Thrombotic Microangiopathy Secondary to COVID-19 in a Recent ABO-Incompatible Kidney Transplant
Article Information
Zaira Castaneda1, Joana Sellares1, Irina B Torres1, Manel Perello1, Maria Meneghini1, Ignacio Cidraque1, Ester Marquez-Algaba2, Oscar Len2, Veronica Pons3, Rafael Parra3, Juliana Esperalba4, Laura Donadeu5, Oriol Bestard1, Francesc Moreso1*
1Nephrology department, Vall d'Hebron University Hospital, Barcelona, Spain
2Infectious disease department, Vall d'Hebron University Hospital, Barcelona, Spain
3Blood bank department, Vall d'Hebron University Hospital City, Barcelona, Spain
4Microbiology department, Vall d'Hebron University Hospital, City, Barcelona, Spain
5Nephrology and Transplantation Experimental Laboratory, Vall d’Hebron Institute of Research (VHIR), Barcelona, Spain
*Corresponding Author: Francesc Moreso, Nephrology department, Vall d'Hebron University Hospital, Barcelona, Spain.
Received: 19 January 2022; Accepted: 16 February 2022; Published: 04 March 2022
Citation: Zaira Castaneda, Joana Sellares, Irina B Torres, Manel Perello, Maria Meneghini, Ignacio Cidraque, Ester Marquez-Algaba, Oscar Len, Veronica Pons, Rafael Parra, Juliana Esperalba, Laura Donadeu, Oriol Bestard, Francesc Moreso. Thrombotic Microangiopathy Secondary to COVID-19 in a Recent ABO-Incompatible Kidney Transplant. Archives of Clinical and Medical Case Reports 6 (2022): 173-181.
Share at FacebookAbstract
De novo Thrombotic Microangiopathy (TMA) after renal transplantation is an uncommon complication which has been related with viral infections like the coronavirus disease (COVID-19). Severely immunosuppressed patients who acquire COVID-19 are at high risk of serious complications that should be carefully managed. We report a case of biopsy-proven TMA secondary to COVID-19 associated with Acute Respiratory Distress Syndrome (ARDS) in a recent ABO-incompatible kidney transplant. Eculizumab was initiated; and after 4 weekly doses, she recovered her renal function. Two months later, she was readmitted due to suspected organizing pneumonia, and received treatment with steroids. During the second week of admission, ARDS and TMA with neurological involvement were observed. Positive tests in blood and broncho-alveolar lavage for SARS-CoV-2 were obtained. A new cycle of eculizumab was started and convalescent plasma was associated due to persistent infection. The patient experienced progressive clinical improvement with resolution of respiratory function, neurological symptoms and hemolysis. Our case confirms that TMA could be triggered by COVID-19 and eculizumab is useful to control hemolysis and attenuate organ damage. Convalescent plasma should be considered in immune deficient high-risk patients with COVID-19.
Keywords
ABO incompatible kidney transplant; Convalescent plasma; COVID- 19; Eculizumab; Thrombotic microangiopathy
ABO incompatible kidney transplant articles; Convalescent plasma articles; COVID- 19 articles; Eculizumab articles; Thrombotic microangiopathy articles
Article Details
Abbreviations:
ARDS: Acute respiratory distress syndrome; aHUS: Atypical uremic hemolytic syndrome; ABOi: ABO-incompatible; BAL: Bronchoalveolar lavage; COVID-19: Coronavirus disease 2019; DM: Diabetes mellitus; HTN: Hypertension; NAAT: Nucleic acid amplification test; SCr: Serum creatinine; Tx: Transplant; TMA: Thrombotic microangiopathy
1. Introduction
Thrombotic microangiopathy (TMA) is an uncommon complication after solid organ transplantation that usually develops in the early post-transplant period. It can occur as a recurrent atypical hemolytic uremic syndrome (aHUS) or as de novo TMA [1]. Since the new coronavirus disease 2019 (COVID-19) outbreak, many complications have been described. Few reports of long-term kidney transplant patients that developed TMA-associated to COVID-19 have been published in the literature [2, 3]. We report a case of biopsy-proven TMA secondary to COVID-19 in a recent ABO-Incompatible (ABOi) kidney transplant recipient who developed neurological involvement.
2. Case Report
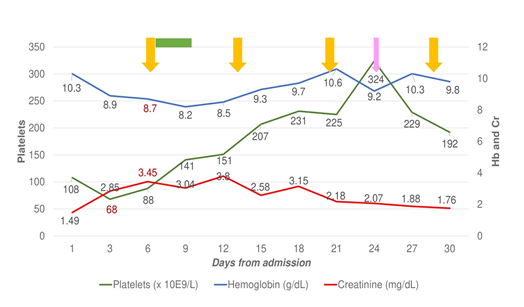
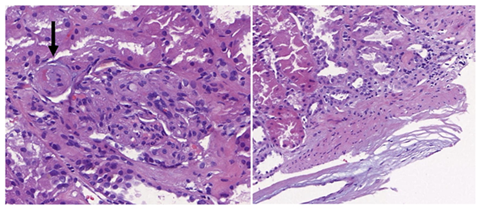
We present a 35-year-old woman with end-stage chronic kidney disease secondary to Schönlein-Henoch purpura who received a preemptive ABOi kidney transplant from her father. No features of TMA were observed before transplantation. Due to high anti-A isoagglutinin titers (1/512), a total of 10 immunoadsorption sessions and 2 doses of Rituximab (375 mg/m2) were required for desensitization. Induction was with basiliximab and maintenance with tacrolimus, mycophenolate and corticosteroids. The evolution after transplant was unremarkable with serum creatinine 1.5 mg/dL at discharge. Because of an in-hospital outbreak of COVID-19, SARS-CoV-2 was detected by NAAT (nucleic acid amplification test) in nasopharyngeal swab done at discharge; and she was readmitted four days after due to febrile syndrome. She rapidly evolved to acute respiratory failure with bilateral interstitial pneumonia requiring mechanical ventilation. SARS-CoV-2 was detected in the Bronchoalveolar Lavage (BAL). Laboratory tests revealed anemia, schistocytes in the peripheral blood smear, thrombocytopenia (68 x 103/ mL), elevated lactate dehydrogenase (615 UI/L), haptoglobin <0.3 g/L and a negative Coombs test. Acute renal failure (creatinine 3.4 mg/dL) with proteinuria (1.7 g/gCr) was detected. Determination of anti-A isoagglutinins (1/4) and anti-HLA antibodies were negative. Complement factors and ADAMTS-13 levels were normal. Blood and urine cultures were negative. Serological tests for HIV, HCV, HBV, and whole blood viral load for cytomegalovirus, parvovirus and Epstein-Barr virus were negative. Tacrolimus trough levels were in the therapeutic range (8-12 ng/mL). At admission, mycophenolate was withdrawn, tacrolimus dose was reduced and dexametasone (6 mg/day) was started. We interpreted the clinical and analytical findings as de novo TMA secondary to COVID-19 and treatment with Eculizumab was started (loading dose of 1200 mg and 900 mg weekly). A rapid recovery of hemolytic parameters and progressive clinical improvement was observed allowing mechanical ventilator weaning (Figure 1). A graft biopsy displayed features compatible with TMA without T-cell or antibody-mediated rejection (Figure 2). After 4 doses, eculizumab was discontinued and the patient was discharged with a serum creatinine of 1.8 mg/dL, a negative SARS-CoV-2 NAAT in the nasopharyngeal swab and a negative test for IgG and IgM antibodies, and receiving immunosuppression with tacrolimus (target trough levels 4-6) and low dose steroids.
Figure 2: Renal biopsy showed mesangiolysis, fibrous thickening of the intima with subendothelial edema and hematic extravasation (black arrow), as well as interstitial edema and acute tubular damage, compatible with TMA. Immunofluorescence revealed the deposit of C4d in the peritubular capillaries according to the ABOi allograft. The immunohistochemical technique to detect SARS-CoV-2 in kidney tissue was negative.
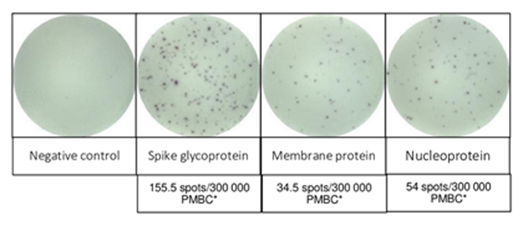
Two months later, she was readmitted due to fever and dyspnea with bilateral pulmonary infiltrates. The NAAT from the nasopharyngeal swab was negative but positive in the BAL with a low Cycle Threshold (Ct) value (26-31). The pulmonary biopsy findings were non-specific and SARS-CoV-2 was not detected. Under the diagnosis of Secondary Organizing Pneumonia (SOP), methylprednisolone (1 mg/Kg/d) was initiated. The SARS-CoV-2 NAAT in blood was positive with a Ct value of 35. Renal function impaired (creatinine 3.3 mg/dL and proteinuria 4 g/gCr) and laboratory findings were consistent with TMA. After 15 days, she presented a sudden left faciobrachiocrural hemiparesis. An MRI-angiography revealed a vasospasm on the middle cerebral artery and an arteriography was performed with the administration of intrarterial nimodipine. Afterwards, her respiratory condition worsened requiring mechanical ventilation. Treatment with eculizumab due to ongoing TMA was reinitiated and three cycles of treatment with convalescent plasma (CP; IgG titer >20.000 AU/mL) due to persistent COVID-19 was added. The patient experienced a progressive clinical improvement with resolution of neurological symptoms, respiratory function and haemolysis parameters. After discharge, transfusion of one unit of ABO compatible CP every 21 days was prescribed and she has remained asymptomatic. The SARS-CoV-2 NAAT in the BAL and blood persisted negative. Her renal function never returned to baseline values (creatinine 2.7 mg/dL), and the proteinuria progressively improved (0.6 g/g). Analyses of the complement genes did not identify any pathogenic variant related to aHUS [4]. Polymorphisms in heterozygosis in the CFH (CFH-H3) and MCP (MCPggaac) genes were detected. A heterozygous variant in PLG gene encoding plasminogen protein was found (c.505C>A; p.Pro169Thr). Before transplantation and during follow-up, CD19+ B cells were absent in peripheral blood. A severe deficiency of circulating IgG (< 300 mg/dL) and IgM (< 30 mg/dL) levels was detected during follow-up. She had not developed specific antibodies against nucleocapsid either spike antigens of SARS-CoV-2. The interferon gamma T cell enzyme-linked Immunospot (IFN-γ ELISpot) [5] was performed after receiving two cycles of CP and showed a strong positive response (Figure 3).
Figure 3: SARS-CoV-2-reactive T cell response of the patient was evaluated using interferon gamma T cell enzyme-linked Immunospot (IFN- γ T cell ELISpot). The main structural SARS-CoV-2 proteins were used for stimulation: Spike Glycoprotein (S), Membrane Protein (M) and Nucleoprotein (N). This was performed after receiving two cycles of CP and showed a strong response. *PMBC: Peripheral blood mononuclear cells.
3. Discussion
We describe an ABOi kidney transplant recipient who presented a TMA secondary to COVID-19 early after transplantation. Treatment with eculizumab led to an initial favourable response, but its discontinuation associated with relapsing infection required a new cycle of eculizumab alongside with CP to clinical recovery. Post-transplant de novo TMA may be triggered by factors that contribute to endothelial damage and complement activation; such as antibody-mediated rejection, immunosuppressive drugs or viral infections [1]. There are few reports of patients with TMA-associated to COVID-19 [6-11], and only 2 cases in renal transplants recipients have been described [2, 3] (Table 1). Our patient was severely immunosuppressed and unable to develop a humoral response against SARS-CoV-2. A major factor that determines the establishment of persistent RNA virus infections is the competence of the immune response [12], and persistent COVID-19 in patients with adaptive immune deficits have already been described [13].
Table 1: Case reports of patients who developed TMA in the context of COVID-19.
Endothelial injury and complement system activation associated to hyper immune response have been extensively described in patients with severe COVID-19 [14, 15]. The presence of micro vascular thrombosis in addition to C5b-9 and C4d deposition supports the participation of the complement system in the pathogenesis of COVID-19 [16]. Thus, the predominant mechanism by which patients with COVID-19 develop TMA seems to be mainly related to complement activation, but could also be triggered by the direct toxic effect of the virus to the vascular endothelium [17]. Genetic variants of complement proteins have been described in about 30% of recipients with de novo TMA [1], but no one was detected in our patient. However, we detected polymorphisms in the CFH and MCP genes which have been described to contribute to secondary TMA in other settings. Complement blockade with eculizumab is an effective therapy in de novo post-transplant TMA. This approach could also have a promising role in severe COVID-19-related complications [18] and different clinical trials are evaluating its safety and efficacy (NCT04346797, NCT04355494, NCT04288713). Recent evidences suggest that treatment with CP for COVID-19 may be useful in mildly ill infected older adults and immunodeficient high-risk patients [19, 20]. Its efficacy has been reported in small case series in patients with primary or secondary immunodeficiencies following rituximab therapy. In kidney transplant recipients, some reports have shown its safety, but its efficacy should be evaluated in large registries or clinical trials [21]. Besides providing neutralizing antibodies against SARS-CoV-2, CP may increase the magnitude of antiviral T-cell responses [22]. In our patient, we observed a strong specific memory T-cell response after 2 doses of CP, suggesting an additional benefit in patients with humoral immunodeficiencies. However, the success of CP therapy may be temporary and monitorization of the viral load and the antiviral immunity are recommended for treatment individualization [23].
4. Conclusion
We report a case of biopsy-proven TMA associated with COVID-19 in an early ABOi kidney transplant recipient with rituximab-induced B-cell deficiency. Treatment with eculizumab and CP and was associated with clinical recovery.
Statement of Ethics
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors declare that no funding was obtained.
Author Contributions
Z.C., J.S. and F.M have participated in the research design and in the writing of the paper. L. D. have developed the ELIspot assay.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- Garg N, Rennke HG, Pavlakis M, et al. De novo thrombotic microangiopathy after kidney transplantation. Transplant Rev (Orlando) 32 (2018): 58-68.
- Akilesh S, Nast CC, Yamashita M, et al. Multicenter clinicopathologic correlation of kidney biopsies performed in COVID-19 patients presenting with acute kidney injury or proteinuria. Am J Kidney Dis 77 (2021): 82-93.
- Bascunana A, Mijaylova A, Vega A, et al. Thrombotic Microangiopathy in a Kidney Transplant Patient with COVID-19. Kidney Med 3 (2021): 124-127.
- Test for atypical hemolytic uremic syndrome (2021).
- Fava A, Donadeu L, Sabe N, et al. SARS-CoV-2 specific serological and functional T cell immune responses during acute and early COVID-19 convalescence in solid organ transplant patients. Am J Transplant 21 (2021): 2749-2761.
- Ville S, Le Bot S, Chapelet-Debout A, et al. Atypical HUS relapse triggered by Covid-19. Kidney Int. 99 (2021): 267-268.
- Jhaveri KD, Meir LR, Flores Chang BS, et al. Thrombotic microangiopathy in a patient with COVID-19. Kidney Int 98 (2020): 505-516.
- Mat O, Ghisdal L, Massart A, et al. Kidney thrombotic microagiopathy after COVID-19 associated with C3 gene mutation. Kidney Int Rep 6 (2021): 1732-1737.
- Kaufeld J, Reinhardt M, Schroder C, et al. Atypical HUS triggered by infection with SARS-CoV2. Kidney Int Rep 6 2021): 2709-2712.
- Sharma P, Uppal NN, Wanchoo R, et al. COVID-19-associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol 31 (2020): 1948-1958.
- Korotchaeva J, Chebotareva N, Andreeva E, et al. Thrombotic Microangiopathy triggered by COVID-19: Case Reports. Nephron (2021): 1-6.
- Randall RE, Griffin DE. Within host RNA virus persistence: mechanism and consequences. Curr Opin Virol 23 (2017): 35-42.
- Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med 383 (2020): 2291-2293.
- Perico L, Benigni A, Casiraghi F, et al. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol 17 (2021): 46-64.
- Noris M, Benigni A, Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int 98 (2020): 314-322.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Trasl Res 220 (2020): 1-13.
- Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 395 (2020): 1417-1418.
- Diurno F, Numis FG, Porta G, et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci 24 (2020): 4040-4047.
- Katz LM. (A little) Clarity on convalescent plasma for COVID-19. N Eng J Med 384 (2021): 666-668.
- Libster R, Perez Marc G, Wappner D, et al. Early High-titer plasma therapy to prevent COVID-19 in older adults. N Engl J Med 384 (2021): 610-618.
- Gupta A, Kute VB, Patel HV, et al. Feasibility of convalescent plasma therapy in kidney transplant recipients with severe COVID-19: A single-center prospective cohort study. Exp Clin Transplant 19 (2021): 304-309.
- Fung M, Nambiar A, Pandey S, et al. Treatment of immunocompromised COVID-19 patients with convalescent plasma. Transpl Infect Dis 23 (2021): 13477.
- Lindermann M, Krawczyk A, Dolff S, et al. SARS-CoV-2-specific and cellular immunity in two renal transplants and two hemodialysis patients treated with convalescent plasma. J Med Virol 93 (2021): 3047-3054.