The Potential Role of Interleukin-11 in Epithelial Ovarian Cancer
Article Information
Nikhil Arora1, Nuzhat Ahmed2,3,4, Rodney B. Luwor1*
1Department of Surgery, The Royal Melbourne Hospital, The University of Melbourne, Victoria 3052, Australia
2Fiona Elsey Cancer Research Institute, Ballarat, Victoria 3353, Australia
3Federation University Australia, Ballarat, Victoria 3010, Australia
4The Hudson Institute of Medical Research, Victoria 3168, Australia
*Corresponding Author: Dr. Rodney B. Luwor , Level 5, Clinical Sciences Building, Department of Surgery, The Royal Melbourne Hospital, The University of Melbourne, Parkville, Victoria 3050, Australia
Received: 13 February 2019; Accepted: 21 February 2019; Published: 28 February 2019
Citation: Nikhil Arora, Nuzhat Ahmed, Rodney B. Luwor. The Potential Role of Interleukin-11 in Epithelial Ovarian Cancer. Journal of Cancer Science and Clinical Therapeutics 3 (2019): 28-47.
Share at FacebookAbstract
Interleukin-11 (IL-11) has recently gained attention in cancer biology, and there is now an upsurge in IL-11 research with studies implicating a role of IL-11 in several human cancers of epithelial and hematopoietic origins. The identification of the pro-tumorigenic activities elicited by IL-11 has placed a new focus on generating therapeutic agents that will inhibit the IL-11 signaling pathway. However, the precise role of IL-11 signaling remains elusive in epithelial ovarian cancer (EOC). The purpose of this review is to describe the ovarian tumor microenvironment and delineate the possible sources and functions of IL-11 in EOC. Taking a holistic view of the dynamics of IL-11 in other cancer types, we have elucidated the potential role of IL-11 signaling in ovarian tumor cell biology and have also provided future recommendations to exactly decipher and target the signaling pathway in EOC. Improved understanding of the ovarian tumor microenvironment, particularly the IL-11 biology, would shed more light on EOC progression and enable the design of better-targeted therapies to manage this disease effectively.
Keywords
Interleukin-11, Epithelial ovarian cancer, Tumor microenvironment
Interleukin-11 articles Interleukin-11 Research articles Interleukin-11 review articles Interleukin-11 PubMed articles Interleukin-11 PubMed Central articles Interleukin-11 2023 articles Interleukin-11 2024 articles Interleukin-11 Scopus articles Interleukin-11 impact factor journals Interleukin-11 Scopus journals Interleukin-11 PubMed journals Interleukin-11 medical journals Interleukin-11 free journals Interleukin-11 best journals Interleukin-11 top journals Interleukin-11 free medical journals Interleukin-11 famous journals Interleukin-11 Google Scholar indexed journals Epithelial ovarian cancer articles Epithelial ovarian cancer Research articles Epithelial ovarian cancer review articles Epithelial ovarian cancer PubMed articles Epithelial ovarian cancer PubMed Central articles Epithelial ovarian cancer 2023 articles Epithelial ovarian cancer 2024 articles Epithelial ovarian cancer Scopus articles Epithelial ovarian cancer impact factor journals Epithelial ovarian cancer Scopus journals Epithelial ovarian cancer PubMed journals Epithelial ovarian cancer medical journals Epithelial ovarian cancer free journals Epithelial ovarian cancer best journals Epithelial ovarian cancer top journals Epithelial ovarian cancer free medical journals Epithelial ovarian cancer famous journals Epithelial ovarian cancer Google Scholar indexed journals Tumor microenvironment articles Tumor microenvironment Research articles Tumor microenvironment review articles Tumor microenvironment PubMed articles Tumor microenvironment PubMed Central articles Tumor microenvironment 2023 articles Tumor microenvironment 2024 articles Tumor microenvironment Scopus articles Tumor microenvironment impact factor journals Tumor microenvironment Scopus journals Tumor microenvironment PubMed journals Tumor microenvironment medical journals Tumor microenvironment free journals Tumor microenvironment best journals Tumor microenvironment top journals Tumor microenvironment free medical journals Tumor microenvironment famous journals Tumor microenvironment Google Scholar indexed journals Ovarian cancer articles Ovarian cancer Research articles Ovarian cancer review articles Ovarian cancer PubMed articles Ovarian cancer PubMed Central articles Ovarian cancer 2023 articles Ovarian cancer 2024 articles Ovarian cancer Scopus articles Ovarian cancer impact factor journals Ovarian cancer Scopus journals Ovarian cancer PubMed journals Ovarian cancer medical journals Ovarian cancer free journals Ovarian cancer best journals Ovarian cancer top journals Ovarian cancer free medical journals Ovarian cancer famous journals Ovarian cancer Google Scholar indexed journals cancer biology articles cancer biology Research articles cancer biology review articles cancer biology PubMed articles cancer biology PubMed Central articles cancer biology 2023 articles cancer biology 2024 articles cancer biology Scopus articles cancer biology impact factor journals cancer biology Scopus journals cancer biology PubMed journals cancer biology medical journals cancer biology free journals cancer biology best journals cancer biology top journals cancer biology free medical journals cancer biology famous journals cancer biology Google Scholar indexed journals hematopoietic origins articles hematopoietic origins Research articles hematopoietic origins review articles hematopoietic origins PubMed articles hematopoietic origins PubMed Central articles hematopoietic origins 2023 articles hematopoietic origins 2024 articles hematopoietic origins Scopus articles hematopoietic origins impact factor journals hematopoietic origins Scopus journals hematopoietic origins PubMed journals hematopoietic origins medical journals hematopoietic origins free journals hematopoietic origins best journals hematopoietic origins top journals hematopoietic origins free medical journals hematopoietic origins famous journals hematopoietic origins Google Scholar indexed journals pro-tumorigenic activities articles pro-tumorigenic activities Research articles pro-tumorigenic activities review articles pro-tumorigenic activities PubMed articles pro-tumorigenic activities PubMed Central articles pro-tumorigenic activities 2023 articles pro-tumorigenic activities 2024 articles pro-tumorigenic activities Scopus articles pro-tumorigenic activities impact factor journals pro-tumorigenic activities Scopus journals pro-tumorigenic activities PubMed journals pro-tumorigenic activities medical journals pro-tumorigenic activities free journals pro-tumorigenic activities best journals pro-tumorigenic activities top journals pro-tumorigenic activities free medical journals pro-tumorigenic activities famous journals pro-tumorigenic activities Google Scholar indexed journals therapeutic agents articles therapeutic agents Research articles therapeutic agents review articles therapeutic agents PubMed articles therapeutic agents PubMed Central articles therapeutic agents 2023 articles therapeutic agents 2024 articles therapeutic agents Scopus articles therapeutic agents impact factor journals therapeutic agents Scopus journals therapeutic agents PubMed journals therapeutic agents medical journals therapeutic agents free journals therapeutic agents best journals therapeutic agents top journals therapeutic agents free medical journals therapeutic agents famous journals therapeutic agents Google Scholar indexed journals ovarian tumor articles ovarian tumor Research articles ovarian tumor review articles ovarian tumor PubMed articles ovarian tumor PubMed Central articles ovarian tumor 2023 articles ovarian tumor 2024 articles ovarian tumor Scopus articles ovarian tumor impact factor journals ovarian tumor Scopus journals ovarian tumor PubMed journals ovarian tumor medical journals ovarian tumor free journals ovarian tumor best journals ovarian tumor top journals ovarian tumor free medical journals ovarian tumor famous journals ovarian tumor Google Scholar indexed journals Epithelial ovarian cancer articles Epithelial ovarian cancer Research articles Epithelial ovarian cancer review articles Epithelial ovarian cancer PubMed articles Epithelial ovarian cancer PubMed Central articles Epithelial ovarian cancer 2023 articles Epithelial ovarian cancer 2024 articles Epithelial ovarian cancer Scopus articles Epithelial ovarian cancer impact factor journals Epithelial ovarian cancer Scopus journals Epithelial ovarian cancer PubMed journals Epithelial ovarian cancer medical journals Epithelial ovarian cancer free journals Epithelial ovarian cancer best journals Epithelial ovarian cancer top journals Epithelial ovarian cancer free medical journals Epithelial ovarian cancer famous journals Epithelial ovarian cancer Google Scholar indexed journals
Article Details
1. Introduction
Ovarian cancer is known to be the seventh most common cancer in human females worldwide [1], and approximately 1,500 Australian women are diagnosed with the disease (mostly with an advanced stage) per annum. The five-year survival rate for ovarian cancer patients is only 43% [2]. About 9 out of 10 tumors of the ovary diagnosed (90%) are epithelial ovarian tumors [3].
Epithelial ovarian cancer (EOC) is the most frequent cause of gynecological cancer-related deaths [4]. Epithelial ovarian cancer (EOC) tumors have been largely categorized into two distinct groups with unique histological, clinical and molecular profiles. Type I ovarian cancers represent a minority of epithelial lesions and include low-grade and borderline serous cancers, endometrioid, mucinous and clear-cell cancers. The group has more frequent PTEN, PI3K catalytic subunit-α (PIK3CA), KRAS, BRAF and β-catenin (CTNNB1) mutations [5]. In general, these tumors are slow-growing, are confined to the ovary, and are less responsive to the conventional chemotherapy. In contrast, type II ovarian cancers represent a bulk of epithelial ovarian cancers and comprise high-grade serous cancers, mixed malignant mesodermal tumors, carcinosarcomas, and undifferentiated cancers. These tumors possess TP53 mutations in a vast majority of cases along with remarkable genomic instability and originate more commonly from the Fallopian tubes and the ovarian surface epithelium [5]. These tumors are clinically aggressive and are often widely metastatic at the time of diagnosis. Ovarian cancers in women with inherited BRCA1 and BRCA2 mutations are typically type II.
Ovarian cancer remains difficult to treat owing to the high recurrence rate. Platinum and taxane-based chemotherapy is the first line of treatment for all EOC patients after debulking surgery. Around 40-60% of patients attain a full clinical response to first-line chemotherapy. However, nearly 50% of these patients relapse within five years and only 10-15% of patients diagnosed with the advanced stage of the disease attain long-standing remission [6]. It is clear that clinical recurrence due to chemoresistance is inevitable in the vast majority of cases. Therefore, a detailed understanding of acquired and innate chemoresistance is required for better management of this lethal disease.
An appropriate understanding of the IL-11 biology would shed more light on the crosstalk between the EOC and its stroma and unravel its role in EOC progression. This advanced knowledge would establish whether or not the IL-11 signaling is associated with chemoresistance in EOC and would also enable the design of better-targeted therapies. The review compiles, discusses and consolidates our current perception of interleukin-11 and STAT3 biology in epithelial ovarian cancer and other cancer types. Finally, the review attempts to elucidate the possible sources and roles of IL-11 in EOC by mimicking its protumorigenic nature seen in other cancer types.
2. IL-11 in Human Physiology
IL-11 is a member of the IL-6 family of cytokines that comprises nine secreted soluble ligands: ciliary neurotrophic factor (CNTF), cardiotrophin-1 (CT-1), cardiotrophin-like cytokine (CLC), IL-6, IL-11, IL-27, IL-31, leukaemia inhibitory factor (LIF) and oncostatin-M (OSM) [15]. Each ligand binds to a specific non-catalytic transmembrane receptor alpha chain. The family also uses the ubiquitously expressed transmembrane glycoprotein-130 (GP130, also known as IL6ST or CD130) beta-subunit [15]. IL-6 and IL-11 are the only family members to utilize GP130 as a homodimeric complex [16].
IL-11 was derived from bone marrow stromal cell line supernatants as a 19 kDa soluble factor that stimulated the proliferation of a plasmacytoma cell line that was otherwise IL-6 dependent [17]. The characteristic type 1 four-helix bundle conformation was revealed by the crystallization of the 178 amino acid human IL-11 protein. Its structure is somewhat distinct from its closest relative IL-6 [18]. The 7 kb human IL-11 gene is localized to chromosome 19q13.3–19q13.4 and includes five coding exons [19]. The identifiable sources of IL-11 are B cells, cardiac myocytes, chondrocytes, fibroblasts, gastrointestinal epithelial cells, hepatocytes, macrophages, osteoblasts, synoviocytes, T cells and trophoblasts among others. However, the main source of IL-11 secretion is still unclear [20]. Although low levels of IL-11 mRNA are present throughout the body, it is hardly detected in the serum of healthy individuals. However, IL-11 levels are readily detectable in many inflammatory diseases and cancers [20].
IL-11 is a pleiotropic cytokine with demonstrated multiple actions: enhances platelet recovery following chemotherapy-induced thrombocytopenia, supports erythropoiesis, regulates macrophage proliferation and differentiation, modulates antigen-antibody responses and regulates bone cell proliferation and differentiation [16]. It also has functions in many other tissues, including the brain, gut, kidney, lung, and liver [16]. Although regarded as an anti-inflammatory cytokine with evidence supporting the efficacy of IL-11 in inflammatory diseases, few studies have demonstrated the pro-inflammatory function of IL-11. IL-11 may promote the inflammatory response observed in multiple sclerosis by stimulating the differentiation of naive CD4+ T cells into Th17 cells [21]. IL-11 may participate in mast cell hyperplasia in various inflammatory skin conditions [22].
IL-11 has cyto-protective effects too. These actions are well reported in the gastrointestinal tract of rodents where IL-11 guards against various forms of mucosal injury. IL-11 shields small intestinal cells from collective radiation, chemotherapy, and ischemia in mice [23, 24]. The mechanism(s) of protective actions of IL-11 on mucosae are not entirely known. The reduction of injury may be due to both anti-inflammatory and direct cytoprotective effects of IL-11. IL-11 stimulates endothelial cells to diminish inflammation-associated injuries in both in vitro and in vivo models and, thus, plays a protective role in immune-mediated injuries [25]. This protective action of IL-11 has been attributed to its ability to activate the expression of antiapoptotic protein survivin through the STAT3 pathway in endothelial cells [25]. IL-11 may also play a key role in neurogenesis through direct stimulation of and acting as a differentiation factor for neuronal progenitor cells [26] (Table 1).
|
Pleiotropic Effects of IL-11 |
||
|
Effect |
Observation |
Reference |
|
Cytoprotective & Prosurvival |
Mucosal (small intestinal) recovery Stem cell survival (small intestinal) Megakaryocytopoiesis Erythropoiesis Neurogenesis Oligodendrocyte survival & maturation Osteoclast development Decidua & fetoplacental development Reduces hepatocyte necrosis & apoptosis Protects against renal ischemia and reperfusion injury |
[102] [23] [24] [103] [24] [104] [105] [26] [106] [107] [27] [108] [109] |
|
Anti-Inflammatory Effects |
Inhibits macrophage TNF-α, IL-1β, IL-6 and IL-12 production Reduces Th1 cytokines Decreases IFN-γ |
[110] [111] [112] [111] [111] |
|
Pro-Inflammatory Effects |
Induces differentiation of naive CD4+ T cells into Th17 cells (observed in multiple sclerosis) Promotes mast cell growth |
[21] [22] |
|
Cell Migration |
Stimulates human extravillous trophoblast migration |
[28] |
Table 1: Summarizes the important effector functions of IL-11.
IL-11 is also a key factor in human trophoblast function and placentation. Bilinski et al. [27] had earlier shown that maternal IL-11Rα signaling is essential for normal organization of the decidua and fetoplacental development. A recent study by Paiva et al. [28] has demonstrated that IL-11 induces human extravillous trophoblast (EVT) migration, but not proliferation, possibly via STAT-3 which indicates an important role for IL-11 in placentation. Elevated IL-11 levels result in physiological alterations at the maternal-fetal interface, leading to abnormal placentation and pregnancy complications including preeclampsia, intrauterine growth restriction (IUGR) and preterm birth [29].
3. Multiple Facets of IL-11 in Cancer Biology
Cytokines exert diverse biological effects and are an important component of innate immunity. As a result, cytokines engaged in cancer-related inflammation have been a subject matter of intensive research and therapeutic applications. Among cytokines that are associated with cancer-related inflammation, greater emphasis has traditionally been placed on IL-6 that links chronic inflammation and cancer development [30]. However, unlike IL-6, the role of IL-11 in various inflammation-associated cancers is newly emerging. Surprisingly, IL-11 has generally been deemed as an anti-inflammatory cytokine, in contrast with the pro-inflammatory IL-6, having direct effects on macrophages and other effector cells at the inflammation site [12].
IL-11 is a prominent tumor-promoting cytokine. Emerging evidence implicates a role of IL-11 in several human cancers of epithelial origin; including breast [31], gastric [14] and colon [32] cancers; and of hematopoietic origin [33]. Elevated IL-11 expression levels are linked to poor prognosis in human cancers. For example, in endometrial and gastric adenocarcinomas the expression of IL-11 escalates with tumor grade while in breast cancer the level of IL-11 can predict the development of metastatic spread to the bone [16, 34]. A new study has suggested an important role of IL-11 in NRF2-addicted tumorigenesis and that IL-11 is a promising therapeutic target for NRF2-driven breast cancers [35].
Appraisal of the recent literature strongly indicates a complex multi-faceted protumorigenic role for IL-11. IL-11 can increase the tumorigenic capacity of cells. Putoczki et al. [14] conclusively demonstrated that IL-11 signaling is essential for tumor onset and progression showing that mice lacking IL-11Rα developed very few tumors in colitis-associated cancer and sporadic colon cancer models. IL-11 signaling has been recognized to lead to the growth of osteosarcoma cells via the upregulation of IL-11Rα [36]. In addition, IL-11 signaling may enhance cell motility of chondrosarcoma cells, further suggesting its role in cancer progression [37]. Interestingly, in a study by Wu et al. decreased levels of IL-11 are linked to poorer prognosis in transitional cell carcinoma (TCC) of the bladder [38]. The authors theorized that the downregulation of IL-11 diminished the protective, anti-inflammatory actions of IL-11 and, as a result, may promote bladder TCC tumorigenesis and progression [38]. While most cancers are associated with an upregulation of IL-11, the bladder TCC is correlated with low expression of IL-11.
In a recent study, Pasqualini et al. [39] have reported the results of preclinical and early clinical evaluations of a novel therapy targeting IL-11 receptor α (IL-11Rα) in metastatic prostate cancer. Pasqualini and colleagues developed a ligand-directed agent—bone metastasis-targeting peptidomimetic-11 (BMTP-11)—containing the CGRRAGGSC peptide motif that has previously been found to bind to IL-11Rα in the tumor vascular endothelium, and a pro-apoptotic motif, D(KLA-KLAK)2, internalization of which induces apoptosis of the cell in preclinical models of cancer. Lewis et al. [40] too have evaluated the efficacy of IL-11Rα–targeted proapoptotic BMTP-11 in preclinical models of primary intratibial osteosarcomas and have observed a clear reduction of both tumor growth and lung metastases. Their findings corroborate the importance of developing IL-11Rα-targeted approaches and present BMTP-11 as a leading drug candidate for clinical translation for the benefit of high-risk osteosarcoma patients.
IL-11 has also been implicated in several other aspects of tumor biology. These include the stimulation of angiogenesis, survival under hypoxia, chemoresistance, as well as expansion and survival of early micrometastatic colonies in bone and soft tissues including liver and lung [31]. The latest study emphasizing the function of IL-11 in cancer development is that of Pastor et al. [41], who established that IL-11 in bronchoalveolar lavage fluid (BALF) is a highly specific diagnostic biomarker for lung adenocarcinoma (Table 2).
4. IL-11 and Epithelial Ovarian Cancer
Normal ovarian tissue is rich in cytokines and chemokines, which are essential in the ovarian physiology and ovulation. They regulate the growth, differentiation, and apoptosis of various cellular components of the ovary. Campbell et al. [13] investigated IL-11 and the IL-11 receptor subunits’ expression in primary human benign and malignant tumors as well as in normal ovaries. They found that the malignant epithelial cells in the majority of the primary ovarian carcinoma samples expressed IL-11Rα. Co-expression of IL-11Rα and GP130 was also found in most primary ovarian carcinoma samples (Table 2). Moreover, benign ovarian tumors, normal ovarian epithelium, and ovarian stromal cells also expressed IL-11Rα and GP130 (Table 3). However, IL-11 mRNA was expressed in only 14.3% malignant samples studied. Although it was demonstrated that IL-11Rα and GP130 subunits were commonly expressed within ovarian epithelial cells of both malignant and nonmalignant primary tissues, the exact role of the IL-11 receptor system in ovarian epithelial cell biology was not established [13].
|
Gynaecological and Other Cancers Associated with IL-11 Signaling |
||
|
Cancers Associated with Enhanced IL-11 Signaling |
||
|
Cancer |
Observation |
Reference |
|
Endometrial |
Positively associated with higher tumor grades Contribute to migration and metastasis of high-grade cancer |
[48] [115] [49] [51] |
|
Ovarian |
Presence of IL-11Rα |
[13] |
|
Bone |
Cell lines and primary tumors express IL-11 Promotes cell motility |
[116] [36] [40] [37] |
|
Breast |
Required for primary tumor growth and metastasis |
[117] [31] [34] |
|
Colorectal |
Required for primary tumor growth and Metastasis |
[88] [14] |
|
Glioblastoma |
Cell lines express IL-11 |
[118] |
|
Hodgkin’s lymphoma |
Presence of IL-11Rα |
[119] |
|
Liver |
Associated with bone metastasis |
[120] [121] |
|
Leukemia |
Elevated IL-11 and IL-11Rα |
[33] |
|
Lung |
Elevated IL-11 IL-11 as a highly specific diagnostic biomarker Associated with chemoresistance |
[122] [41] [89] |
|
Pancreatic |
Elevated in primary and advanced tumors |
[123] [124] |
|
Prostate |
Associated with progression |
[101] [125] |
|
Renal |
Cell lines express IL-11 |
[126] |
|
Skin |
Cell lines express IL-11 |
[127] |
|
Stomach |
Required for primary tumor growth and Metastasis |
[128] [14] |
|
Thyroid |
Positively correlated with distant metastasis |
[74] |
|
Cancers Associated with Decreased IL-11 Signaling |
||
|
Cancer |
Observation |
Reference |
|
Bladder transitional cell carcinoma (TCC) |
Reduced levels of IL-11 correlated with poorer prognosis |
[38] |
Table 2: Shows at a glance all cancer types that are associated with IL-11 signaling.
A key question is what role the IL-11/IL-11 receptor axis might play in epithelial ovarian cancer biology. Perhaps the answer lies in the ovarian physiology. There is strong evidence that IL-11 is present in periovulatory follicular fluid (FF). Branisteanu et al. [42] demonstrated that IL-11 is present in FF and in conditioned medium from cultured granulosa cells, with higher concentrations in atretic follicles. The report raised the question of IL-11 involvement in the process of atresia.
The role IL-11 plays in folliculogenesis and/or oocyte development was not clear until Jang et al. [43] examined the regulation of IL-11 expression as well as the role of IL-11 during ovulation. They observed that LH stimulated the expression of IL-11 protein in theca cells and LH-stimulated IL-11 mRNA levels were repressed by protein kinase A and mitogen-activated protein kinase inhibitors (Table 4). Moreover, the treatment of preovulatory follicles with IL-11 stimulated progesterone production. This is by far the only study that has revealed for the first time the regulation and the role of IL-11 expression during ovulation. IL-11 in theca cells is activated by mitogen-activated protein kinase signaling and TLR4 activation, leading to enhanced progesterone production during ovulation [43] (Table 3). Interestingly, progesterone protects the ovary from neoplastic transformation [44]. Nonetheless, an intriguing question of what role IL-11R might play in ovarian epithelial cell biology is valid and is still unanswered given that it is commonly expressed within ovarian epithelial cells.
Mice made deficient in IL-11Rα via gene targeting appear to have functional ovaries although these mice are infertile, due to a defect in the ability of the uterus to undergo decidualization, resulting in failure of implantation [45]. Winship et al. [46] studied the autocrine and paracrine effect of IL-11 on human decidual and trophoblast cells during placental development. Insufficient IL-11 levels may disrupt the balance of decidual restraint and trophoblast invasion necessary for normal placentation, whereas elevated levels may obstruct trophoblast invasion necessary for a healthy pregnancy.
The expression of IL-11 has been observed in both human endometrial epithelial and stromal cells [47] (Table 3) (Table 4). IL-11 levels are elevated in uterine lavage fluid from endometrial cancer patients, and enhanced levels are positively correlated with higher tumor grades [48]. Moreover, elevated IL-11 levels may promote migration and metastasis of high-grade endometrial cancer cells via the STAT3 pathway [49] (Table 2). Targeting IL-11 receptor-alpha attenuates proliferation and invasion of human endometrial cancer cell in vitro and reduces tumor growth and metastasis in vivo [50, 51].
|
Gynaecological Tissues and Organs That Respond to IL-11 Signaling |
|||
|
Tissue/Organ |
Cell Type |
Response |
Reference |
|
Endometrium |
Epithelial cells Endothelial cells Smooth muscle |
Increase TNF-α N/A N/A |
[114] [48] [48] |
|
Ovary |
Epithelial cells Stromal cells Granulosa cells Theca cells |
N/A N/A Increase progesterone production & StAR gene expression Increase progesterone production & StAR gene expression |
[13] [13] [43] [43] |
Table 3: Gynaecological Tissues and Organs That Respond to IL-11 Signaling.
|
Gynaecological Tissues and Organs That Produce IL-11 |
|||
|
Tissue/Organ |
Cell Type |
Stimulators |
Reference |
|
Endometrium |
Epithelial cells Stromal cells |
Endogenous |
[113] |
|
Ovary |
Theca cells |
Luteinizing hormone (LH) |
[43] |
Table 4: Gynaecological Tissues and Organs That Produce IL-11.
Having known a clear source of IL-11 production in the ovarian milieu from Jang et al. [43] study, what now has to be determined is whether IL-11R of the ovarian epithelium responds to IL-11 from theca cells or to IL-11 derived from an extra-ovarian source, and what role IL-11R might play in ovarian epithelial cell biology. Perhaps the answer might come from the report that suggests the potential involvement of IL-11 in the normal growth controls in the intestinal epithelium [52].
Whatever the role of IL-11/IL-11R axis in epithelial ovarian cell biology might be, however, the importance of IL-11Rα to epithelial ovarian cancer cannot be underestimated given its high frequency of expression [13]. It is possible that the dysregulation of the IL-11 signaling in the ovarian epithelium might be one of the factors in epithelial ovarian tumorigenesis. It is reasonable to assume that these tumor cells are able to disturb the homeostasis of cytokine expression including IL-11 in the ovary in such a way that the ovarian cytokine expression favors the growth of the tumor. Therefore, IL-11 deserves attention in EOC, and additional investigations are warranted to determine the precise role(s) of the IL-11 receptor system and associated autocrine/paracrine interactions in epithelial ovarian cancer biology.
5. IL-11 Signaling
IL-11 initiates signaling upon binding to the cognate IL-11Rα. The IL-11/IL-11Rα complex then interacts with the transmembrane glycoprotein β-receptor subunit GP130 leading to the formation of a hexameric complex in a 2:2:2 pattern (IL-11/IL-11Rα/GP130) [16]. The resulting receptor complex is called the interleukin-11 receptor, a type 1 cytokine receptor with GP130 receptor as a signal-transducing subunit. Since the expression of GP130 is ubiquitous, with the exception of pre-B-cells, the ability of cells to respond to IL-11 is governed by the expression of α-receptor subunits.
The hexameric complex facilitates juxta-positioning of the Janus (JAK) family tyrosine kinases JAK1, JAK2, and TYK2, which are constitutively coupled with a proline-rich intracellular domain of GP130, to permit trans-phosphorylation and kinase activation [53]. Activated JAK kinases, in turn, phosphorylate the various cytoplasmic tyrosine residues in GP130 to provide docking sites for the SH2 domain-containing signaling molecules [53]. Specifically, the four membrane distal pY residues in GP130 act as docking sites for the latent STAT1 and STAT3 (signal transducer and activator of transcription 1/3) transcription factors [16]. This allows JAK-mediated phosphorylation of a conserved Y residue in the carboxyl-terminal portion of these STAT proteins. The resultant STAT homo- or heterodimers, upon active translocation into the nucleus, bind to DNA in a sequence-specific manner to regulate gene transcription (Figure 1) [16].
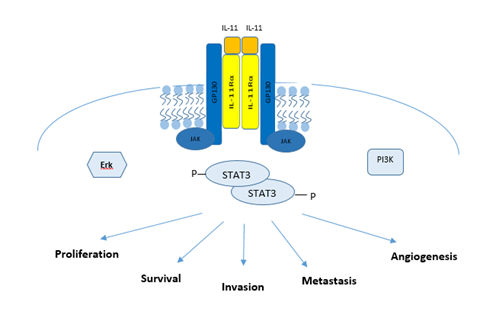
Figure 1: The IL-11/gp130/STAT3 signaling Axis: Formation of an IL-11 signaling complex leads to the recruitment of JAKs which permit the phosphorylation of STAT3. Activated STAT3 when bound to DNA results in increased pro-cancerous gene transcription.
Among the transcriptional target genes for STAT3, SOCS3 (suppressor of cytokine signaling 3) is an important negative regulator of GP130 signaling. Through its SH2-domain, SOCS3 binds to GP130 and interacts simultaneously with a JAK molecule to occlude access to the substrate binding pocket of JAKs. GP130 also serves as a docking site for the Y-phosphatase SHP2/PTPN11, which provides the framework for GP130-dependent activation of the RAS/ERK signaling cascade [16]. Finally, GP130 is able to activate the phosphatidylinositol 3' kinase (PI3K)–AKT–mTORC1 pathway that does not require tyrosine phosphorylation of GP130 [54]. Genetic evidence suggests that STAT3 activation is the most important event to transduce a majority of GP130-family cytokines’ signals [55].
6. STAT3 Signaling
IL-11 can confer most of the ‘hallmark’ capabilities to neoplastic cells, provided they express the cognate IL-11Rα receptor subunit, through the STAT3 signaling pathway. STAT3 is associated with a majority of the six crucial alterations in cellular physiology (i.e. self-sufficiency in growth signals, insensitivity to growth-inhibiting signals, resistance to apoptosis, unrestricted cellular replication, sustained angiogenesis and tissue invasion and metastasis) identified by Hanahan and Weinberg [56].
Indeed, aberrant STAT3 activation is a common characteristic in many human cancers of epithelial origin (i.e., breast, head and neck, stomach, colon) as well as hemopoietic origin (i.e., leukemias, multiple myeloma) [57]. Abundant evidence highlights that STAT3 is an ideal target for cancer treatment. Wen et al. [58] demonstrated that knockdown of JAK1/STAT3 by using shRNA or a small-molecule inhibitor effectively restrained ovarian tumor progression. The results were consistent with the findings by Gritsina et al. [59], who showed the interruption of functions necessary for ovarian tumor growth and progression following the pharmacologic inhibition of the JAK2/STAT3 pathway. RNAi-mediated knockdown of STAT3 repressed the growth of human ovarian cancer through the downregulation of cyclin D1, c-Myc and Bcl-2 [60].
In addition to its known roles in promoting tumor cell proliferation, survival, invasion, angiogenesis and immunosuppression, STAT3 oncogenic pathway has recently been shown to confer drug-resistance, radio-resistance, and cancer stem cell-like phenotype. Autocrine interleukin-6 production has been shown to confer cisplatin and paclitaxel resistance in ovarian cancer [82]. However, no study has yet reported the presence of IL-11 in the ascites. It is surprising that even though IL-11Rα is commonly expressed by ovarian tumor cells, the IL-11 function is not well-characterized in ovarian cancer biology [13]. Nonetheless, the role of IL-11 in ovarian malignancy cannot be discounted. Castells et al. [83] proposed that mesenchymal stem cells (MSCs) activated the release of IL-6 and IL-8 by macrophages that mediate the acquisition of chemoresistance in human ovarian cancers. Yu et al. [84] studied the cell stemness capacity of a set of interleukins and identified that IL-3, IL-6, and IL-11 stimulated while IL-10 and IL-24 repressed the growth, invasion and migration of human prostate cancer cell lines. Furthermore, IL-3, IL-6 or IL-11 treatment reduced the chemosensitivity to docetaxel while IL-10 or IL-24 treatment enhanced the sensitivity to docetaxel. Therefore, IL-11 deserves attention in the EOC setting as well.
Recently, an unexpected function of IL-11 in colitis-associated cancer (CAC) has been reported [88]. Secreted TGF-β from the cancer cells stimulates IL-11 secretion (from cancer-associated fibroblasts) that activates STAT3 signaling via GP130 in tumor cells. This circuit increases the survival rate of metastatic cells, thereby increasing metastasis [88]. The crosstalk between cancer-associated fibroblasts (CAFs) and cancer cells through IL-11 is also seen in lung adenocarcinoma. The upregulation of IL-11 in CAFs post-chemotherapy supports lung adenocarcinoma cell chemoresistance through the activation of the anti-apoptotic IL-11R/STAT3 signaling pathway [89].
The role of IL-11 in cancer cell migration and invasion under the hypoxic environment has also been reported. Lim [100] demonstrated that the hypoxia-induced migration and invasion was attenuated following the inhibition of the IL-11-STAT3 axis in MDA-MB-231 breast cancer cells. Onnis et al. [101] had previously found that IL-11 was a hypoxia-inducible, VHL-regulated gene in human cancer cells and the transcriptional activation of the IL-11 promoter was mediated by the cooperative association between HIF-1 and AP-1. Silencing of IL-11 dramatically abrogated hypoxia-induced anchorage-independent growth and significantly diminished tumor growth in xenograft models [101]. Hence, an appropriate understanding of molecular cues that facilitate the crosstalk between the tumor and its stroma is necessary to design novel targeted therapeutics disrupting the prostemness tumor-stroma interaction.
7. Conclusions
The ovarian tumor microenvironment (TME) is now perceived as the pivotal factor in multiple phases of disease progression, particularly local resistance, immune evasion, and distant metastasis. Comprehensive understanding of the TME will permit the evaluation and selection of novel drug candidates to target malignancies at both primary and metastatic sites.
Cytokines and chemokines play an important role in the physiology of the ovaries, for example in the regulation of ovulation. It is interesting to note that most of the cytokines that are expressed in normal ovarian tissue are also expressed in the microenvironment of ovarian cancer and are associated with malignancy. Traditionally much emphasis has been put on IL-6. The role of IL-6 in conferring cisplatin and paclitaxel resistance in ovarian cancer cells is well-characterized [82]. However, the role of IL-11/IL-11R axis remains elusive in epithelial ovarian cancer milieu although the IL-11R expression has been reported in EOC [13].
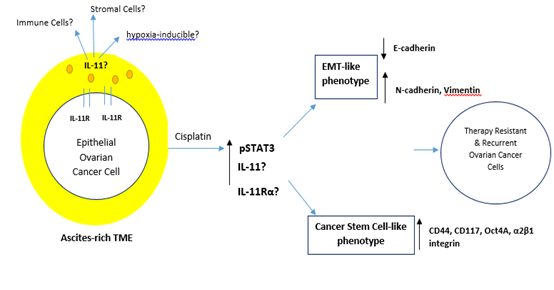
Figure 2: Potential Working Model of the Biology of Il-11 in EOC. The ascites-rich TME constitutes a complex reservoir of cellular components (CAFs, endothelial cells, MSCs and immune cells) and extracellular solutes (growth factors, cytokines and chemokines). Chemotherapy or hypoxia-induced IL-11 signaling in EOC may be correlated with EMT-like and/or Cancer stem cell-like phenotypes through the activation of STAT3.
There has been an upsurge in IL-11 research recently demonstrating that autocrine and paracrine IL-11 signaling is pro-tumorigenic in other cancer types. Roles of IL-11 in cyto-protection and cell migration in both physiological and pathological situations are now known. It is worth noting that cancer cells utilize these cytokines to their advantage by manipulating the TME. Understanding the biology of IL-11 in EOC is therefore imperative given the advancements in IL-11 research that reflect its biology similar to the more prominently studied IL-6. We have demonstrated a potential working model of its biology in Figure 2. Further investigations into the critical role of IL-11 signaling will also assist in the design of rational treatment strategies for women with EOC.
References
- World Cancer Research Fund International. Ovarian cancer statistics (2015).
- The Ovarian Cancer Research Foundation (OCRF). STATISTICS (2015).
- Cancer Research UK. Types of ovarian cancer (2015).
- Jayson GC, Kohn EC, Kitchener HC, et al. Ovarian cancer. The Lancet 384 (2014): 1376-1388.
- Bast RC, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer 9 (2009): 415-428.
- Lloyd KL, Cree IA, Savage RS. Prediction of resistance to chemotherapy in ovarian cancer: a systematic review. BMC Cancer 15 (2015): 117.
- Abubaker K, Luwor RB, Zhu H. Inhibition of the JAK2/STAT3 pathway in ovarian cancer results in the loss of cancer stem cell-like characteristics and a reduced tumor burden. BMC Cancer 14 (2014): 317.
- Chan E, Luwor R, Burns C, et al. Momelotinib decreased cancer stem cell associated tumor burden and prolonged disease-free remission period in a mouse model of human ovarian cancer. Oncotarget 9 (2018): 16599-16618.
- Latifi A, Luwor RB, Bilandzic M, et al. Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: molecular phenotype of chemoresistant ovarian tumors. PLoS One 7 (2012): e46858.
- Sheng WJ, Jiang H, Wu DL, et al. Early responses of the STAT3 pathway to platinum drugs are associated with cisplatin resistance in epithelial ovarian cancer. Braz J Med Biol Res 46 (2013): 650-658.
- Ahmed N, Abubaker K, Findlay J, et al. Epithelial mesenchymal transition and cancer stem cell-like phenotypes facilitate chemoresistance in recurrent ovarian cancer. Current Cancer Drug Targets 10 (2010): 268-278.
- Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol 26 (2014): 54-74.
- Campbell CL, Guardiani R, Ollari C, et al. Interleukin-11 receptor expression in primary ovarian carcinomas. Gynecol Oncol 80 (2001): 121-127.
- Putoczki TL, Thiem S, Loving A, et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell 24 (2013): 257-271.
- Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol 15 (1997): 797-819.
- Putoczki TL, Ernst M. IL-11 signaling as a therapeutic target for cancer. Immunotherapy 7 (2015): 441-453.
- Paul SR, Bennett F, Calvetti JA, et al. Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc Natl Acad Sci USA 87 (1990): 7512-7516.
- Putoczki TL, Dobson RC, Griffin MD. The structure of human interleukin-11 reveals receptor-binding site features and structural differences from interleukin-6. Acta Crystallogr D Biol Crystallogr 70 (2014): 2277-2285.
- Yin T, Yasukawa K, Taga T, et al. Identification of a 130-kilodalton tyrosine-phosphorylated protein induced by interleukin-11 as JAK2 tyrosine kinase, which associates with gp130 signal transducer. Exp Hematol 22 (1994): 467-472.
- Xu DH, Zhu Z, Wakefield MR, et al. The role of IL-11 in immunity and cancer. Cancer Letters 373 (2016): 156-163.
- Zhang X, Tao Y, Chopra M, et al. IL-11 Induces Th17 Cell Responses in Patients with Early Relapsing-Remitting Multiple Sclerosis. J Immunol 194 (2015): 5139-5149.
- Palmqvist P, Persson E, Conaway HH, et al. The IL-6 family cytokines, interleukin-6, interleukin-11, oncostatin M, and leukemia inhibitory factor, enhance mast cell growth through fibroblast-dependent pathway in mice. Arch Dermatol Res 293 (2001): 508-514.
- Du X, Liu Q, Yang Z, et al. Protective effects of interleukin-11 in a murine model of ischemic bowel necrosis. Am J Physiol 272 (1997): G545-G552.
- Orazi A1, Du X, Yang Z, et al. Interleukin-11 prevents apoptosis and accelerates recovery of small intestinal mucosa in mice treated with combined chemotherapy and radiation. Lab Invest 75 (1996): 33-42.
- Mahboubi K, Biedermann BC, Carroll JM, et al. IL-11 activates human endothelial cells to resist immune-mediated injury. J Immunol 164 (2000): 3837-3846.
- Mehler MF, Rozental R, M Dougherty, et al. Cytokine regulation of neuronal differentiation of hippocampal progenitor cells. Nature 362 (1993): 62-65.
- Bilinski P, Roopenian D, Gossler A. Maternal IL-11Ralpha function is required for normal decidua and fetoplacental development in mice 12 (1998): 2234-2243.
- Paiva P, Salamonsen LA, Manuelpillai U, et al. Interleukin-11 promotes migration, but not proliferation, of human trophoblast cells, implying a role in placentation. Endocrinology 148 (2007): 5566-5572.
- Winship AL, Sorby K, Correia J, et al. Interleukin-11 up-regulates endoplasmic reticulum stress induced target, PDIA4 in human first trimester placenta and in vivo in mice. Placenta 53 (2017): 92-100.
- Ernst M, Putoczki TL. Molecular pathways: IL11 as a tumor-promoting cytokine-translational implications for cancers. Clin Cancer Res 20 (2014): 5579-5588.
- Johnstone CN, Chand A, Putoczki TL, et al. Emerging roles for IL-11 signaling in cancer development and progression: Focus on breast cancer. Cytokine Growth Factor Rev 26 (2015): 489-498.
- Ernst M, Putoczki TL. Targeting IL-11 signaling in colon cancer. Oncotarget 4 (2013): 1860-1861.
- Tsimanis A, Shvidel L, Klepfish A, et al. Over-expression of the functional interleukin-11 alpha receptor in the development of B-cell chronic lymphocytic leukemia. Leuk Lymphoma 42 (2001): 195-205.
- rawan C, Atmakusumah D, Siregar NC, et al. Expression of biomarkers CXCR4, IL11-RA, TFF1, MLF1P in advanced breast cancer patients with bone metastatic: a diagnostic study. Acta Med Indones 48 (2016): 261-268.
- Kitamura H, Onodera Y, Murakami S, et al. IL-11 contribution to tumorigenesis in an NRF2 addiction cancer model. Oncogene 36 (2017): 6315-6324.
- Lewis VO, Ozawa MG, Deavers MT, et al. The interleukin-11 receptor alpha as a candidate ligand-directed target in osteosarcoma: consistent data from cell lines, orthotopic models, and human tumor samples. Cancer Res 69 (2009): 1995-1999.
- Li TM, Wu CM, Huang HC, et al. Interleukin-11 increases cell motility and up-regulates intercellular adhesion molecule-1 expression in human chondrosarcoma cells. J Cell Biochem 113 (2012): 3353-3362.
- Wu D, Tao J, Ding J, et al. Interleukin-11, an interleukin-6-like cytokine, is a promising predictor for bladder cancer prognosis. Mol Med Rep 7 (2013): 684-688.
- Pasqualini R, Millikan RE, Christianson DR, et al. Targeting the interleukin-11 receptor alpha in metastatic prostate cancer: A first-in-man study. Cancer 121 (2015): 2411-2421.
- Lewis VO, Devarajan E, Cardó-Vila M, et al. BMTP-11 is active in preclinical models of human osteosarcoma and a candidate targeted drug for clinical translation. Proc Natl Acad Sci U S A 114 (2017): 8065-8070.
- Pastor MD, Nogal A, Molina-Pinelo S, et al. IL-11 and CCL-1: Novel Protein Diagnostic Biomarkers of Lung Adenocarcinoma in Bronchoalveolar Lavage Fluid (BALF). J Thorac Oncol 11 (2016): 2183-2192.
- Branisteanu I, Pijnenborg R, Spiessens C, et al. Detection of immunoreactive interleukin-I 1 in human follicular fluid: correlations with ovarian steroid, insulin-like growth factor I levels, and follicular maturity. 67 (1997): 1054-1058.
- Jang YJ1, Park JI2, Jeong SE, et al. Regulation of interleukin-11 expression in ovulatory follicles of the rat ovary. Reprod Fertil Dev 29 (2017): 2437-2445.
- Diep CH, Daniel AR, Mauro LJ, et al. Progesterone action in breast, uterine, and ovarian cancers. Journal of molecular endocrinology 54 (2015): R31-R53.
- Robb L, Li R, Hartley L, Nandurkar HH, et al. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med 4 (1998): 303-308.
- Winship A, Menkhorst E, Van Sinderen M, et al. Interleukin 11: similar or opposite roles in female reproduction and reproductive cancer? Reprod Fertil Dev 28 (2016): 395-405.
- Cork BA, Li TC, Warren MA, Laird SM, et al. Interleukin-11 (IL-11) in human endometrium: expression throughout the menstrual cycle and the effects of cytokines on endometrial IL-11 production in vitro. J Reprod Immunol 50 (2001): 3-17.
- Yap J, Salamonsen LA, Jobling T, et al. Interleukin 11 is upregulated in uterine lavage and endometrial cancer cells in women with endometrial carcinoma. Reprod Biol Endocrinol 8 (2010): 63.
- Lay V, Yap J, Sonderegger S, et al. Interleukin 11 regulates endometrial cancer cell adhesion and migration via STAT3. Int J Oncol 41 (2012): 759-764.
- Winship A, Van Sinderen M, Rainczuk K, et al. Therapeutically blocking Interleukin-11 Receptor-alpha enhances doxorubicin cytotoxicity in high grade type I endometrioid tumours. Oncotarget 8 (2017): 22716-22729.
- Winship AL, Van Sinderen M, Donoghue J, et al. Targeting Interleukin-11 Receptor-alpha Impairs Human Endometrial Cancer Cell Proliferation and Invasion In Vitro and Reduces Tumor Growth and Metastasis In Vivo. Mol Cancer Ther 15 (2016): 720-730.
- Booth C, Potten CS. Effects of IL-11 on the growth of intestinal epithelial cells in vitro. Cell Proliferation 28 (1995): 581-594.
- Ernst M, Jenkins BJ. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet 20 (2004): 23-32.
- Winship AL, Van Sinderen M, Donoghue J, et al. mTORC1 inhibition restricts inflammation-associated gastrointestinal tumorigenesis in mice. J Clin Invest 123 (2013): 767-781.
- Jenkins BJ, Grail D, Nheu T, et al. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-beta signaling. Nat Med 11 (2005): 845-852.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 144 (2011): 646-674.
- Bowman T, Garcia R, Turkson J, et al. STATs in oncogenesis. Oncogene 19 (2000): 2474-2488.
- Wen W, Liang W, Wu J, et al. Targeting JAK1/STAT3 signaling suppresses tumor progression and metastasis in a peritoneal model of human ovarian cancer. Mol Cancer Ther 13 (2014): 3037-3048.
- Gritsina G, Xiao F, O'Brien SW, et al. Targeted Blockade of JAK/STAT3 Signaling Inhibits Ovarian Carcinoma Growth. Mol Cancer Ther 14 (2015): 1035-1047.
- Shu-Hua Zhao, Fan Zhao, Jing-Ying Zheng, et al. Knockdown of stat3 expression by RNAi inhibits in vitro growth of human ovarian cancer. Radiology and Oncology 45 (2011): 196-203.
- Ji T, Gong D, Han Z, et al. Abrogation of constitutive Stat3 activity circumvents cisplatin resistant ovarian cancer. Cancer Lett 341 (2013): 231-239.
- L'Espérance S, Bachvarova M, Tetu B, et al. Global gene expression analysis of early response to chemotherapy treatment in ovarian cancer spheroids. BMC Genomics 9 (2008): 99.
- Konnikova L, Simeone MC, Kruger MM, et al. Signal transducer and activator of transcription 3 (STAT3) regulates human telomerase reverse transcriptase (hTERT) expression in human cancer and primary cells. Cancer Res 65 (2005): 6516-6520.
- Abubaker K, Latifi A, Luwor R, et al. Short-term single treatment of chemotherapy results in the enrichment of ovarian cancer stem cell-like cells leading to an increased tumor burden. Mol Cancer 12 (2013): 24.
- Latifi A, Abubaker K, Castrechini N, et al. Cisplatin treatment of primary and metastatic epithelial ovarian carcinomas generates residual cells with mesenchymal stem cell-like profile. J Cell Biochem 112 (2011): 2850-2864.
- Steg AD, Bevis KS, Katre AA, et al. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clinical Cancer Research 18 (2012): 869-881.
- Abubaker K, Luwor RB, Escalona R, et al. Targeted Disruption of the JAK2/STAT3 Pathway in Combination with Systemic Administration of Paclitaxel Inhibits the Priming of Ovarian Cancer Stem Cells Leading to a Reduced Tumor Burden. Front Oncol 4 (2014): 75.
- Patch AM, Christie EL, Etemadmoghadam D, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 521 (2015): 489-494.
- Colomiere M, Ward AC, Riley C, et al. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial–mesenchymal transition in ovarian carcinomas. Nature Publishing Group: Great Britain 100 (2009): 134-144.
- Bose D, Zimmerman LJ, Pierobon M, et al. Chemoresistant colorectal cancer cells and cancer stem cells mediate growth and survival of bystander cells. British Journal of Cancer 105 (2011): 1759-1767.
- Sherry MM, Reeves A, Wu JK, et al. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem cells (Dayton, Ohio) 27 (2009): 2383-2392.
- Wendt MK, Balanis N, Carlin CR, et al. STAT3 and epithelial-mesenchymal transitions in carcinomas. JAKSTAT 3 (2014): e28975.
- Silver DL, Naora H, Liu J, et al. Activated signal transducer and activator of transcription (STAT) 3: localization in focal adhesions and function in ovarian cancer cell motility. Cancer Res 64 (2004): 3550-3558.
- Zhong Z, Hu Z, Jiang Y, et al. Interleukin-11 promotes epithelial-mesenchymal transition in anaplastic thyroid carcinoma cells through PI3K/Akt/GSK3beta signaling pathway activation. Oncotarget 7 (2016).
- McLean K, Gong Y, Choi Y, et al. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest 121 (2011): 3206-3219.
- Thibault B, Castells M, Delord JP, et al. Ovarian cancer microenvironment: implications for cancer dissemination and chemoresistance acquisition. Cancer Metastasis Rev 33 (2014): 17-39.
- Cojoc M, Mäbert K, Muders MH, et al. A role for cancer stem cells in therapy resistance: cellular and molecular mechanisms. Semin Cancer Biol 31 (2015): 16-27.
- Gilbert LA, Hemann MT. Chemotherapeutic resistance: surviving stressful situations. Cancer Res 1 (2011): 5062-5066.
- Sun Y. Translational horizons in the tumor microenvironment: harnessing breakthroughs and targeting cures. Med Res Rev 35 (2015): 408-436.
- Ahmed N, Stenvers KL. Getting to know ovarian cancer ascites: opportunities for targeted therapy-based translational research. Front Oncol 3 (2013): 256.
- Matte I, Lane D, Laplante C, et al. Profiling of cytokines in human epithelial ovarian cancer ascites. Am J Cancer Res 2 (2012): 566-580.
- Wang Y, Niu XL, Qu Y, et al. Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cancer Lett 295 (2010): 110-123.
- Castells M, Thibault B, Mery E, et al. Ovarian ascites-derived Hospicells promote angiogenesis via activation of macrophages. Cancer Lett 326 (2012): 59-68.
- Yu D, Zhong Y, Li X, et al. ILs-3, 6 and 11 increase, but ILs-10 and 24 decrease stemness of human prostate cancer cells in vitro. Oncotarget 6 (2015): 42687-42703.
- Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 7 (2007): 41-51.
- Groner B, Lucks P, Borghouts C. The function of Stat3 in tumor cells and their microenvironment. Semin Cell Dev Biol 19 (2008): 341-350.
- Jia ZH, Jia Y, Guo FJ, et al. Phosphorylation of STAT3 at Tyr705 regulates MMP-9 production in epithelial ovarian cancer. PLoS One 12 (2017): e0183622.
- Calon A, Espinet E, Palomo-Ponce S, et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer cell 22 (2012): 571-584.
- Tao L, Huang G, Wang R, et al. Cancer-associated fibroblasts treated with cisplatin facilitates chemoresistance of lung adenocarcinoma through IL-11/IL-11R/STAT3 signaling pathway. Sci Rep 6 (2016): 38408.
- Yan H, Guo BY, Zhang S. Cancer-associated fibroblasts attenuate Cisplatin-induced apoptosis in ovarian cancer cells by promoting STAT3 signaling. Biochem Biophys Res Commun 470 (2016): 947-954.
- Bollrath J, Phesse TJ, von Burstin VA, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 15 (2009): 91-102.
- Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell 129 (2007): 465-472.
- Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist 9 (2004): 4-9.
- Nozawa-Suzuki N, Nagasawa H, Ohnishi K, et al. The inhibitory effect of hypoxic cytotoxin on the expansion of cancer stem cells in ovarian cancer. Biochem Biophys Res Commun 457 (2015): 706-711.
- Liang D, Ma Y, Liu J, et al. The hypoxic microenvironment upgrades stem-like properties of ovarian cancer cells. BMC Cancer 12 (2012): 201.
- McCann GA, Naidu S, Rath KS, et al. Targeting constitutively-activated STAT3 in hypoxic ovarian cancer, using a novel STAT3 inhibitor. Oncoscience 1 (2014): 216-228.
- Pawlus MR, Wang L, Hu CJ. STAT3 and HIF1alpha cooperatively activate HIF1 target genes in MDA-MB-231 and RCC4 cells. Oncogene 33 (2014): 1670-1679.
- McEvoy LM, O'Toole SA, Spillane CD, et al. Identifying novel hypoxia-associated markers of chemoresistance in ovarian cancer. BMC Cancer 15 (2015): 547.
- Jung HY, Fattet L, Yang J. Molecular pathways: linking tumor microenvironment to epithelial-mesenchymal transition in metastasis. Clin Cancer Res 21 (2015): 962-968.
- Lim JH. Inhibition of the Interleukin-11-STAT3 Axis Attenuates Hypoxia-Induced Migration and Invasion in MDA-MB-231 Breast Cancer Cells. Korean J Physiol Pharmacol 18 (2014): 391-396.
- Onnis B, Fer N, Rapisarda A, et al. Autocrine production of IL-11 mediates tumorigenicity in hypoxic cancer cells. J Clin Invest 123 (2013): 1615-1629.
- Du XX, Doerschuk CM, Orazi A, et al. A bone marrow stromal-derived growth factor, interleukin-11, stimulates recovery of small intestinal mucosal cells after cytoablative therapy. Blood 83 (1994): 33-37.
- Potten CS. Interleukin-11 protects the clonogenic stem cells in murine small-intestinal crypts from impairment of their reproductive capacity by radiation. International Journal Of Cancer 62 (1995): 356-361.
- Weich NS, et al. Recombinant Human Interleukin-11 Directly Promotes Megakaryocytopoiesis In Vitro. 1997, W B SAUNDERS CO: United States (1997): 3893.
- de Haan G, Dontje B, Engel C, et al. In vivo effects of interleukin-11 and stem cell factor in combination with erythropoietin in the regulation of erythropoiesis. British Journal of Haematology 90 (1995): 783.
- Zhang Y, Taveggia C, Melendez-Vasquez C, et al. Interleukin-11 Potentiates Oligodendrocyte Survival and Maturation, and Myelin Formation. 2006, Society for Neuroscience: United States (2006):12174.
- Romas E, Udagawa N, Zhou H, et al. The Role of gp130-mediated Signals in Osteoclast Development: Regulation of Interleukin 11 Production by Osteoblasts and Distribution of Its Receptor in Bone Marrow Cultures. The Journal of Experimental Medicine 183 (1996): 2581.
- Maeshima K , Takahashi T , Nakahira K , et al. A Protective Role of Interleukin 11 on Hepatic Injury in Acute Endotoxemia. Biomedical Press: United States 21 (2004): 134-138.
- Lee HT, Won Park S, Kim M, et al. Interleukin-11 protects against renal ischemia and reperfusion injury. American Journal Of Physiology. Renal Physiology 303 (2012): F1216-F1224.
- Trepicchio WL, Wang L, Bozza M, et al. IL-11 Regulates Macrophage Effector Function Through the Inhibition of Nuclear Factor-kappaB. J Immunol 159 (1997): 5661-5670.
- Trepicchio WL, Bozza M, Pedneault G, et al. Recombinant Human IL-11 Attenuates the Inflammatory Response Through Down-Regulation of Proinflammatory Cytokine Release and Nitric Oxide Production. J Immunol 157 (1996): 3627-3634.
- Leng SX, Elias JA. Interleukin-11 Inhibits Macrophage Interleukin-12 Production. J Immunol 159 (1997): 2161-2168.
- Cork BA, Li TC, Warren MA, et al. Interleukin-11 (IL-11) in human endometrium: expression throughout the menstrual cycle and the effects of cytokines on endometrial IL-11 production in vitro. J Reprod Immunol 50 (2001): 3-17.
- Cork BA, Tuckerman EM, Li TC, et al. Expression of interleukin (IL)-11 receptor by the human endometrium in vivo and effects of IL-11, IL-6 and LIF on the production of MMP and cytokines by human endometrial cells in vitro. Mol Hum Reprod 8 (2002): 841-848.
- Sales KJ, Grant V, Cook IH, et al. Interleukin-11 in endometrial adenocarcinoma is regulated by prostaglandin F2alpha-F-prostanoid receptor interaction via the calcium-calcineurin-nuclear factor of activated T cells pathway and negatively regulated by the regulator of calcineurin-1. The American Journal Of Pathology 176 (2010): 435-445.
- Elias JA, Tang W, Horowitz MC, Cytokine and hormonal stimulation of human osteosarcoma interleukin-11 production. Endocrinology 136 (1995): 489-498.
- Sotiriou C, et al. Interleukins-6 and -11 expression in primary breast cancer and subsequent development of bone metastases. Elsevier Scientific Publishers Ireland Ltd: Netherlands (2001): 87.
- Murphy GM, Bitting L, Majewska A, et al. Expression of interleukin-11 and its encoding mRNA by glioblastoma cells. Neuroscience Letters 196 (1995): 153-156.
- Karube K, Ohshima K, Suzumiya J, et al. Gene expression profile of cytokines and chemokines in microdissected primary Hodgkin and Reed-Sternberg (HRS) cells: high expression of interleukin-11 receptor alpha. Annals Of Oncology: Official Journal Of The European Society For Medical Oncology 17 (2006): 110-116.
- Gao YB, Xiang ZL, Zhou LY, et al. Enhanced production of CTGF and IL-11 from highly metastatic hepatoma cells under hypoxic conditions: an implication of hepatocellular carcinoma metastasis to bone. Journal Of Cancer Research And Clinical Oncology 139 (2013): 669-679.
- Xiang Z-L, Zeng Z-C, Tang Z-Y, et al. Potential prognostic biomarkers for bone metastasis from hepatocellular carcinoma. The Oncologist 16 (2011): 1028-1039.
- Kratz JR, et al. IL11 is prognostic of survival in lung adenocarcinoma. Journal of the American College of Surgeons 213 (2011): S38-S39.
- Fukuda A, Wang SC, Morris JP, et al. Article: Stat3 and MMP7 Contribute to Pancreatic Ductal Adenocarcinoma Initiation and Progression. Cancer Cell 19 (2011): 441-455.
- Bellone G1, Smirne C, Mauri FA, et al. Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival. Cancer Immunology, Immunotherapy: CII 55 (2006): 684-698.
- Zurita AJ, Troncoso P, Cardó-Vila M, et al. Combinatorial screenings in patients: the interleukin-11 receptor alpha as a candidate target in the progression of human prostate cancer. Cancer Research 64 (2004): 435-439.
- Knoefel B, Nuske K, Steiner T, et al. Renal cell carcinomas produce IL-6, IL-10, IL-11, and TGF-beta 1 in primary cultures and modulate T lymphocyte blast transformation. J Interferon Cytokine Res 17 (1997): 95-102.
- Paglia D1, Oran A, Lu C, et al. Expression of Leukemia Inhibitory Factor and Interleukin-11 by Human Melanoma Cell Lines: LIF, IL-6, and IL-11 Are Not Coregulated. J Interferon Cytokine Res 15 (1995): 455-460.
- Necula LG1, Chivu-Economescu M, Stanciulescu EL, et al. IL-6 and IL-11 as markers for tumor aggressiveness and prognosis in gastric adenocarcinoma patients without mutations in Gp130 subunits. J Gastrointestin Liver Dis 21 (2012): 23-29.
