The Relationship Between Positive Sentinel Lymph Node Biopsy and Residual Axillary Lymph Nodes Status in Early Stage Breast Cancer
Article Information
Maher H. Ibraheem1*, Mona Ali2, Amr Hafez1, Ghada Mohamed2, Rimoun Boutrus3, Omnia Talaat4, Mohamed Gamil1
1Department of surgical oncology, National cancer institute, Cairo University, Egypt
2Department of pathology, National cancer institute, Cairo University, Egypt
3Department of radiation oncology, National cancer institute, Cairo University, Egypt
4Department of nuclear medicine, National cancer institute, Cairo University, Egypt
*Corresponding Author: Dr. Maher H. Ibraheem, Department of surgical oncology, National cancer institute, Cairo University, Egypt
Received: 02 November 2019; Accepted: 12 November 2019 Published: 02 January 2020
Citation: Maher H. Ibraheem, Mona Ali, Amr Hafez, Ghada Mohamed, Rimoun Boutros, Omnia Talaat, Mohamed Gamil. The Relationship Between Positive Sentinel Lymph Node Biopsy and Residual Axillary Lymph Nodes Status in Early Stage Breast Cancer. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 001-014.
Share at FacebookAbstract
Introduction: For patients with clinically negative axilla, sentinel lymph node biopsy (SLNB) is the standard method for axillary staging Because the SLBNs are the only positive nodes in approximately 40–70% of patients with pathologically proven positive axillae after completion axillary lymph node dissection (ALND), the treatment of patients with a positive SLBN has been reconsidered and the development of predictive tools that select the patients whom routine ALND could be avoided safely.
Purpose: to characterize the patients in whom completion ALND can be avoided in spite of positive SLNB. Patients and methods: This retrospective study included all patients who had SLNB at the National Cancer Institute, Cairo, Egypt, between January 2013 and December 2015. The characteristics of the special group with positive SLNB and node-negative upon completion ALND were studied.
Results: out of 66 patients with clinically negative axillae, SLNB was negative in 36 patients, with no more ALND, and SLNB was positive in 30 patients for whom completion ALND was done, and revealed that 63.4% (19 out of 30 patients) had no other positive nodes after completion ALND.
Conclusion: In patients with clinically negative axillae and positive sentinel SLNB, A combination of multiple predictive parameters as, the number of positive SLNs, the ratio between metastatic SLNs and total number of SLN retrieved, extracapsular invasion, and lymphovascular invasion were significant predictors for the risk of non- SLN involvement and can identify the patients with positive SLNB for whom routine ALND could be safely avoided.
Keywords
Positive SLN; Safely avoided ALND
Positive SLN articles, Safely avoided ALND articles
Positive SLN articles Positive SLN Research articles Positive SLN review articles Positive SLN PubMed articles Positive SLN PubMed Central articles Positive SLN 2023 articles Positive SLN 2024 articles Positive SLN Scopus articles Positive SLN impact factor journals Positive SLN Scopus journals Positive SLN PubMed journals Positive SLN medical journals Positive SLN free journals Positive SLN best journals Positive SLN top journals Positive SLN free medical journals Positive SLN famous journals Positive SLN Google Scholar indexed journals Safely avoided ALND articles Safely avoided ALND Research articles Safely avoided ALND review articles Safely avoided ALND PubMed articles Safely avoided ALND PubMed Central articles Safely avoided ALND 2023 articles Safely avoided ALND 2024 articles Safely avoided ALND Scopus articles Safely avoided ALND impact factor journals Safely avoided ALND Scopus journals Safely avoided ALND PubMed journals Safely avoided ALND medical journals Safely avoided ALND free journals Safely avoided ALND best journals Safely avoided ALND top journals Safely avoided ALND free medical journals Safely avoided ALND famous journals Safely avoided ALND Google Scholar indexed journals negative axilla articles negative axilla Research articles negative axilla review articles negative axilla PubMed articles negative axilla PubMed Central articles negative axilla 2023 articles negative axilla 2024 articles negative axilla Scopus articles negative axilla impact factor journals negative axilla Scopus journals negative axilla PubMed journals negative axilla medical journals negative axilla free journals negative axilla best journals negative axilla top journals negative axilla free medical journals negative axilla famous journals negative axilla Google Scholar indexed journals sentinel lymph node biopsy articles sentinel lymph node biopsy Research articles sentinel lymph node biopsy review articles sentinel lymph node biopsy PubMed articles sentinel lymph node biopsy PubMed Central articles sentinel lymph node biopsy 2023 articles sentinel lymph node biopsy 2024 articles sentinel lymph node biopsy Scopus articles sentinel lymph node biopsy impact factor journals sentinel lymph node biopsy Scopus journals sentinel lymph node biopsy PubMed journals sentinel lymph node biopsy medical journals sentinel lymph node biopsy free journals sentinel lymph node biopsy best journals sentinel lymph node biopsy top journals sentinel lymph node biopsy free medical journals sentinel lymph node biopsy famous journals sentinel lymph node biopsy Google Scholar indexed journals axillary staging articles axillary staging Research articles axillary staging review articles axillary staging PubMed articles axillary staging PubMed Central articles axillary staging 2023 articles axillary staging 2024 articles axillary staging Scopus articles axillary staging impact factor journals axillary staging Scopus journals axillary staging PubMed journals axillary staging medical journals axillary staging free journals axillary staging best journals axillary staging top journals axillary staging free medical journals axillary staging famous journals axillary staging Google Scholar indexed journals predictive tools articles predictive tools Research articles predictive tools review articles predictive tools PubMed articles predictive tools PubMed Central articles predictive tools 2023 articles predictive tools 2024 articles predictive tools Scopus articles predictive tools impact factor journals predictive tools Scopus journals predictive tools PubMed journals predictive tools medical journals predictive tools free journals predictive tools best journals predictive tools top journals predictive tools free medical journals predictive tools famous journals predictive tools Google Scholar indexed journals prognosis articles prognosis Research articles prognosis review articles prognosis PubMed articles prognosis PubMed Central articles prognosis 2023 articles prognosis 2024 articles prognosis Scopus articles prognosis impact factor journals prognosis Scopus journals prognosis PubMed journals prognosis medical journals prognosis free journals prognosis best journals prognosis top journals prognosis free medical journals prognosis famous journals prognosis Google Scholar indexed journals radiologically articles radiologically Research articles radiologically review articles radiologically PubMed articles radiologically PubMed Central articles radiologically 2023 articles radiologically 2024 articles radiologically Scopus articles radiologically impact factor journals radiologically Scopus journals radiologically PubMed journals radiologically medical journals radiologically free journals radiologically best journals radiologically top journals radiologically free medical journals radiologically famous journals radiologically Google Scholar indexed journals axillary recurrence articles axillary recurrence Research articles axillary recurrence review articles axillary recurrence PubMed articles axillary recurrence PubMed Central articles axillary recurrence 2023 articles axillary recurrence 2024 articles axillary recurrence Scopus articles axillary recurrence impact factor journals axillary recurrence Scopus journals axillary recurrence PubMed journals axillary recurrence medical journals axillary recurrence free journals axillary recurrence best journals axillary recurrence top journals axillary recurrence free medical journals axillary recurrence famous journals axillary recurrence Google Scholar indexed journals axillary metastases articles axillary metastases Research articles axillary metastases review articles axillary metastases PubMed articles axillary metastases PubMed Central articles axillary metastases 2023 articles axillary metastases 2024 articles axillary metastases Scopus articles axillary metastases impact factor journals axillary metastases Scopus journals axillary metastases PubMed journals axillary metastases medical journals axillary metastases free journals axillary metastases best journals axillary metastases top journals axillary metastases free medical journals axillary metastases famous journals axillary metastases Google Scholar indexed journals
Article Details
1. Introduction
Staging of the axilla is one of the most prognostic factors, for prognosis and local control in patients with breast [1]. For patients with negative axilla clinically and radiologically, sentinel lymph node biopsy (SLNB) is the standard method of staging of the axilla with less morbidity than axillary lymph node dissection (ALND) [2]. False-negative result is one of the drawbacks of SLNB, which may increase the risk for axillary recurrence. However, despite of about 5 to 10 percent false-negative rate with SLNB found in studies in which completion ALND has been done, several studies still conclude that axillary recurrence rates are low after a negative SLNB alone in early-stage breast cancer patients [3]. SLNB is highly accurate in patients with early tumors due to the low risk of axillary metastases, and no reports of false-negative SLN biopsy for lesions less than 1.5 cm [4]. Because the SLNs are the only positive nodes in approximately 40-70% of patients with pathologically proven positive axillae after completion ALND, the treatment of patients with a positive SLN has been reconsidered and the development of predictive tools that select the patients whom routine ALND could be avoided safely [5].
2. Method
2.1 Methodology of SLNB2.1.1 Radioactive Colloid and Mobile Gamma Camera &Gamma Probe: Preoperative injection of TC99 labeled albumin nano-colloid peri-areolar sub- dermal corresponding to site of the lesion 12-24 hours prior to operative time. Intra-operatively prior to sterilization; the camera detector was placed over the breast and axilla vertically at distance of 15 cm to acquire an overview image of the field and to assess radio-active uptake by the SLN(s) this take nearly 60-120 seconds (Figure 1). Then this detector was placed nearer to the field to precisely locate the detected SLN uptake using a cross laser pointer on the field that is matched to the hotspot on the camera screen and this site of laser cross is marked. Confirmation of the proper site of the SLN using the gamma probe, after scanning internal mammary region, infraclavicular region and axilla (Figure 2). With field sterilization the detector of mobile gamma camera was sterile-draped in such a way as to allow placement and movement above and within the surgical field. Surgical incision was done guided by the marked site. In some cases when the breast tumor was too close to the SLN, we have a radio-active shine through effect and we couldn`t differentiate which is which, we used the portable gamma camera hiding option to hide the radio-activity of the tumor for better visualization of the SLN. After incision the hand held gamma probe was used to detect the lymph node with high tracer uptake in (SLN) (Figure 3). After retrieval of SLN, then it was imaged again using gamma camera and gamma probe to be sure that it`s the SLN(s) (Figure 4). The surgical field is checked again using the mobile gamma camera and hand held gamma probe to be sure that all radio-active nodes have been removed. The retrieved SLN(s) was sent for frozen section. The SLN(s) was send finally for paraffin section for the final pathology result.
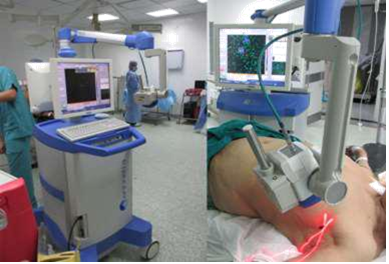
Figure 1: Rapid pre-operative scanning, to properly localize the site of the SLN(s).
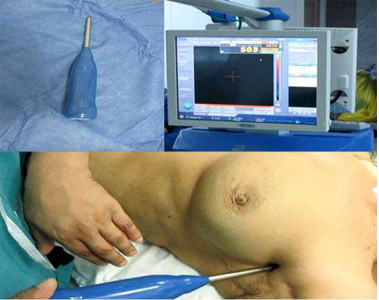
Figure 2: Gamma probe used in SLN detection pre operatively with corresponding high count on the screen.
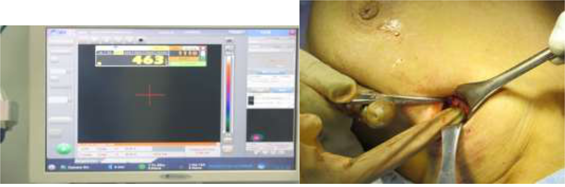
Figure 3: Gamma probe inside surgical field detecting the SLN, with high count on the screen.
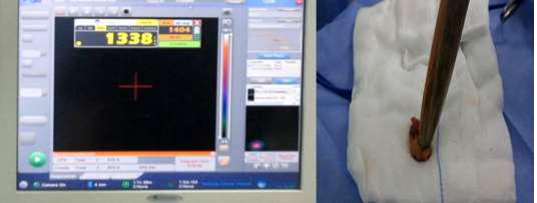
Figure 4: Gamma probe confirming retrieval of SLN EX. Vivo with high count.
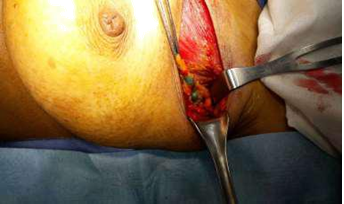
Figure 5: A blue afferent lymphatic vessel draining into a blue-stained node.
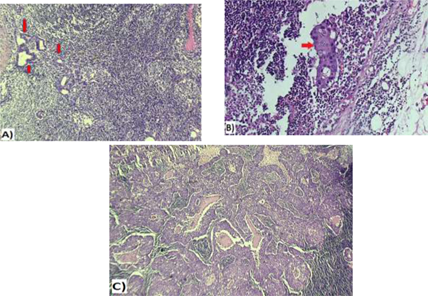
Figure 6: A&B: Micrometastatic deposit of duct carcinoma involving sentinel lymph node (red arrows), measuring < 2 mm in greatest dimension (A&B 20x); C: Macrometastatic duct carcinoma within a sentinel lymph node (> 2 mm, 20x).
Patent blue dye is injected subdrmally in circumareolar region, once the patient is asleep in theatre. This dye travels to and stains the sentinel nodes blue, thereby assisting the surgeon to find the correct lymph nodes (Figure 5). The retrieved SLN(s) was sent for frozen section. The SLN(s) was send finally for paraffin section for the final pathology result (Figure 6).
3. Results
A total of 66 patients who presented with clinically negative nodes, underwent SLN biopsy. According to Frozen section results of detected SLN(s):
- SLNB was negative in 36 patients out of 66, for all of them SLNB was enough with no more axillary dissection.
- SLNB was positive in 30 patients for whom ALND was done and the number of SLN taken as a biopsy was ≤ 2 except in 2 patients who had 3 SLNs taken.
3.1 SLNB characteristics
ALND was done in patients with positive SLNB and revealed that 63.4% (19 out of 30 patients) had no other positive nodes after completion ALND in the final pathology results (group 1) in spite of, the number of lymph nodes that were dissected, ranged from 9-21 lymph nodes. Among those, 12 patients had only one positive sentinel node & 6 patients had two positive sentinel nodes and one had three positive sentinel nodes (Table 1A). It has been found that 36.6% (11patients) had other positive nodes after completion ALND in the final pathological results (group 2) Among those, 7 patients had only one positive sentinel node & 3 patients had two positive sentinel nodes and one had three positive sentinel nodes (Table 1B).
|
No. of +ve SLN. |
No. of patients with –ve non-SLN |
|
1 |
12 |
|
2 |
6 |
|
3 |
1 |
Table 1A: Relation of number of patients with –ve non-SLN (completion ALND) to number of positive sentinel nodes.
|
No. of +ve SLN. |
No. of patients with +ve non SLN |
|
1 |
7 |
|
2 |
3 |
|
3 |
1 |
Table 1B: Relation of number of patients with +ve non-SLN (completion ALND) to number of positive sentinel nodes.
In group 1: Ratio of positive SLN to total number of SLN was =1 in 10 patients (52.6%) and ? 1 in 9 patients (47.4%) (Figure 7).
In group 2: Ratio of positive SLN to total number of SLN was =1 in 10 patients (90.9%) and ? 1 in one patient %) (Figure 7).
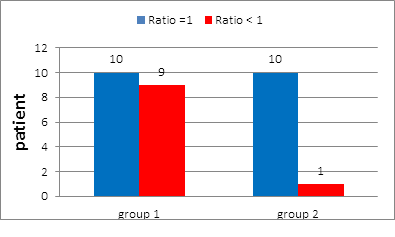
Figure 7: Ratio of +ve SLN to total number of SLN in group 1 and in group 2.
|
Patient. |
No. of SLNB. |
No. of |
Ratio of +ve SLN |
No. of nodes |
NO. of +ve nodes |
|
1 |
2 |
1 |
=0.5 |
14 |
0 |
|
2 |
3 |
1 |
<0.5 |
14 |
0 |
|
3 |
2 |
1 |
=0.5 |
13 |
0 |
|
4 |
3 |
3 |
=1 |
18 |
0 |
|
5 |
2 |
2 |
=1 |
16 |
0 |
|
6 |
1 |
1 |
=1 |
14 |
0 |
|
7 |
2 |
1 |
=0.5 |
20 |
0 |
|
8 |
2 |
2 |
=1 |
14 |
0 |
|
9 |
1 |
1 |
=1 |
11 |
0 |
|
10 |
2 |
2 |
=1 |
9 |
0 |
|
11 |
2 |
1 |
=0.5 |
12 |
0 |
|
12 |
2 |
2 |
=1 |
15 |
0 |
|
13 |
2 |
1 |
=0.5 |
13 |
0 |
|
14 |
1 |
1 |
=1 |
12 |
0 |
|
15 |
2 |
1 |
=0.5 |
10 |
0 |
|
16 |
3 |
1 |
<0.5 |
13 |
0 |
|
17 |
3 |
2 |
>0.5 |
21 |
0 |
|
18 |
1 |
1 |
=1 |
15 |
0 |
|
19 |
2 |
2 |
=1 |
14 |
0 |
Table 2: Relationship Between ratio of Positive Sentinel Lymph Node Biopsy & the pathological results of axillary lymph node dissection (group 1).
|
Patient. |
No. of SLNB. |
No. of +ve SLN. |
Ratio of +ve |
No. of nodes in |
NO. of +ve nodes |
|
1 |
1 |
1 |
=1 |
16 |
2 |
|
2 |
1 |
1 |
=1 |
14 |
7 |
|
3 |
2 |
2 |
=1 |
14 |
3 |
|
4 |
1 |
1 |
=1 |
12 |
6 |
|
5 |
2 |
2 |
=1 |
24 |
8 |
|
6 |
3 |
2 |
>0.5 |
13 |
2 |
|
7 |
3 |
3 |
=1 |
11 |
4 |
|
8 |
1 |
1 |
=1 |
20 |
2 |
|
9 |
2 |
2 |
=1 |
21 |
7 |
|
10 |
1 |
1 |
=1 |
21 |
3 |
|
11 |
1 |
1 |
=1 |
13 |
3 |
Table 3: Relationship Between ratio of +ve Sentinel Lymph Node Biopsy & the pathological results of axillary lymph node dissection (in group 2).
In group 1: 3 patients (15.7%) had SLN with Focal or minimal extracapsular extension, 2 patients (10.5%) had extracapsular invasion (ECI) (Figure 8), and all positive SLNs had macrometastases.
In group 2: 2 patients (18.2%) had SLN with Focal or minimal extracapsular extension, 7 patients (63.6%) had extracapsular invasion (ECI) (Figure 8), and all positive SLNs had macrometastases.
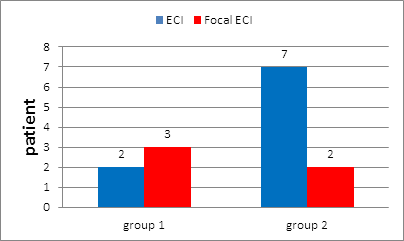
Figure 8: Extracapsular invasion of +ve SLN in group 1 and in group 2.
In group 1: Patients' age ranged from 35-66 years with the mean age at 52.5 Among these patients 57.9 % (11 patients) were post-menopausal while 42.1% (8 patients) were premenopausal (Figure 9).
In group 2: Among these patients 54.5% (6 patients) were post-menopausal, 45.5% (5 patients) were premenopausal (Figure 9). Patients & tumour characteristics were illustrated in Table 4.

Figure 9: Age distribution in group 1 and in group 2.
In group1: Conservative breast surgery (CBS) has been done for 13 patients (68.4%) while 6 patients (31.6%) underwent modified radical mastectomy (MRM) (Figure 10).
In group 2: CBS have been done for 7 patients (63.6%) while 4 patients (36.4%) underwent MRM (Figure 10).
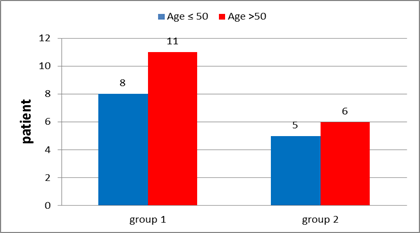
Figure 9: Age distribution in group 1 and in group 2.
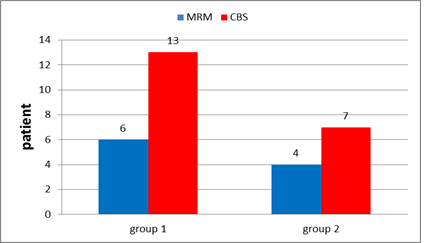
Figure 10: Type of surgery in group 1 and in group 2.
|
|
Group 1 |
Group 2 |
|
Age Premenopausal post-menopausal |
8 patients (42.1%) 11 patients (57.9 %) |
5 patients (45.5%) 6 patients (54.5 %) |
|
Type of surgery CBS MRM |
13 patients (68.4%) 6 patients (31.6%) |
7 patients (63.6%) 4 patients (36.4%) |
|
Tumor size (Figure 22) Tis T1 T2 |
one patient (5.3%) one patient (5.3%) 17 patients (89.5%) |
________ one patient (9.1%) 10 patients (90.9%) |
|
Tumour histopathology (Figure 23) DCIS IDC ILC Mixed IDC & ILC Mucinous |
one patient (5.3 %) 14 patients (73.7%) 2 patients (10.5%) one patient (5.3 %) one patient (5.3 %) |
_____________ 8 patients (72.7%) 2 patients (18.2%) one patient (9.1 %) ____________ |
|
Tumour grade (Figure 24) Grade I Grade II Grade III No grade |
2 patients (10.5%) 12 patients (63.2%) 1 patient (5.3 %) 4 patients (21 %) |
___________ 7 patients (63.6%) 1 patient (9.1 %) 3 patients (27 %) |
|
Lymphovascular invasion |
3 patients (15.7%) |
6 patients (54.5%) |
Table 4: Patient and tumour characteristics in both group 1 and 2.
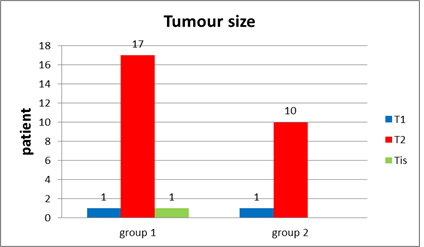
Figure 11: T stage (size of tumor) in group 1 and in group 2.
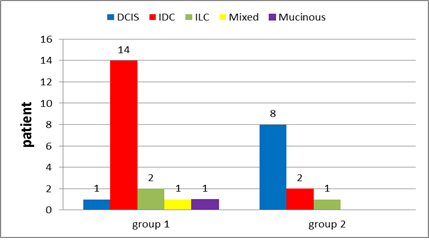
Figure 12: Tumor histopathology in group 1 and in group 2.
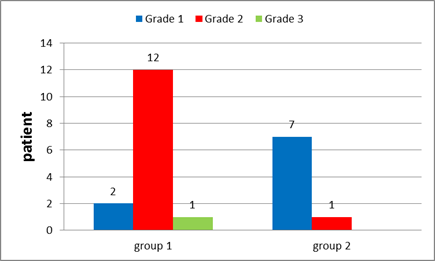
Figure 13: Tumour Grade in group 1and in group 2.
In group 1: Hormonal status were done to all patients with ER positive found in 78.9% of the patients (Figure 14), PR positive in 63.2% (Figure 15), negative HER 2neu in 73.7% (Figure 16) and KI 67 is high in 5 patients 26.4%. After interpretation of data according to molecular classification, 84.2% were Luminal A&B, with 9 patients in the Luminal A group &7 patients in the Luminal B group (Table 4).
|
No of +ve SLN. |
Group 1 |
||||
|
Luminal A |
Luminal B |
Triple negative |
HER2 |
TOTAL |
|
|
1 |
5 |
5 |
1 |
1 |
12 |
|
2 |
3 |
2 |
0 |
1 |
6 |
|
3 |
1 |
0 |
0 |
0 |
1 |
Table 5: No. of +ve SLN in relation to tumour molecular biology in group 1.
In group 2: Hormonal status were done to all patients with ER positive found in 81.8% of the patients (Figure 14), PR positive in 81.8% (Figure 15), negative HER 2neu in 36.4% (Figure 16) and KI 67 is high in 4 patients 36%. After interpretation of data according to molecular classification, 81.8% were Luminal A&B, with 2 patients in the Luminal A group &7 patients in the Luminal B group (Table 5).
|
No of +ve SLN. |
Group 2 |
||||
|
Luminal A |
Luminal B |
Triple negative |
HER2 |
TOTAL |
|
|
1 |
2 |
3 |
1 |
1 |
7 |
|
2 |
0 |
3 |
0 |
0 |
3 |
|
3 |
0 |
1 |
0 |
0 |
1 |
Table 6: No of +ve SLN in relation to tumour molecular biology in group 2.
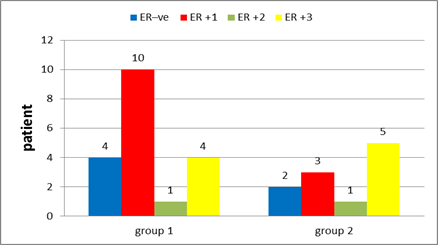
Figure 14: Estrogen receptors in group 1 and in group 2.
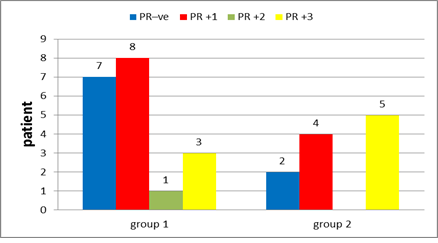
Figure 15: Progesterone receptors in group 1 and in group 2.
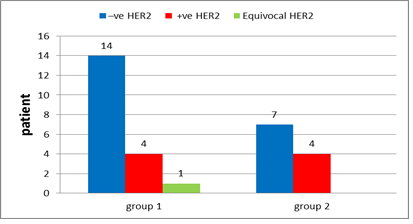
Figure 16: Her2-neu receptors in group 1 and in group 2.
4. Discussion
SLNB has become the cornerstone in evaluation of axillary lymph node status instead of the conventional ALND with significant lower morbidity. SLNB is a staging modality rather than a therapeutic technique, and it is mainly depends on surgical and pathologic accuracy, with a low false-negative rate [5]. Because the SLNs are the only positive nodes in approximately 40-70% of patients with pathologically proven positive axillae after completion ALND, the treatment of patients with a positive SLN has been reconsidered and the development of predictive tools that select the patients whom routine ALND could be avoided safely [5]. In this study, more than 63.4% of our patients with a positive SLNs had no other positive nodes in ALND suggesting that the majority of patients with positive SLNs did not benefit from ALND. Based on these results, it has been found that, the number of SLN, the proportion of involved SLNs among all removed SLNs, the presence of extracapsular invasion and the presence of lympho-vascular invasion were important predictors of non-SLN (completion ALND) status.
In this study, it has been found that the more the number of removed SLN, the more the number of positive nodes in SLN, which resulted in increase the ratio of positive SLNs to the total number of removed SLNs up to one, there was subsequently increase in non SLN ( completion ALND) positivity as in group 2, ratio equal to 1was found in 10 out of 11 patients, who had other non SLN (completion ALND) metastases.
Van Zee et al, in a study of 702 patients with a tumor-involved SLN, found that the number of negative SLNs was significantly associated with non-SLN (completion ALND) status [6]. Barranger et al, also used this parameter as the third predictor of non-SLN (completion ALND) status [7]. It is also supported by another study by Swanson and Kennecke which shows a higher percentage of positive SLNs to be predictive of higher likelihood of axillary lymph node (ALN) involvement as well as decreased survival [8]. In this study, the presence of extracapsular invasion in the positive SLN the more the number of positive LN that has been found in axillary evacuation as 7 out of 9 patients with extracapsular invasion had other non SLN (completion ALND) metastases. Saidi et al., also developed a score based on data for 34 patients with positive SLN biopsy findings, taking in consideration the extracapsular extension of SN metastasis, which is a powerful predictor of non-SLN(completion ALND) metastasis [9].
Choi et al. consider that completion ALND is the standard of care for patients with extra nodal extension of sentinel node metastasis, regardless of the number of positive nodes
[10]. It is also supported by Stranzl et al who recommended that all patients with extra nodal extension in any number of sentinel nodes should perform completion ALND because of the high possibility of axillary nodal metastasis associated with extra nodal extension [11]. In this study, the presence of lympho-vascular invasion attributes to increase in the number of positive LN as in group 2, lympho-vascular invasion was found in 6 out of 11 patients. Barranger et al. stated that lymph vascular invasion is poor prognostic factor and correlates with high incidence of residual axillary nodal metastasis in patients with positive SLN metastasis [7].
Chu et al., used categorical data on the TNM system to classify SLN metastases and tumor size. They concluded that T1a tumors or T1/T2 tumors with micro metastases to the SLN could avoid completion ALND. They found 6% and 10% of non-SLN ( completion ALND) metastasis in patients with SLN-micrometastatic T1 and T2 tumors, respectively [12]. Interestingly, in this study the degree of nodal involvement in the largest node was not a predictor of non SLN metastases as, all patients had SLN with macrometastases.
Reynolds et al., found that tumors with SLN micro metastases had no metastasis in non-SLNs (completion ALND [13]. Mazzarol et al., from Milan also detected low incidence of metastatic non-SLNs (completion ALND) in cases of micrometastatic SLNs (18%) than in cases of macrometastatic SLNs (59%) [14]. Cserni suggested that micrometastases confined to the sinuses of only one SLN, with tumors ≤ 1.8 cm, are the most likely to have no further axillary nodal metastasis. However, the number of patients in this group was very low [15].
In this study, tumor characteristics did not appear to influence the decision to perform ALND. as, tumor size, grade and histological subtype. All of which are factors deemed to be important in determining further ALN involvement, were not significantly predictors for non SLN metastases. This results are also supported by data from Swanson & Kennecke, There was lack of difference in characteristics between groups (SLNB and SLNB with completion ALND) and the surgical decision based mainly on the status of the axilla itself [8]. Multivariate analysis also showed that primary tumor size also significantly influenced the risk of non-SLN involvement, the risk was 0% in patients with pT1a, b tumors, 17% in those with pT1c tumors, and 67% in those with tumors measuring more than 20 mm. In the series of 157 cases published by Chu et al., the rate of non-SLN( completion ALND) involvement increased from 13 to 38% from stage T1b to stage T2 tumors [12]. Results of this study are in keeping with those reported in the Z0011 trial which did not demonstrate an advantage for completion ALND in patients with early breast cancer who had 1–2 positive SLNs [16]. As patients in group 1 in this study had clinical T1-T2 invasive breast cancer, no palpable axillary lymph nodes, 1 to 2 SLNs containing metastases identified by frozen section, no other positive non SLN and positive hormonal receptors, and most of the patients were in the luminal A group in comparison with group 2 in which most of the patients (63.6%) were in the luminal B group, but data are limited due to the low number of patients.
5. Conclusion
In patients with early breast cancer with clinically negative axillary lymph nodes and positive sentinel lymph nodes by frozen section, it has been found that the number of positive SLNs, the ratio between metastatic SNs and total number of sentinel lymph nodes retrieved, extracapsular invasion, and lymphovascular invasion were significant predictors for the risk of non- SLN involvement. Combination of these predictive parameters can identify patients with a positive SLNB for whom routine ALND could be safely avoided.
References
- Edge S, Carlson R. Breast cancer staging: predicting outcome and response to treatment. Breast Surgical Techniques and Interdisciplinary (2010): 269-285.
- Mansel RE1, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. Journal of the National Cancer Institute 98 (2006): 599-609.
- Mabry H, Giuliano AE. Sentinel Node Mapping for Breast Cancer: Progress to Date and Prospects for the Future. Surgical Oncology Clinics of North America 16 (2007): 55-70.
- Bedrosian I, Reynolds C, Mick R, et al. Accuracy of sentinel lymph node biopsy in patients with large primary breast tumors. Cancer 88 (2000): 2540-2545.
- Stitzenberg KB, Meyer AA, Stern SL, et al. Extracapsular extension of the sentinel lymph node metastasis: a predictor of nonsentinel node tumor burden. Ann Surg 237 (2003): 607-612.
- Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Annals of surgical 10 (2003): 1140-1151.
- Barranger E, Coutant C, Flahault A, et al. An axilla scoring system to predict non-sentinel lymph node status in breast cancer patients with sentinel lymph node involvement. Breast Cancer Research and Treatment 91 (2005): 113-119.
- Aslani N1, Swanson T, Kennecke H, et al. Factors that determine whether a patient receives completion axillary lymph node dissection after a positive sentinel lymph node biopsy for breast cancer in British. Can J Surg 54 (2011): 237-242.
- Saidi RF, Dudrick PS, Remine SG, et al. Nonsentinel Lymph Node Status After Positive Sentinel Lymph Node Biopsy in Early Breast Cancer. Am Surg 70 (2004): 101-105.
- Choi AH, Surrusco M, Rodriguez S, et al. Extranodal extension on sentinel lymph node dissection: why should we treat it differently?. Am Surg 80 (2014): 932-935.
- Stranzl H, Ofner P, Peintinger F. Postoperative irradiation in breast cancer patients with one to three positive axillary lymph nodes. Strahlenther Onkol 182 (2006): 583-588.
- Chu KU, Turner RR, Hansen NM, et al. Do all patients with sentinel node metastasis from breast carcinoma need complete axillary node dissection?. Ann Surg 229 (1999): 536-541.
- Reynolds C, Mick R, Donohue JH, et al. Sentinel lymph node biopsy with metastasis: can axillary dissection be avoided in some patients with breast cancer?. of Clinical Oncology 17 (1999): 1720-1726.
- Mazzarol G, Orvieto E, Citelli G, et al. Micrometastases of breastcarcinoma in axillary sentinel nodes and the nonsentinel nodestatus. Eur J Nucl Med (1999).
- Cserni G. Sentinel lymph-node biopsy-based prediction of further breast cancer metastases in the axilla. European Journal of Surgical Oncology (EJSO) 27 (2001): 532-538.
- Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305 (2011): 569-575.
