Therapeutic Role of Marijuana in Migraine
Article Information
Simrat Kaur Batth*
Review Article
Punjab Institute of Medical Sciences, Jalandhar, India
*Corresponding author: Simrat Kaur Batth, Punjab Institute of Medical Sciences, Amritsar, Jalandhar, India
Received: 27 March 2021; Accepted: 06 April 2021; Published: 16 April 2021
Citation:
Simrat Kaur Batth. Therapeutic Role of Marijuana in Migraine. Archives of Internal Medicine Research 4 (2021): 054-061.
Share at FacebookAbstract
Marijuana has been known for a long time to have a variety of therapeutic implications in pain management. The role of the cannabinoid system in regulation of migraine headaches highlights the potential of marijuana as a treatment option. Marijuana has the ability to modulate the migraine associated pain pathway via the cannabinoid receptors. Endocannabinoid deficiency has been theorized to contribute to the pathophysiology of migraine. People who have used marijuana for their migraine headaches have reported a decrease in the frequency of their headaches, especially the population who failed to show response with the conventional treatment options. Also, marijuana has a more tolerable side effect profile and administering this along with conventional treatment options can help reduce their dosages and side effects. Large scale clinical trials are needed to further find out about the different strains, formulations, prefer- red delivery method and doses of marijuana required for effective treatment.
Keywords
Marijuana, Migraine
Article Details
1. Introduction
Migraine is a highly prevalent neurological condition with substantial impact on individuals through associated complications, comorbidities, and increased healthcare costs. Globally, the percentage of the adult population with an active headache disorder is 47% for headache in general, 10% for migraine, 38% for tension-type headache, and 3% for chronic headache that lasts for more than 15 days per month. The large costs of headache to society, which are mostly indirect through loss of work time, have been reported. On the individual level, headaches cause disability, suffering, and loss of quality of life that is on a par with other chronic disorders [1]. Severe headache and migraine remain important public health problems that are more common and burdensome for women, particularly women of childbearing age, and other historically disadvantaged segments of the population [2]. Migraine patients worldwide are still not receiving adequate treatment and there remains a significant unmet need in migraine care [3].
For thousands of years, Cannabis sativa has been utilized as a medicine and for recreational and spiritual purposes. Phytocannabinoids are a family of compounds that are found in the cannabis plant, which is known for its psychotogenic and euphoric effects; the main psychotropic constituent of cannabis is Δ9-tetrahydrocannabinol (Δ9-THC). The pharmacological effects of cannabinoids are a result of interactions between those compounds and cannabinoid receptors, CB1 and CB2, located in many parts of the human body [4]. Several studies have suggested that the endocannabinoid system is centrally and peripherally involved in the processing of pain signals. It is imperative to understand the patterns of cannabis use and its associated relief as compared to non-cannabis products in migraineurs. A number of migraine patients are refractory to conventional treatments (e.g., NSAIDs, tricyclic antidepressants, and triptans), and as such, individuals with more severe, treatment-resistant migraines may be more likely to turn to alternative or complementary treatment strategies such as cannabis use.
Medical Cannabis is becoming a popular alternative due, in part, to rapidly changing marijuana laws and increased availability. Currently, there is not enough evidence from well-designed clinical trials to support the use of marijuana for headache, but there are sufficient anecdotal and preliminary results, as well as plausible neurobiological mechanisms, to warrant properly designed clinical trials [5].
2. Pathophysiology
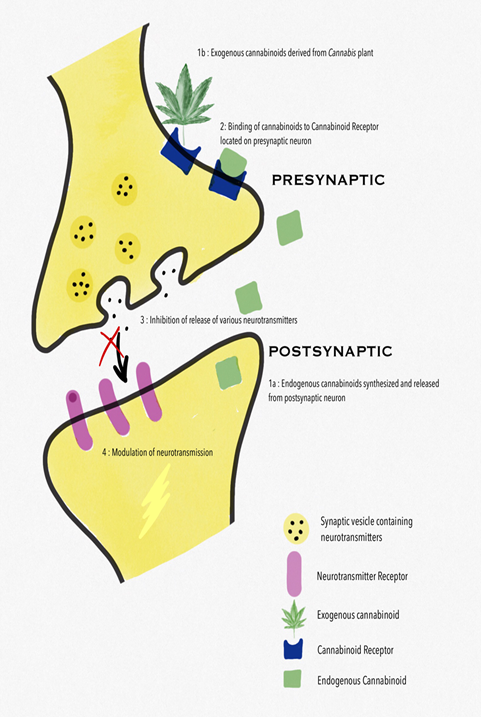
Emerging evidence from preclinical studies suggests that endocannabinoids modulate migraine-associated pain pathways and an endocannabinoid deficiency is theorized to contribute to the pathophysiology of migraine. The CB1 receptor is predominantly located in the CNS but has also been detected on the terminals of peripheral nerves, the gastrointestinal system and the reproductive system. Localized to the terminals of nerve fibres, the CB1 receptor has been implicated in inhibiting retrograde signalling resulting in the inhibition of neurotransmitter release and synaptic transmission (Figure 1).
CB1 receptors have also been discovered on various immune cells and can promote both pro-inflammatory and anti-inflammatory actions. The CB2 receptor was previously known as the peripheral cannabinoid receptor and originally thought to be only located on the cell membrane of immune cells and immune-mediating organs. However, CB2 receptors have also been found in the CNS and on sensory nerve terminals in the periphery. The presence of CB1 and CB2 receptors on sensory nerve terminals and their inhibitory effect on neuronal activity suggest that the endocannabinoid system has the potential to moderate neurogenic inflammation and pain. Physiological characterization of the phytocannabinoids is still in its infancy with the vast majority of research focusing on the psychoactive compound Δ9-tetrahydrocannabinol (THC) and the nonpsychotropic cannabinoid, cannabidiol (CBD) [6-8].
Arachidonylethanolamide (anandamide, AEA) is believed to be the endogenous ligand of the CB1 and CB2 receptors. CB1 receptors have been found localized on fibers in the spinal trigeminal tract and spinal trigeminal nucleus caudalis. Anandamide acts both presynaptically, to prevent calcitonin gene related peptide (CGRP) release from trigeminal sensory fibers, and postsynaptically to inhibit the CGRP-induced nitric oxide (NO) release in the smooth muscle of dural arteries. CB1 receptors seem to be involved in the NO/CGRP relationship that exists in causing headache and dural blood vessel dilation. AEA also activates the transient receptor potential vanilloid receptor on trigeminal ganglion neurons, modulating the release of CGRP, and influencing vasomotor tone [7, 9]. Since endogenous cannabinoids have been shown to be involved in migraine headaches, administering marijuana exogenously clearly has the potential for regulating the processes involved in migraine headache development.
CB2 receptor selective agonists also have an analgesic effect, the main advantage of which is the lack of the typical psychotropic side effects of CB1 receptor activation [10]. Cannabinoids can alter the transmission of various neurotransmitters. Endocannabinoids exert a critical control on cerebrovascular tone, by interacting with serotonergic transmission, NO production, and CGRP release [11]. It has been clearly demonstrated that endocannabinoids regulate these brain functions acting as retrograde neurotransmitters, since they are synthesized and released from postsynaptic neurons and bind to CB1 receptor in the presynaptic terminal (Figure 1). In particular CB1 receptor, by inhibiting the release of neurotransmitters such as γ- aminobutyric acid (GABA), glutamate, dopamine, noradrenaline and acetylcholine, are involved in transmission and modulation of pain signals [12]. Modulation of serotonergic pain transmission is also well established in migraine treatment, particularly with the mechanism of action of the triptans. Endocannabinoids interact with serotonergic neurons in the brainstem dorsal raphe to modulate pain mechanisms. AEA and other cannabinoid agonists have also been shown to have inhibitory effects on serotonin receptors, which further suggests its role as an antiemetic and in analgesia [7]. The endogenous endocannabinoid AEA, the phytocannabinoid-THC, and synthetic cannabinoids suppress glutamatergic neurotransmission via inhibitory modulation of the N-methyl-D-aspartate (NMDA) receptors, mediated by CB1 receptors. Activation of CB1 receptors suppresses cortical spreading depression (CSD). This is suspected to be due to decreased glutamatergic transmission via inhibitory NMDA modulation, although modulation of NO, CGRP, or lipoxygenase and cyclooxygenase pathways are also possible contributors to the suppressive effect of cannabinoids on CSD [13].
N-Arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG), the best characterized ECs, are amides and esters of arachidonic acid (AA). The best characterized enzymes involved in AEA and 2-AG metabolism are the N-acylphosphatidyl-ethanolamine-phospholipase D (NAPE-PLD) and the sn-1-specific diacylglycerol lipase (DAGL), respectively which synthesize them from phospholipids membrane and fatty acid amide hydrolase (FAAH) which releases AA and ethanolamine and AA and glycerol, respectively. Nevertheless, 2-AG degradation is mainly due to a cytosolic monoacylglycerol lipase (MAGL) [7, 8]. Medicine continues to struggle in its approaches to numerous common subjective pain syndromes that lack objective signs and remain treatment resistant. Foremost among these are migraine, fibromyalgia, and irritable bowel syndrome, disorders that may overlap in their affected populations and whose sufferers have all endured the stigma of a psychosomatic label, as well as the failure of endless pharmacotherapeutic interventions with substandard benefit [14]. The commonality in symptomatology in these conditions displaying hyperalgesia and central sensitization with possible common underlying pathophysiology suggests that a clinical endocannabinoid deficiency might characterize their origin. Its base hypothesis is that all humans have an underlying endocannabinoid tone that is a reflection of levels of the endocannabinoids, anandamide (arachidonylethanolamide), and 2-arachidonoylglycerol, their production, metabolism, and the relative abundance and state of cannabinoid receptors. Its theory is that in certain conditions, whether congenital or acquired, endocannabinoid tone becomes deficient and productive of pathophysiological syndromes [14].
3. Use of Marijuana in Migraine
Cannabinoids including medical marijuana are widely used for pain control either with, or without, prescriptions from physicians. A study examined, for the first time, the effectiveness of using dried Cannabis flower, the most widely used type of Cannabis product in the United States, for the treatment of headache- and migraine-related pain and the associations between different product characteristics and changes in symptom intensity following Cannabis use. The results suggested that whole dried Cannabis flower may be an effective medication for treatment of migraine- and headache-related pain, but the effectiveness differs according to characteristics of the Cannabis plant, the combustion methods, and the age and gender of the patient. In this study, all groups experienced symptom relief primarily associated with THC levels 10% and over, with joints potentially offering more relief than pipes. The analgesic effects of Cannabis varied primarily with age, with younger patients experiencing greater benefits in general [15].
A cross-sectional questionnaire-based study aimed to investigate the associations between phytocannabinoid treatment and migraine frequency was conducted. The findings indicate that medical cannabis results in long-term reduction of migraine frequency in 60% of treated patients and is associated with less disability and lower antimigraine medication intake. The study also elucidated the associations between specific cannabinoids consumed over a monthly dose and the clinical response of migraine frequency reduction following medical cannabis treatment initiation [16]. Another study determined the monthly frequency of migraine headache in patients diagnosed with migraine headache who used medical marijuana. Patients using medical marijuana for migraine headache reported a statistically significant decrease in the number of migraine headaches per month. Almost all patients used marijuana daily for migraine headache prevention. Inhaled forms of marijuana were commonly used for acute migraine treatment and were reported to abort the headache. Overall, more positive than negative effects were reported with medical marijuana use. Edible marijuana was reported to cause more negative effects compared with other forms [17].
One study conducted on a sample of cannabis users provided an insight into the prevalence of cannabis use for migraine relief in them, and suggested that these migraineurs reported a higher level of migraine relief from cannabis than non-cannabis products [17]. But headache is very common in cannabis users and cannabis withdrawal so it is possible that using cannabis simply relieves headache caused by cannabis withdrawal. Another study showed that here were significant reductions in headache and migraine ratings by 50% after cannabis use. Men reported larger reductions in headache than women and use of concentrates was associated with larger reductions in headache than flower. Further, there was evidence of tolerance to these effects [18]. To date, however, there has only been one randomized, double-blind study of cannabinoid treatment for headache or migraine demonstrating the benefits of nabilone on headache, analgesic consumption and the quality of life in patients with intractable medication overuse headache. Larger scale studies are needed to confirm these preliminary findings [19].
4. Side Effects
As per one study negative effects, notably somnolence and difficulty controlling drug effects, were reported in 11.6 % of participants, and were experienced only after the use of edible, rather than inhaled, cannabis [20]. Another study explored the possibility that exposure to cannabinoids could, like many acute migraine medications, result in latent trigeminal sensitization and vulnerability to typical migraine triggers. Prolonged periods of exposure with the cannabinoid agonists produced a long-lasting increased sensitivity to stress, supporting the possibility that sustained CB1 receptor activation may increase the risk of medication overuse headache in vulnerable populations [21]. It is important to remember that none of the studies or reported adverse effects of cannabis specifically compare and take into account many potential variables. These include route of administration, patient age, concurrent medications being taken, patient comorbidities, standardized dosing, type of cannabis strain, ratio of the phytocannabinoids in the cannabis strain (particularly the CBD:THC ratio), sterility of cannabis growing conditions, cannabis analyzation for commonly encountered issues of toxins, pesticides, and fungal and bacterial microbial contaminants, among others [7].
Adverse effect profile of marijuana in case of controlled medical use is different from those with heavy maijuana intake. Adverse reactions have been reported in the central nervous system, cardiovascular system, respiratory system, gastrointestinal, reproductive, and immune systems in case of high doses [7]. Detailed estimated dose amounts and percentages of Δ9-THC between various routes of administration, including conversion factors between smoked and oral forms can be seen in Health Canada’s publication of Information for Health Care Professionals: Cannabis (marihuana,marijuana) and the cannabinoids. There are no standard clinical guidelines in terms of contraindications for use of cannabis and cannabinoids, although Health Canada has outlined some suggestions [22]. Tolerability is a concern in the use of cannabinoids. Dosing is individualized and requires titration. Psychoactive effects occur at doses above the individual consumer's psychotropic threshold. They are generally pleasurable and relaxing. However, this can turn into dysphoria, anxiety, or even panic. Impairment of memory, reductions in psychomotor and cognitive performance and disordered perception of the passage of time can occur. Common physical effects are tiredness, dizziness, tachycardia, orthostatic hypotension, dry mouth, reduced lacrimation, muscle relaxation, and increased appetite. Tolerance develops to many of these undesired effects of cannabinoids particularly tiredness, dizziness, and cardiovascular and psychoactive effects over a period of days or weeks [23]. There is an association between cannabis use and the development of psychosis, at least in vulnerable patients, with the highest risk among the most frequent users [24].
5. What Still Needs to be Determined?
Most studies have been using a single-compound, single-target approach to clinical problem-solving. This is wholly different to medical cannabis treatment, which is oftentimes multi-compound, whole-plant treatment. The cannabis plant contains hundreds of different active components, including phytocannabinoids, terpenes, and flavonoids. While THC and CBD are among the most well-known phytocannabinoids, others are likely to have biological activity as well [16]. Hence, it is conceivable that various combinations of phytocannabinoids differ in their anti-migraine activity. This multi-compound effect of cannabis has been called the “entourage effect”, which suggests that studies examining the role of single-molecule cannabinoids in disease may not necessarily capture the synergy at play in multi-compound medical cannabis treatment. To add to the complexity of medical cannabis treatment with multiple compounds, there are hundreds of different cannabis cultivars, each with its own unique chemical composition [16]. Ultimately, studying each isolated constituent is mandatory to determine each compound’s individual therapeutic benefits. Also, the use of cannabinoids in combination with other traditional medication might help to reduce their dosages and hence side effects. Medicinal marijuana can be a possible alternative to narcotics with less potential for dependence, addiction, and abuse. These interactions also raise the question of a theoretical role in helping patients to wean down or off of opiates.
6. Conclusion
Medical marijuana, and its possible role in migraine treatment deserves proper scientific examination, both biochemically and clinically. More research needs to be done on the therapeutic potential of cannabis in migraine like use of different strains, formulations, preferred delivery method and doses of marijuana to better understand the effects of medical marijuana on migraine headache treatment and prophylaxis. Further research should be performed to determine the potential long-term effects of medical marijuana. As legal barriers fall and scientific bias fades this will become more apparent. Future double-blind, placebo controlled clinical trials are warranted and will help to rule out placebo effects and provide a more controlled examination of dose, type of cannabis, THC, CBD, and THC x CBD interactions.
References
- Jensen R, Stovner LJ. Epidemiology and comorbidity of headache. Lancet Neurol 7 (2008): 354-361.
- Burch R, Rizzoli P, Loder E. The Prevalence and Impact of Migraine and Severe Headache in the United States: Figures and Trends From Government Health Studies. Headache 58 (2018): 496-505.
- Brandes JL. Global trends in migraine care: results from the MAZE survey. CNS Drugs 1 (2002): 13-18.
- Breijyeh Z, Jubeh B, Bufo SA, et al. Cannabis: A Toxin-Producing Plant with Potential Therapeutic Uses. Toxins 13 (2021).
- Lochte BC, Beletsky A, Samuel NK, et al. The Use of Cannabis for Headache Disorders. Cannabis Cannabinoid Res 2 (2017): 61-71.
- McKenna M, McDougall JJ. Cannabinoid control of neurogenic inflammation. Br J Pharmacol. 177 (2020): 4386-4399.
- Baron EP. Comprehensive Review of Medicinal Marijuana, Cannabinoids, and Therapeutic Implications in Medicine and Headache: What a Long Strange Trip It’s Been …. Headache 55 (2015): 885-916.
- Greco R, Gasperi V, Maccarrone M, et al. The endocannabinoid system and migraine. Exp Neurol 224 (2010): 85-91.
- Akerman S, Kaube H, Goadsby PJ. Anandamide is able to inhibit trigeminal neurons using an in vivo model of trigeminovascular-mediated nociception. J Pharmacol Exp Ther 309 (2004): 56-63.
- Beltramo M. Cannabinoid type 2 receptor as a target for chronic - pain. Mini Rev Med Chem 9 (2009): 11-25.
- Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol 63 (2001): 569-611.
- Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med 14 (2008): 923-930.
- Kazemi H, Rahgozar M, Speckmann E-J, et al. Effect of Cannabinoid Receptor Activation on Spreading Depression. Iran J Basic Med Sci 15 (2012): 926-936.
- Russo EB. Clinical Endocannabinoid Deficiency Reconsidered: Current Research Supports the Theory in Migraine, Fibromyalgia, Irritable Bowel, and Other Treatment-Resistant Syndromes. Cannabis Cannabinoid Res 1 (2016): 154-165.
- Stith SS, Diviant JP, Brockelman F, et al. Alleviative effects of Cannabis flower on migraine and headache. J Integr Med 18 (2020): 416-424.
- Aviram J, Vysotski Y, Berman P, et al. Migraine Frequency Decrease Following Prolonged Medical Cannabis Treatment: A Cross-Sectional Study. Brain Sci 10 (2020).
- Rhyne DN, Anderson SL, Gedde M, et al. Effects of Medical Marijuana on Migraine Headache Frequency in an Adult Population. Pharmacotherapy 36 (2016): 505-510.
- Cuttler C, Spradlin A, Cleveland MJ, et al. Short- and Long-Term Effects of Cannabis on Headache and Migraine. J Pain 21 (2020): 722-730.
- Pini LA, Guerzoni S, Cainazzo MM, et al. Nabilone for the treatment of medication overuse headache: results of a preliminary double-blind, active-controlled, randomized trial. J Headache Pain 13 (2012): 677-684.
- Gibson LP, Hitchcock LN, Bryan AD, et al. Experience of migraine, its severity, and perceived efficacy of treatments among cannabis users. Complement Ther Med 56 (2021): 102619.
- Kopruszinski CM, Navratilova E, Vagnerova B, et al. Cannabinoids induce latent sensitization in a preclinical model of medication overuse headache. Cephalalgia Int J Headache 40 (2020): 68-78.
- For health care professionals: Cannabis and cannabinoids - Canada.ca (2021).
- Tassorelli C, Greco R, Silberstein SD. The endocannabinoid system in migraine: from bench to pharmacy and back. Curr Opin Neurol 32 (2019): 405-412.
- Crocker CE, Carter AJE, Emsley JG, et al. When Cannabis Use Goes Wrong: Mental Health Side Effects of Cannabis Use That Present to Emergency Services. Front Psychiatry 12 (2021): 640222.