The Role of Hedgehog Pathway in Female Cancers
Article Information
Natalia Garcia, Mara Ulin, Ayman Al-Hendy, Qiwei Yang*
Department of Surgery, University of Illinois at Chicago, Chicago, Illinois, USA
*Corresponding Author: Dr. Qiwei Yang, Department of Surgery, University of Illinois at Chicago, Chicago, Illinois, USA
Received: 02 August 2020; Accepted: 01 September 2020; Published: 09 October 2020
Citation:
Natalia Garcia, Mara Ulin, Ayman Al-Hendy, Qiwei Yang. The Role of Hedgehog Pathway in Female Cancers. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 487-498.
Share at FacebookAbstract
Background: The hedgehog pathway (HH) is one of the key regulators involved in many biological events. Malfunction of this pathway is associated with a variety of diseases including several types of cancers.
Methods: We collected data from public databases and conducted a comprehensive search linking the HH pathway with female cancers. In addition, we overviewed clinical trials of targeting HH pathway in female cancers.
Results: The activation of HH pathway and its role in female cancers, including breast cancer, ovarian cancer, cervical cancer, endometrial cancer, and uterine leiomyosarcoma were summarized. Treatment options targeting SMO and GLI in HH pathway were reviewed and discussed.
Conclusions: The hedgehog pathway was shown to be activated in several types of female cancers. Therefore, targeting HH pathway may be considered as a therapeutic option to be acknowledged in the treatment of female cancers.
Keywords
Hedgehog pathway; Female cancer; Ovarian cancer; Cervical cancer; Endometrial cancer; Uterine leiomyosarcoma; Therapeutic option
Hedgehog pathway articles; Female cancer articles; Ovarian cancer articles; Cervical cancer articles; Endometrial cancer articles; Uterine leiomyosarcoma articles; Therapeutic option articles
Hedgehog pathway articles Hedgehog pathway Research articles Hedgehog pathway review articles Hedgehog pathway PubMed articles Hedgehog pathway PubMed Central articles Hedgehog pathway 2023 articles Hedgehog pathway 2024 articles Hedgehog pathway Scopus articles Hedgehog pathway impact factor journals Hedgehog pathway Scopus journals Hedgehog pathway PubMed journals Hedgehog pathway medical journals Hedgehog pathway free journals Hedgehog pathway best journals Hedgehog pathway top journals Hedgehog pathway free medical journals Hedgehog pathway famous journals Hedgehog pathway Google Scholar indexed journals Female cancer articles Female cancer Research articles Female cancer review articles Female cancer PubMed articles Female cancer PubMed Central articles Female cancer 2023 articles Female cancer 2024 articles Female cancer Scopus articles Female cancer impact factor journals Female cancer Scopus journals Female cancer PubMed journals Female cancer medical journals Female cancer free journals Female cancer best journals Female cancer top journals Female cancer free medical journals Female cancer famous journals Female cancer Google Scholar indexed journals Ovarian cancer articles Ovarian cancer Research articles Ovarian cancer review articles Ovarian cancer PubMed articles Ovarian cancer PubMed Central articles Ovarian cancer 2023 articles Ovarian cancer 2024 articles Ovarian cancer Scopus articles Ovarian cancer impact factor journals Ovarian cancer Scopus journals Ovarian cancer PubMed journals Ovarian cancer medical journals Ovarian cancer free journals Ovarian cancer best journals Ovarian cancer top journals Ovarian cancer free medical journals Ovarian cancer famous journals Ovarian cancer Google Scholar indexed journals Cervical cancer articles Cervical cancer Research articles Cervical cancer review articles Cervical cancer PubMed articles Cervical cancer PubMed Central articles Cervical cancer 2023 articles Cervical cancer 2024 articles Cervical cancer Scopus articles Cervical cancer impact factor journals Cervical cancer Scopus journals Cervical cancer PubMed journals Cervical cancer medical journals Cervical cancer free journals Cervical cancer best journals Cervical cancer top journals Cervical cancer free medical journals Cervical cancer famous journals Cervical cancer Google Scholar indexed journals Endometrial cancer articles Endometrial cancer Research articles Endometrial cancer review articles Endometrial cancer PubMed articles Endometrial cancer PubMed Central articles Endometrial cancer 2023 articles Endometrial cancer 2024 articles Endometrial cancer Scopus articles Endometrial cancer impact factor journals Endometrial cancer Scopus journals Endometrial cancer PubMed journals Endometrial cancer medical journals Endometrial cancer free journals Endometrial cancer best journals Endometrial cancer top journals Endometrial cancer free medical journals Endometrial cancer famous journals Endometrial cancer Google Scholar indexed journals Uterine leiomyosarcoma articles Uterine leiomyosarcoma Research articles Uterine leiomyosarcoma review articles Uterine leiomyosarcoma PubMed articles Uterine leiomyosarcoma PubMed Central articles Uterine leiomyosarcoma 2023 articles Uterine leiomyosarcoma 2024 articles Uterine leiomyosarcoma Scopus articles Uterine leiomyosarcoma impact factor journals Uterine leiomyosarcoma Scopus journals Uterine leiomyosarcoma PubMed journals Uterine leiomyosarcoma medical journals Uterine leiomyosarcoma free journals Uterine leiomyosarcoma best journals Uterine leiomyosarcoma top journals Uterine leiomyosarcoma free medical journals Uterine leiomyosarcoma famous journals Uterine leiomyosarcoma Google Scholar indexed journals Therapeutic option articles Therapeutic option Research articles Therapeutic option review articles Therapeutic option PubMed articles Therapeutic option PubMed Central articles Therapeutic option 2023 articles Therapeutic option 2024 articles Therapeutic option Scopus articles Therapeutic option impact factor journals Therapeutic option Scopus journals Therapeutic option PubMed journals Therapeutic option medical journals Therapeutic option free journals Therapeutic option best journals Therapeutic option top journals Therapeutic option free medical journals Therapeutic option famous journals Therapeutic option Google Scholar indexed journals basal cell nevus syndrome articles basal cell nevus syndrome Research articles basal cell nevus syndrome review articles basal cell nevus syndrome PubMed articles basal cell nevus syndrome PubMed Central articles basal cell nevus syndrome 2023 articles basal cell nevus syndrome 2024 articles basal cell nevus syndrome Scopus articles basal cell nevus syndrome impact factor journals basal cell nevus syndrome Scopus journals basal cell nevus syndrome PubMed journals basal cell nevus syndrome medical journals basal cell nevus syndrome free journals basal cell nevus syndrome best journals basal cell nevus syndrome top journals basal cell nevus syndrome free medical journals basal cell nevus syndrome famous journals basal cell nevus syndrome Google Scholar indexed journals breast cancer articles breast cancer Research articles breast cancer review articles breast cancer PubMed articles breast cancer PubMed Central articles breast cancer 2023 articles breast cancer 2024 articles breast cancer Scopus articles breast cancer impact factor journals breast cancer Scopus journals breast cancer PubMed journals breast cancer medical journals breast cancer free journals breast cancer best journals breast cancer top journals breast cancer free medical journals breast cancer famous journals breast cancer Google Scholar indexed journals medulloblastoma articles medulloblastoma Research articles medulloblastoma review articles medulloblastoma PubMed articles medulloblastoma PubMed Central articles medulloblastoma 2023 articles medulloblastoma 2024 articles medulloblastoma Scopus articles medulloblastoma impact factor journals medulloblastoma Scopus journals medulloblastoma PubMed journals medulloblastoma medical journals medulloblastoma free journals medulloblastoma best journals medulloblastoma top journals medulloblastoma free medical journals medulloblastoma famous journals medulloblastoma Google Scholar indexed journals
Article Details
1. Introduction
The hedgehog (HH) gene was described by Christiane Nusslein-Volhard and Eric Wieschausin in 1980 during gene screening in Drosophila [1]. The HH signaling pathway plays an essential role in embryonic and normal tissue development along with patterning and tissue differentiation [2]. The role of dysregulated Hedgehog Signaling pathway in cancer was first identified in basal cell nevus syndrome, a genetic disorder characterized by an increased risk of basal cell carcinoma and medulloblastoma. From these findings, it is understandable that mutations in PTCH1 caused aberrant activation of HH pathway and further predisposed patients to develop cancer [3].
2. Hedgehog Pathway
In vertebrates, HH signaling pathway consists of 3 ligands which are Indian hedgehog (IHH), Desert hedgehog (DHH) and Sonic hedgehog (SHH), a receptor called Patched 1 (PTCH1), a signal transducer called Smoothened (SMO), a cytoplasmic protein named SUFU and 3 transcription factor (GLI1, GLI2 and GLI3) [4] (Figure 1). Alteration in HH signaling pathway promotes GLI translocation into the nucleus leading to overactivation of several target genes which regulate cell differentiation (INSM1, SOX2, OCT4 and NANOG) [5-7], proliferation (c-MYC and n-MYC) [8, 9], apoptosis (BCL2, CASPASE 3, BAX, CASPASE 9 and BAK) [8-12], cell cycle (CCND1 and P21) [8, 11, 13-15], DNA damage (RAD51 and TP53) [15, 16], angiogenesis (c-MET, VEGFR2) [11, 17] and adhesion (N-CADHERIN, E-CADHERIN and SNAIL 1) [13, 18, 19] contributing to the pathogenesis of cancer. The hedgehog signaling pathway is tightly associated with embryogenesis as well as with the development of several female cancers. This review summarizes the findings of the role of HH signaling in female cancers and outlines the treatment options.
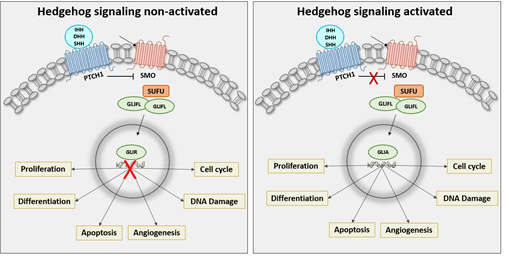
Figure 1: Non-activated or Activated State of Sonic Hedgehog Signaling Pathway. Left panel: With the absence of HH ligand, PTCH1 inhibits SMO activity. GLI activity is phosphorylated, converting GLI full- length (GLIFL) to repressor form (GLIR). GLIR translocates into the nucleus, which binds to HH target gene promoters and suppresses their expression. Right panel: The activation of the signaling occurs when HH ligands bind to PTCH, HH relieves the inhibition of PTCH to activate the signal transduction. SMO transmits a signal to the cytoplasm in a phosphorylation cascade leading translocation of GLI activator (GLIA) to the nucleus and binding to the target gene promoters and activates the transcription of the target genes.
3. Hedgehog in Female Cancers
HH signaling pathway has been reported to be involved in the pathogenesis of several types of female cancer, including breast [20], ovarian [21], endometrium [22], cervical [23], and uterine leiomyosarcoma [24] (Figure 2).
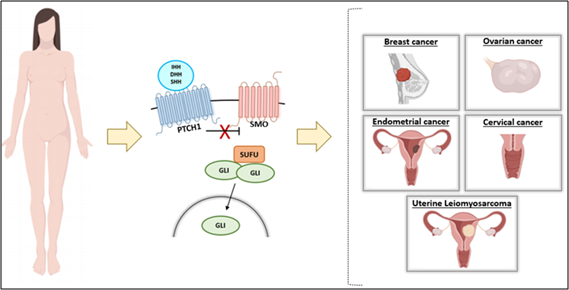
Figure 2: Abnormal Hedgehog Signaling Pathway Leads to Female Cancers.
3.1 Breast cancer
Breast cancer is the most frequently diagnosed cancer in women and the second leading cause of death in women diagnosed with cancer [25]. The HH signaling pathway plays an essential role in mammary gland development. Throughout a lifetime, this pathway activity varies. For instance, in the early phases of embryogenesis, this pathway is repressed to allow proper mammary gland parenchyma formation. During puberty there is ductal morphogeneisis and the HH signaling pathway is required for activation to promote the elongation of the terminal buds. Soon after puberty, in the mammary glands, the HH signaling pathway activity decreases [26]. In breast cancer HH signaling pathway activation has been associated with younger age presentation (<50 years), larger tumor size, lymph node metastasis, progesterone receptor-negative status, high proliferation index of Ki67, and poor overall survival [27-29]. Studies have shown that the expression of GLI1 [29-31] along with GLI1, 2 and 3 protein levels are upregulated in breast tumor compared to normal tissue [28]. Furthermore, the GLI expression level is associated with a higher tumor grade [29]. It is reported that targeting HH pathway in breast cancer showed promising results in several clinical trials (Table 1).
Table 1: Clinical Trials Targeting SHH Pathways in Female Cancers.
3.2 Ovarian Cancer
Ovarian cancer is the leading cause of death from gynecologic malignancies in the United States [32]. Epithelial ovarian cancer accounts for over 90% of all ovarian malignancies and comprises five histological subtypes: serous, mucinous, endometrioid, undifferentiated and clear cell type [33]. Aberrant activation of the HH signaling pathway is mediated through increased endogenous ligand-dependent expression of HH or ligand-independent mutations of PTCH, SMO and SUFU [34, 35]. Accumulating evidence suggests that the deregulation of the HH signaling pathway also contributes to the malignancy of ovarian cancer [36-40]. The expression of SHH, DHH, GLI, PTCH and SMO is absent in normal ovary [38, 41]. Elevated expression of PTCH1 and GLI1 is correlated with poor prognosis in ovarian cancer [41, 42]. In addition, the presence of SHH, DHH, PTCH, SMO and GLI1 proteins are associated with abnormal cell proliferation [38]. Moreover, the HH pathway is involved in regulating cancer stem cells leading to tumor formation, progression and invasion in ovarian cancer [43, 44]. It is well demonstrated that the strategy for blocking this pathway has been used in several clinical trials (Table 1) with a promising outcome.
3.3 Cervical cancer
Cervical cancer is the third most common malignant neoplasm in females, representing the fourth cause of cancer deaths among females worldwide [45]. Persistent infection by high-risk HPVs (16,18,31 and 33) is a risk factor for the development of cervical cancer [23, 46-48]. High expression of the HH signaling pathway regulates proliferation, migration and invasion of cervical cancer cell lines [23]. Reports in the literature have demonstrated that inhibition of the HH signaling pathway with Cyclopamine and Gant 58 decreased invasion and enhanced apoptosis, demonstrating this treatment can be effective in treating cervical cancer [49].
- Endometrial cancer
Endometrial cancer is the most common malignancy of the female reproductive tract, with a substantial increase in incidence and mortality rate in developed countries. This type of cancer predominantly affects postmenopausal women. However, 15-25% of cases are diagnosed before menopause. Many risk factors have been identified to predispose women with endometrial cancer, including polycystic ovarian syndrome, obesity and endometrial hyperplasia [50]. Moreover, several pathways have been identified to be altered in endometrial carcinoma including HH signaling pathway. Interestingly, PTCH1 has been found to be expressed in patients with endometrial hyperplasia, and GLI 1, GLI2, cytoplasmic GLI3 and SUFU have also been identified to be overexpressed in patients with endometrial carcinoma [51-53].
3.5 Uterine leiomyosarcoma
Uterine Leiomyosarcoma (LMS) is the most common type of uterine sarcoma. This tumor can be present at any age. However, it is frequently diagnosed in the perimenopausal years. It represents around 3-7% of all uterine cancers [54]. LMS is an extremely aggressive tumor that shows a challenge for treatment. LMS exhibits resistance to standard therapy [55]. The involvement of the HH signaling pathway in uterine leiomyosarcoma was first described in 2016 [24]. Elevated expression of SMO and GLI 1 was observed in leiomyosarcoma when compared to normal myometrium and uterine fibroids tissue. In addition, SUFU and SHH proteins were correlated with poor prognosis in leiomyosarcoma patients [24]. Recently, we demonstrated that uterine leiomyosarcoma cells exhibited an upregulation of SMO and GLI1 members concomitantly with an increase in nuclear translocation of GLI-1 and 2 compared to uterine smooth muscle cells. Uterine cells showed a decrease in proliferation, migration, invasion and exhibited an increase in apoptosis in response to treatment with SMO and GLI inhibitors, respectively [56, 57]. Identifying the HH pathway in relation to this aggressive cancer might allow better treatment options for women suffering from this devastating condition.
4. Therapeutic Options to Block the Hedgehog Pathway
Several compounds have been identified to inhibit the HH signaling pathway and can be categorized as HH ligand inhibitors (HH neutralizing antibodies and small molecule Robotnikinin, SMO antagonists, cyclopamine and its derivatives (IPI-926 and Cyc-T), synthetic compounds such as Vismodegib (GDC0449), Sonidegib (LDE225), and GLI transcriptional inhibitors (Gant 58 and Gant 61) [58] (Figure 3).
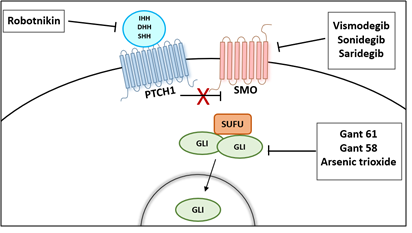
Figure 3: Therapeutic Options to Block the Activation of Hedgehog Signaling Pathway.
4.1 Hedgehog ligand inhibitor
The only ligand inhibitor described in the literature is a small molecule called Robotnikin, which binds to an extracellular sonic HH protein. This molecule is able to bind to the ligand SHH protein, therefore blocking downstream of the signaling [59]. Currently, there are no studies reported in the literature using this drug in female cancers.
4.2 SMO inhibitors
SMO is the first molecule reported in the literature to target the HH pathway. Through suppression of SMO, activation of GLI transcription factors was decreased, leading to the downregulation of the HH target genes [60]. Cyclopamine is the first component described to block SMO [61, 62], its use in vitro and in vivo has shown anticancer activity. However, Cyclopamine has poor bioavailability making the clinical utility limited [63, 64]. Vismodegib (Erivedge Capsule, Genentech, Inc, USA) (GDC0449) was approved by the FDA in 2012 for the treatment of patients who are not candidates for surgery or radiation therapy, locally advanced, metastatic or recurrent basal cell carcinoma [65]. This drug is usually given until the disease progresses or until unacceptable toxicity occurs [66].
Vismodegib is a small molecule showing promising outcomes through inactivating SMO, resulting in decreased downstream target gene expression [67]. In a preclinical trial, Vismodegib, exhibited excellent potency, solubility, and metabolic stability. In addition, Phase I and II clinical trials in patients with various carcinomas have shown to have a positive response to this compound [66]. Currently, there are several clinical trials using this molecule to treat several types of female cancer (Table 1). Unfortunately, there are two known SMO mutations (D473 and E518) that can lead to resistance of vismodegib, thus decreased the ability of vismodegib to bind to SMO leading to decrease efficacy [68, 69].
Sonidegib (LDE225) was first identified in 2010 during screening biphenyl carboxamides that displayed potent antitumor activity against a medulloblastoma model [70]. In July 2015, this drug was marketed as Odomzo by Novartis. Its approval by the FDA has been used for the treatment of recurrent basal cell carcinoma in patients who are not eligible for surgery or radiotherapy. Sonidegib interacts with SMO, acting as an antagonist, preventing downstream activation of the HH pathway signaling pathway [71-73]. It has favorable blood-brain barrier penetration and high tissue penetration, making it a viable treatment for medulloblastoma [70]. Unfortunately, SMO mutations in Q476 and D473, prevent Sonidegib binding. Other mutations, including S533 and W535, confer resistance to Sonidegib [73, 74]. Currently, there are 2 ongoing clinical trials using this drug to treat breast and ovarian cancer (Table 1).
Saridegib is a potent SMO inhibitor, also known as IPI-926, a cyclopamine derivative. Studies have been shown to benefit from saridegib treatment in medulloblastoma, chondrosarcoma, and ovarian cancer [75-78]. The reduction of tumor mass in the preclinical model is explained by the decrease in the expression of GLI1 and PTCH1 [79].
4.3 GLI inhibitors
Gants, are the first GLI inhibitors, reported in the literature by the National Cancer Institute during GLI assay screening in HEK923 cells [80]. GLI antagonists can directly bind to GLI proteins and prevent their translocation into the nucleus. Gant 58 and Gant 61 are the most studied agents that have been used pre-clinically. They inhibit both GLI1 and GLI2 causing a significant decrease in tumor growth (80, 81). Studies have been shown that Gant 61 treatment induces cell cycle arrest by decreasing levels of the HH target such as CCND1 and increasing the expression of p21 [82, 83].
Arsenic trioxide (ATO) is an FDA-approved medication for pro-myelocytic leukemia. ATO has been found to inhibit HH signaling pathway by binding to GLI1 and GLI2 protein and prevents their binding to DNA as a transcription factor [84, 85]. ATO has been shown to increase apoptosis, reduce tumor cell growth and decrease expression of HH target genes both in vitro and in vivo [86-89].
5. Clinical Trials
The SHH signaling pathway has been related to several types of cancer. Clinical applications of molecules that block the SHH pathway have shown to have a significant benefit in preclinical and clinical studies to treat several types of female cancer (Table 1).
6. Conclusion
Although there is a remarkable growth of knowledge regarding the involvement of HH pathway in female cancer development, the precise mechanism underlying activation of HH contributing to a variety of female cancer phenotypes is mostly unknown. For instance, what are the key HH target genes related to activated canonical and non-canonical HH pathways in a variety of female cancers? What is the difference of HH regulated genes between different types of female cancer? Are there specific GLI response genes for each type of female cancer? A better understanding of these changes will lead to the development of new therapies for women with cancers.
Acknowledgments
This study was supported in part by the National Institutes of Health grants: R01 HD094378 and R01 ES028615. The figures were created using BioRender.com
Conflicts of Interest
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 287 (1980): 795-801.
- Dessaud E, McMahon AP, Briscoe J. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development (Cambridge, England) 135 (2008): 2489-2503.
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 272 (1996): 1668-1671.
- Echelard Y, Epstein DJ, St-Jacques B, et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75 (1993): 1417-1430.
- Tong W, Qiu L, Qi M, et al. GANT-61 and GDC-0449 induce apoptosis of prostate cancer stem cells through a GLI-dependent mechanism. Journal of cellular biochemistry 119 (2018): 3641-3652.
- Kakiuchi S, Minami Y, Miyata Y, et al. NANOG Expression as a Responsive Biomarker during Treatment with Hedgehog Signal Inhibitor in Acute Myeloid Leukemia. International journal of molecular sciences 18 (2017).
- Chen C, Breslin MB, Lan MS. Sonic hedgehog signaling pathway promotes INSM1 transcription factor in neuroendocrine lung cancer. Cellular signalling 46 (2018): 83-91.
- Chaturvedi NK, Kling MJ, Coulter DW, et al. Improved therapy for medulloblastoma: targeting hedgehog and PI3K-mTOR signaling pathways in combination with chemotherapy. Oncotarget 9 (2018): 16619-16633.
- Tang CT, Lin XL, Wu S, et al. NOX4-driven ROS formation regulates proliferation and apoptosis of gastric cancer cells through the GLI1 pathway. Cellular signalling 46 (2018): 52-63.
- Song K, Zheng G, Zhao Y. Liver kinase B1 suppresses growth of lung cancer cells through sonic hedgehog signaling pathway. Cell biology international 42 (2018): 994-1005.
- Di Mauro C, Rosa R, D'Amato V, et al. Hedgehog signalling pathway orchestrates angiogenesis in triple-negative breast cancers. British journal of cancer 116 (2017): 1425-1435.
- Lin Z, Li S, Sheng H, et al. Suppression of GLI sensitizes medulloblastoma cells to mitochondria-mediated apoptosis. Journal of cancer research and clinical oncology 142 (2016): 2469-2478.
- Wang L, Jin JQ, Zhou Y, et al. Gli is activated and promotes epithelial-mesenchymal transition in human esophageal adenocarcinoma. Oncotarget 9 (2018): 853-865.
- Gonnissen A, Isebaert S, McKee CM, et al. The Effect of Metformin and GANT61 Combinations on the Radiosensitivity of Prostate Cancer Cells. International journal of molecular sciences 18 (2017).
- Gonnissen A, Isebaert S, McKee CM, et al. The hedgehog inhibitor GANT61 sensitizes prostate cancer cells to ionizing radiation both in vitro and in vivo. Oncotarget 7 (2016): 84286-84298.
- Li X, Chen F, Zhu Q, et al. Gli-1/PI3K/AKT/NF-kB pathway mediates resistance to radiation and is a target for reversion of responses in refractory acute myeloid leukemia cells. Oncotarget 7 (2016): 33004-33015.
- Williamson AJ, Doscas ME, Ye J, et al. The sonic hedgehog signaling pathway stimulates anaplastic thyroid cancer cell motility and invasiveness by activating Akt and c-Met. Oncotarget 7 (2016): 10472-10485.
- Magistri P, Battistelli C, Strippoli R, et al. SMO Inhibition Modulates Cellular Plasticity and Invasiveness in Colorectal Cancer. Frontiers in pharmacology 8 (2017): 956.
- Li J, Cai J, Zhao S, et al. GANT61, a GLI inhibitor, sensitizes glioma cells to the temozolomide treatment. Journal of experimental & clinical cancer research: CR 35 (2016): 184.
- Kurebayashi J, Kanomata N, Koike Y, et al. Comprehensive immunohistochemical analyses on expression levels of hedgehog signaling molecules in breast cancers. Breast cancer (Tokyo, Japan) (2018).
- Ozretic P, Trnski D, Musani V, et al. Non-canonical Hedgehog signaling activation in ovarian borderline tumors and ovarian carcinomas. International journal of oncology 51 (2017): 1869-1877.
- Liao X, Siu MK, Au CW, et al. Aberrant activation of hedgehog signaling pathway contributes to endometrial carcinogenesis through beta-catenin. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc 22 (2009): 839-847.
- Zhang F, Ren CC, Liu L, et al. SHH gene silencing suppresses epithelial-mesenchymal transition, proliferation, invasion, and migration of cervical cancer cells by repressing the hedgehog signaling pathway. Journal of cellular biochemistry 119 (2018): 3829-3842.
- Garcia N, Bozzini N, Baiocchi G, et al. May Sonic Hedgehog proteins be markers for malignancy in uterine smooth muscle tumors? Hum Pathol 50 (2016): 43-50.
- Wörmann B. Breast cancer: basics, screening, diagnostics and treatment. Med Monatsschr Pharm 40 (2017): 55-64.
- Riobo-Del Galdo NA, Lara Montero Á, Wertheimer EV. Role of Hedgehog Signaling in Breast Cancer: Pathogenesis and Therapeutics. Cells 8 (2019).
- O'Toole SA, Machalek DA, Shearer RF, et al. Hedgehog overexpression is associated with stromal interactions and predicts for poor outcome in breast cancer. Cancer research 71 (2011): 4002-4014.
- Xuan Y, Lin Z. Expression of Indian Hedgehog signaling molecules in breast cancer. Journal of cancer research and clinical oncology 135 (2009): 235-240.
- Souzaki M, Kubo M, Kai M, et al. Hedgehog signaling pathway mediates the progression of non-invasive breast cancer to invasive breast cancer. Cancer science 102 (2011): 373-381.
- Kubo M, Nakamura M, Tasaki A, et al. Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer research 64 (2004): 6071-6074.
- Koga K, Nakamura M, Nakashima H, et al. Novel link between estrogen receptor alpha and hedgehog pathway in breast cancer. Anticancer research. 28 (2008): 731-740.
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a cancer journal for clinicians 67 (2017): 7-30.
- Rosen DG, Yang G, Liu G, et al. Ovarian cancer: pathology, biology, and disease models. Frontiers in bioscience (Landmark edition) 14 (2009): 2089-2102.
- Evangelista M, Tian H, de Sauvage FJ. The hedgehog signaling pathway in cancer. Clinical cancer research: an official journal of the American Association for Cancer Research 12 (2006): 5924-5928.
- Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nature reviews Cancer 3 (2003): 903-911.
- Bhattacharya R, Kwon J, Ali B, et al. Role of hedgehog signaling in ovarian cancer. Clinical cancer research: an official journal of the American Association for Cancer Research 14 (2008): 7659-7666.
- Ray A, Meng E, Reed E, et al. Hedgehog signaling pathway regulates the growth of ovarian cancer spheroid forming cells. International journal of oncology 39 (2011): 797-804.
- Chen X, Horiuchi A, Kikuchi N, et al. Hedgehog signal pathway is activated in ovarian carcinomas, correlating with cell proliferation: it's inhibition leads to growth suppression and apoptosis. Cancer science 98 (2007): 68-76.
- Yang L, He J, Huang S, et al. Activation of hedgehog signaling is not a frequent event in ovarian cancers. Molecular cancer 8 (2009): 112.
- Schmid S, Bieber M, Zhang F, et al. Wnt and hedgehog gene pathway expression in serous ovarian cancer. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society 21 (2011): 975-980.
- Liao X, Siu MK, Au CW, et al. Aberrant activation of hedgehog signaling pathway in ovarian cancers: effect on prognosis, cell invasion and differentiation. Carcinogenesis 30 (2009): 131-140.
- Li Z, Li B, Pan J, et al. TNF-alpha enhances the effect of TGF-beta on Gli2 expression in the KG-1 leukemic cell line. Experimental and therapeutic medicine 8 (2014): 676-680.
- Takebe N, Miele L, Harris PJ, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nature reviews Clinical oncology 12 (2015): 445-464.
- Ferent J, Cochard L, Faure H, et al. Genetic activation of Hedgehog signaling unbalances the rate of neural stem cell renewal by increasing symmetric divisions. Stem cell reports 3 (2014): 312-323.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA: a cancer journal for clinicians 61 (2011): 69-90.
- Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. The New England journal of medicine 348 (2003): 518-527.
- Ueda T, Tsubamoto H, Inoue K, et al. Itraconazole Modulates Hedgehog, WNT/beta-catenin, as well as Akt Signalling, and Inhibits Proliferation of Cervical Cancer Cells. Anticancer research 37 (2017): 3521-3526.
- Bohr Mordhorst L, Ahlin C, Sorbe B. Prognostic impact of the expression of Hedgehog proteins in cervical carcinoma FIGO stages I-IV treated with radiotherapy or chemoradiotherapy. Gynecologic oncology 135 (2014): 305-311.
- Sharma A, De R, Javed S, Srinivasan R, et al. Sonic hedgehog pathway activation regulates cervical cancer stem cell characteristics during epithelial to mesenchymal transition. J Cell Physiol (2019).
- Raglan O, Kalliala I, Markozannes G, et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int J Cancer. 145 (2019): 1719-1730.
- Kim KH, Kim JM, Choi YL, et al. Expression of sonic hedgehog signaling molecules in normal, hyperplastic and carcinomatous endometrium. Pathol Int 59 (2009): 279-287.
- Inoue K, Tsubamoto H, Sakata K, et al. Expression of Hedgehog Signals and Growth Inhibition by Itraconazole in Endometrial Cancer. Anticancer Res 36 (2016): 149-153.
- Polychronidou G, Kotoula V, Manousou K, et al. Mismatch repair deficiency and aberrations in the Notch and Hedgehog pathways are of prognostic value in patients with endometrial cancer. PLoS One 13 (2018): e0208221.
- Amant F, Coosemans A, Debiec-Rychter M, et al. Clinical management of uterine sarcomas. Lancet Oncol 10 (2009): 1188-1198.
- D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol 116 (2010): 131-139.
- Garcia N, Ayman A-H, Baracat CE, et al. Targeting Hedgehog pathway and DNAmethyltransferases supress the phenotype of uterine leiomyosarcoma cells. Research Square (2020).
- Garcia N, Carvalho K, Al-Hendy A, et al. Targeting Sonic Hedgehog Signaling in Human Leiomyosarcoma Cells by SMO andGLI1 Inhibition. Springer: Reproductive Science (2019): 13A.
- Yun JI, Kim HR, Park H, et al. Small molecule inhibitors of the hedgehog signaling pathway for the treatment of cancer. Archives of pharmacal research 35 (2012): 1317-1333.
- Stanton BZ, Peng LF, Maloof N, et al. A small molecule that binds Hedgehog and blocks its signaling in human cells. Nat Chem Biol 5 (2009): 154-156.
- Gan GN, Jimeno A. Emerging from their burrow: Hedgehog pathway inhibitors for cancer. Expert opinion on investigational drugs 25 (2016): 1153-1166.
- Cooper MK, Porter JA, Young KE, et al. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science (New York, NY) 280 (1998): 1603-1607.
- Incardona JP, Gaffield W, Kapur RP, et al. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development (Cambridge, England) 125 (1998): 3553-3562.
- Keeler RF. Cyclopamine and related steroidal alkaloid teratogens: their occurrence, structural relationship, and biologic effects. Lipids 13 (1978): 708-715.
- Taipale J, Chen JK, Cooper MK, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature 406 (2000): 1005-1009.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. The New England journal of medicine. 366 (2012): 2171-2179.
- Abidi A. Hedgehog signaling pathway: a novel target for cancer therapy: vismodegib, a promising therapeutic option in treatment of basal cell carcinomas. Indian journal of pharmacology 46 (2014): 3-12.
- Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. The New England journal of medicine 361 (2009): 1164-1172.
- Rudin CM, Hann CL, Laterra J, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. The New England journal of medicine 361 (2009): 1173-1178.
- Dijkgraaf GJ, Alicke B, Weinmann L, et al. Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer research. 71 (2011): 435-444.
- Pan S, Wu X, Jiang J, et al. Discovery of NVP-LDE225, a Potent and Selective Smoothened Antagonist. ACS medicinal chemistry letters. 1 (2010): 130-134.
- Sharpe HJ, Wang W, Hannoush RN, et al. Regulation of the oncoprotein Smoothened by small molecules. Nature chemical biology. 11 (2015): 246-255.
- Wang C, Wu H, Evron T, et al. Structural basis for Smoothened receptor modulation and chemoresistance to anticancer drugs. Nature communications 5 (2014): 4355.
- Wang C, Wu H, Katritch V, et al. Structure of the human smoothened receptor bound to an antitumour agent. Nature 497 (2013): 338-343.
- Danial C, Sarin KY, Oro AE, et al. An Investigator-Initiated Open-Label Trial of Sonidegib in Advanced Basal Cell Carcinoma Patients Resistant to Vismodegib. Clinical cancer research: an official journal of the American Association for Cancer Research 22 (2016): 1325-1329.
- Keysar SB, Le PN, Anderson RT, et al. Hedgehog signaling alters reliance on EGF receptor signaling and mediates anti-EGFR therapeutic resistance in head and neck cancer. Cancer research 73 (2013): 3381-3392.
- McCann CK, Growdon WB, Kulkarni-Datar K, et al. Inhibition of Hedgehog signaling antagonizes serous ovarian cancer growth in a primary xenograft model. PloS one 6 (2011): e28077.
- Lee MJ, Hatton BA, Villavicencio EH, et al. Hedgehog pathway inhibitor saridegib (IPI-926) increases lifespan in a mouse medulloblastoma model. Proceedings of the National Academy of Sciences of the United States of America 109 (2012): 7859-7864.
- Campbell VT, Nadesan P, Ali SA, et al. Hedgehog pathway inhibition in chondrosarcoma using the smoothened inhibitor IPI-926 directly inhibits sarcoma cell growth. Molecular cancer therapeutics 13 (2014): 1259-1269.
- Armas-Lopez L, Zuniga J, Arrieta O, et al. The Hedgehog-GLI pathway in embryonic development and cancer: implications for pulmonary oncology therapy. Oncotarget 8 (2017): 60684-60703.
- Lauth M, Bergstrom A, Shimokawa T, et al. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proceedings of the National Academy of Sciences of the United States of America 104 (2007): 8455-8460.
- Agyeman A, Jha BK, Mazumdar T, et al. Mode and specificity of binding of the small molecule GANT61 to GLI determines inhibition of GLI-DNA binding. Oncotarget 5 (2014): 4492-4503.
- Mazumdar T, Sandhu R, Qadan M, et al. Hedgehog signaling regulates telomerase reverse transcriptase in human cancer cells. PloS one 8 (2013): e75253.
- Srivastava RK, Kaylani SZ, Edrees N, et al. GLI inhibitor GANT-61 diminishes embryonal and alveolar rhabdomyosarcoma growth by inhibiting Shh/AKT-mTOR axis. Oncotarget 5 (2014): 12151-12165.
- Beauchamp EM, Ringer L, Bulut G, et al. Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway. The Journal of clinical investigation 121 (2011): 148-160.
- List A, Beran M, DiPersio J, et al. Opportunities for Trisenox (arsenic trioxide) in the treatment of myelodysplastic syndromes. Leukemia 17 (2003): 1499-1507.
- Bansal N, Farley NJ, Wu L, et al. Darinaparsin inhibits prostate tumor-initiating cells and Du145 xenografts and is an inhibitor of hedgehog signaling. Molecular cancer therapeutics 14 (2015): 23-30.
- Kerl K, Moreno N, Holsten T, et al. Arsenic trioxide inhibits tumor cell growth in malignant rhabdoid tumors in vitro and in vivo by targeting overexpressed Gli1. International journal of cancer 135 (2014): 989-995.
- Nakamura S, Nagano S, Nagao H, et al. Arsenic trioxide prevents osteosarcoma growth by inhibition of GLI transcription via DNA damage accumulation. PloS one 8 (2013): e69466.
- Yang D, Cao F, Ye X, et al. Arsenic trioxide inhibits the Hedgehog pathway which is aberrantly activated in acute promyelocytic leukemia. Acta haematologica 130 (2013): 260-267.
