jannati M. Optimal Time Repair of Ventricular Septal Rupture Post Myocardial Infarction. Journal of the Saudi Heart Association 32 (2020): 288-294.
- Becker AE, Caruso G. Myocardial disarray. A critical review. British heart journal 47 (1982): 527-538.
- Faccini A, Butera G. Techniques, Timing, and Prognosis of Transcatheter Post Myocardial Infarction Ventricular Septal Defect Repair. Curr Cardiol Rep 21 (2019): 59.
- Boi A, Cocco D, Sanna F, et al. Post-Myocardial Infarction Ventricular Septal Defect Closure by a Percutaneous Septal Occluder Device After Unsuccessful Surgical Closure: Never Lose Hope. Cardiovascular revascularization medicine : including molecular interventions 21 (2020): 65-68.
- Long A, Talreja A, Baran D, et al. Percutaneous Closure of a Large Postinfarct Ventricular Septal Defect With an Atrial Septal Defect Closure Device in a High Surgical Risk Patient. J Invasive Cardiol 33 (2021): 485-486.
- Occlutech pmVD occluder. https://www.occlutech.com/files/brochure/Occlutech_PmVSD_Br.pdf
- Occlutech ASD occluder. https://www.occlutech.com/files/brochure/Occlutech_ASD_Br.pdf