The Importance of Timing in the Application of Mesenchymal Stem Cells in Critically Ill Patients with COVID-19 infection (Retrospective study)
Article Information
Rabia Yilmaz1, Zafer Çukurova1, Gülsüm Oya Hergünsel1, Sinan Asar1, Erdal Karaöz2,4, Nilgün Isiksaçan5, Gökhan Tolga Adas6,7
1Department of Anesthesia and Intensive Care, Bakirkoy Dr. Sadi Konuk Training and Research Hospital, University of Health Seciences, Istanbul/Turkey
2Liv Hospital, Center for Regenerative Medicine and Stem Cell Manufacturing, Istanbul/Turkey
3Istinye University, Faculty of Medicine, Department of Histology&Embryology, Istanbul/Turkey
4Istinye University, Center for Stem Cell and Tissue Engineering Research&Practice, Istanbul/Turkey
5Department of Biochemistry and Immunology, Bakirkoy Dr. Sadi Konuk Training and Research Hospital, University of Health Seciences, Istanbul/Turkey
6Department of Surgery, Bakirkoy Dr. Sadi Konuk Training and Research Hospital, University of Health Seciences, Istanbul/Turkey
7Head of Stem Cell and Gene Therapies Application and Research Center, University of Health Seciences, Istanbul/Turkey
*Corresponding Author: Rabia Yilmaz, Department of Anesthesiology, Division of Intensive Care, Bakirkoy Dr. Sadi Konuk Trainig and Research Hospital, Istanbul, Turkey
Received: 30 July 2022; Accepted: 09 August 2022; Published: xx October 2022
Citation: Rabia Yilmaz, Zafer Çukurova, Gülsüm Oya Hergünsel, Sinan Aşar, Erdal Karaöz, Nilgün Işiksaçan, Gökhan Tolga Adaş, José RA Azevedo. The Importance of Timing in the Application of Mesenchymal Stem Cells in Critically Ill Patients with COVID-19 infection (Retrospective study). Anesthesia and Critical care 4 (2022): 149-156.
Share at FacebookAbstract
Background: The aim of this study is to control the disease by administering MSC treatment to COVID-19 patients who are taken into intensive care unit, and to assess at what time period the MSC transplant has a more positive curative effect on COVID-19 infection.
Methods: Group 1 (n=32): the first administration of MSCs was started within 1-4 days, Group 2 (n=36): the first administration of MSCs was started within 4-8 days, Group 3 (n=36): the first administration of MSCs was started within 8-12 days. The main parameters investigated: COVID-19 inflammation markers, length of hospital stay, and mortality rates.
Results: In our study, 54 (52%) of 104 patients died and 50 patients (48%) were discharged with recovery. When we evaluated the mortality rates between the groups, 10 patients (31%) in group-, 20 patients (56%) in group-2 and 24 patients (67%) in group-3 died, respectively. The groups were compared statistically, the mortality rate was significant in favor of group-1 (p<0,05). When the groups were compared in terms of COVID-19 inflammation markers, CRP and D-dimer levels of group-1 were found to be lower than the other groups (p<0.05).
Conclusions: Administration of MSCs in the early time period reduces mortality in critically ill patients with COVID-19.
Keywords
MSCs timing, COVID-19, Intensive Care
MSCs timing articles; COVID-19 articles; Intensive Care articles
MSCs timing articles MSCs timing Research articles MSCs timing review articles MSCs timing PubMed articles MSCs timing PubMed Central articles MSCs timing 2023 articles MSCs timing 2024 articles MSCs timing Scopus articles MSCs timing impact factor journals MSCs timing Scopus journals MSCs timing PubMed journals MSCs timing medical journals MSCs timing free journals MSCs timing best journals MSCs timing top journals MSCs timing free medical journals MSCs timing famous journals MSCs timing Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Intensive Care articles Intensive Care Research articles Intensive Care review articles Intensive Care PubMed articles Intensive Care PubMed Central articles Intensive Care 2023 articles Intensive Care 2024 articles Intensive Care Scopus articles Intensive Care impact factor journals Intensive Care Scopus journals Intensive Care PubMed journals Intensive Care medical journals Intensive Care free journals Intensive Care best journals Intensive Care top journals Intensive Care free medical journals Intensive Care famous journals Intensive Care Google Scholar indexed journals Angiotensin-Converting Enzyme-2 articles Angiotensin-Converting Enzyme-2 Research articles Angiotensin-Converting Enzyme-2 review articles Angiotensin-Converting Enzyme-2 PubMed articles Angiotensin-Converting Enzyme-2 PubMed Central articles Angiotensin-Converting Enzyme-2 2023 articles Angiotensin-Converting Enzyme-2 2024 articles Angiotensin-Converting Enzyme-2 Scopus articles Angiotensin-Converting Enzyme-2 impact factor journals Angiotensin-Converting Enzyme-2 Scopus journals Angiotensin-Converting Enzyme-2 PubMed journals Angiotensin-Converting Enzyme-2 medical journals Angiotensin-Converting Enzyme-2 free journals Angiotensin-Converting Enzyme-2 best journals Angiotensin-Converting Enzyme-2 top journals Angiotensin-Converting Enzyme-2 free medical journals Angiotensin-Converting Enzyme-2 famous journals Angiotensin-Converting Enzyme-2 Google Scholar indexed journals Mesenchymal stem cells articles Mesenchymal stem cells Research articles Mesenchymal stem cells review articles Mesenchymal stem cells PubMed articles Mesenchymal stem cells PubMed Central articles Mesenchymal stem cells 2023 articles Mesenchymal stem cells 2024 articles Mesenchymal stem cells Scopus articles Mesenchymal stem cells impact factor journals Mesenchymal stem cells Scopus journals Mesenchymal stem cells PubMed journals Mesenchymal stem cells medical journals Mesenchymal stem cells free journals Mesenchymal stem cells best journals Mesenchymal stem cells top journals Mesenchymal stem cells free medical journals Mesenchymal stem cells famous journals Mesenchymal stem cells Google Scholar indexed journals ICU articles ICU Research articles ICU review articles ICU PubMed articles ICU PubMed Central articles ICU 2023 articles ICU 2024 articles ICU Scopus articles ICU impact factor journals ICU Scopus journals ICU PubMed journals ICU medical journals ICU free journals ICU best journals ICU top journals ICU free medical journals ICU famous journals ICU Google Scholar indexed journals alanine transaminase articles alanine transaminase Research articles alanine transaminase review articles alanine transaminase PubMed articles alanine transaminase PubMed Central articles alanine transaminase 2023 articles alanine transaminase 2024 articles alanine transaminase Scopus articles alanine transaminase impact factor journals alanine transaminase Scopus journals alanine transaminase PubMed journals alanine transaminase medical journals alanine transaminase free journals alanine transaminase best journals alanine transaminase top journals alanine transaminase free medical journals alanine transaminase famous journals alanine transaminase Google Scholar indexed journals procalcitonin articles procalcitonin Research articles procalcitonin review articles procalcitonin PubMed articles procalcitonin PubMed Central articles procalcitonin 2023 articles procalcitonin 2024 articles procalcitonin Scopus articles procalcitonin impact factor journals procalcitonin Scopus journals procalcitonin PubMed journals procalcitonin medical journals procalcitonin free journals procalcitonin best journals procalcitonin top journals procalcitonin free medical journals procalcitonin famous journals procalcitonin Google Scholar indexed journals complete blood count articles complete blood count Research articles complete blood count review articles complete blood count PubMed articles complete blood count PubMed Central articles complete blood count 2023 articles complete blood count 2024 articles complete blood count Scopus articles complete blood count impact factor journals complete blood count Scopus journals complete blood count PubMed journals complete blood count medical journals complete blood count free journals complete blood count best journals complete blood count top journals complete blood count free medical journals complete blood count famous journals complete blood count Google Scholar indexed journals reverse transcription polymerase chain reaction articles reverse transcription polymerase chain reaction Research articles reverse transcription polymerase chain reaction review articles reverse transcription polymerase chain reaction PubMed articles reverse transcription polymerase chain reaction PubMed Central articles reverse transcription polymerase chain reaction 2023 articles reverse transcription polymerase chain reaction 2024 articles reverse transcription polymerase chain reaction Scopus articles reverse transcription polymerase chain reaction impact factor journals reverse transcription polymerase chain reaction Scopus journals reverse transcription polymerase chain reaction PubMed journals reverse transcription polymerase chain reaction medical journals reverse transcription polymerase chain reaction free journals reverse transcription polymerase chain reaction best journals reverse transcription polymerase chain reaction top journals reverse transcription polymerase chain reaction free medical journals reverse transcription polymerase chain reaction famous journals reverse transcription polymerase chain reaction Google Scholar indexed journals
Article Details
Introduction
COVID-19 virus enters the human cell binding, through its S spike protein, to Angiotensin-Converting Enzyme-2 (ACE2) receptor present on type-2 alveoli cells, endothelial cells, heart, kidney, liver and other organs [1]. A rapid and well-coordinated innate immune response is the first line of defense against viral infections, but dysregulated and excessive immune responses may cause immunopathology [2,3]. Mesenchymal stem cells (MSCs) can act as immunosuppressants or immunostimulators. In a cell anergy context, they could exhibit a pro-inflammatory phenotype, MSC-1, making it possible to reduce apoptosis and promote T-cell survival. On the other hand, in case of inflammation, they could adoptan immunosuppressive and anti-inflammatory phenotype, called MSC-2 [4]. Previous studies have shown that MSCs plays a role as an immune modulator in cytokine storm developing due to inflammation. Exogenous MSCs have been used as immunomodulators in cytokine storm in conditions such as acute pancreatitis, acute and chronic lung diseases [5,6]. MSCs application proved therapeutic efficiency during infection resulting in reduced impairment of alveolar fluid clearance and lung injury [7]. Furthermore, MSCs improve survival, decrease organ failure, increase bacterial clearance, modulate cytokine production, and improve functions of main organs [4]. In the literature, there are limited studies on the timing of MSCs, especially in critically ill patients. The aim of this study: 1) To investigate the positive effect of transplantation of MSCs on critically ill patients with COVID-19 infection on recovery and mortality 2) To determine in which time period MSC transplantation has a more positive contribution.
Materials and Methods
This study was undertaken in the Bakirkoy Dr. Sadi Konuk Research and Training Hospital at Health Science University and Istinye University. The study protocol was approved by the Ethics Committee (no: 2020/526). In addition, permission has been obtained from the Ministry of Health (no: 56733164/203). In this study, a total of 104 critically ill patients, who were followed up in the intensive care unit with the diagnosis of COVID-19 infection were retrospectively investigated. After intubation in the intensive care unit, the patients were divided into 3 different groups according to the initial time period of stem cell application. All of the patients diagnosed with COVID-19 infection clinically, radiologically, and as a laboratory, also the diagnosis was confirmed by real-time reverse transcription polymerase chain reaction (RT-PCR) analysis.
Group 1 (n=32): Patients with critically conditions, intubated and followed-up in the intensive care unit + the first administration of MSCs was started within 1-4 days in the ICU.
Group 2 (n=36): Patients with critically conditions, intubated and followed-up in the intensive care unit + the first administration of MSCs was started within 4-8 days in the ICU.
Group 3 (n=36): Patients with critically conditions, intubated and followed-up in the intensive care unit + the first administration of MSCs was started within 8-12 days in the ICU.
The study consisted of three parallel groups as illustrated in figure 1.
All patients who had Covid-19 related ARDS were admitted to the ICU due to mechanical respiratory support was required. The diagnosis of severe ARDS was decided in line with the Berlin criteria [8]. The patients were ventilated according to the individualize protective mechanical ventilation strategy with the data obtained from e-CDSS system (Metavision Imd Software). Low tidal volume 4-8 ml / kg PBW was applied with minimum RR to reach pH above 7.25. PEEP relatively high 10-15 cmH2O; driving pressures below 15 cm H2O. If patients PaO2/FiO2 <150 mm Hg were placed in a prone position at least 16-24 hours 7-8 times as needed. Each patient received MSCs suspension of 150 ml of 3x106 cells/kg by intravenous infusion 3 times. MSCs originating from allogeneic umbilical cord produced in the GMP laboratory was systematically (IV) administered to the patients slowly at the same dose on days 1, 4 and 7, respectively.
The inclusion criteria:
1) Having confirmed COVID-19 infection by the RT-PCR analysis,
2) Having confirmed critical stage of ARDS by the CT imaging, laboratory and clinical condition,
3) Obtaining informed consent from patients or their legal relative.
The exclusion criteria:
1) Patients who had pregnant, or puerperium,
2) Patients who had diagnosed malignant tumours before COVID-19 infection,
3) Patients who had history of using immunosuppressive agents.
The conventional treatment:
1- Antivirals (Favipiravir, 2x1600 mg loading dose, and 2x600 mg maintenance 5 days)
2- Dexamethasone (1X6mg IV 5-10days per needed)
3- Enoxaparine (1X0.6 ml)
4- Antibiotics were given according to the results of the antibiogram if there was a secondary infection.
Stem cell processing, quality control and preparation
All samples of Wharton Jelly derived mesenchymal stem cells (WJ-MSCs) as cell therapy medicinal products were isolated, expanded, analysed, and prepared in the cGMP-certified facility at Liv Hospital, Center for Regenerative Medicine and Stem Cell Manufacturing (LivMedCell) as described previously (9,10). The MSCs were slowly drawn into the syringe without pressure having 40 ml volume and suspended in 150 ml of 0.9 % NaCl.
Outcome Measures
- a) Inflammatory biomarkers related to COVID-19
- b) Any allergic reaction, adverse events related to MSCs transplant
- d) Length of stay in hospital
- e) Mortality rates and reasons
Biochemical parameters
Hemogram, alanine transaminase (ALT), aspartate transaminase (AST), total protein, albumin, total bilirubin, direct bilirubin, ferritin, Triglycerides, D Dimer, troponin, myoglobin, procalcitonin (PCT), ammonia, C reactive protein (CRP), creatin kinaz (CK), alkaline phosphatase (ALP) levels were determined using Beckman Coulter AU5800 clinical chemistry analyzer (Beckman Coulter, Brea, CA, USA). A complete blood count (CBC) was analysed with ADVIA 2120i hematology autoanalyzer (Siemens Healthcare Diagnostics, Erlangen, Germany).
Statistical analysis
NCSS (Number Cruncher Statistical System) 2007 (Kaysville, Utah, USA) program was used for statistical analysis. Descriptive statistical methods (mean, standard deviation, median, frequency, percentage, minimum, maximum) were used while evaluating the study data. The conformity of the quantitative data to the normal distribution was tested with the Shapiro-Wilk test and graphical examinations. One-way Anova was used for the comparison of normally distributed variables between three groups. The Kruskal Wallis test was used for the comparison of the non-normally distributed variables between three groups. Pearson chi-square test and Fisher-Freeman-Halton Exact test were used to compare qualitative data. Statistical significance was accepted as p<0,05.
Results
Patients’ characteristics
A total of 104 patients were enrolled in this study, 26 of 104 patients were female (25%) and 78 were male (75%), with a mean age of 54 years. When the groups were compared demographically, there was no statistically significant difference (p> 0,05) (Table 1).
|
Groups |
|
||||
|
Group-1 |
Group-2 |
Group-3 |
P |
||
|
n=32 |
n=36 |
N=36 |
|||
|
Age (years) |
Mean±Sd |
50,8±13,5 |
54,3±11,4 |
56,1±10,9 |
aNS |
|
(Median) Min-Max |
52(23-77) |
53,5 (24-76) |
57 (25-84) |
||
|
Gender |
Male |
23 (72) |
28 (78) |
27 (75) |
bNS |
|
n (%) |
Female |
9 (28) |
8 (22) |
9 (25) |
|
|
Length of Hospital Stay |
Mean±Sd |
27±12,5 |
28±13,4 |
26±11,6 |
cNS |
|
(Median) |
26 (9-58) |
24 (9-70) |
21 (12-55) |
||
|
Min-Max |
|||||
aOneway Anova, b Pearson Chi-Square Test, cKruskal Wallis Test.
Table 1: Distribution of Demographic Characteristics and length of hospital stay by groups. NS: non-significant.
Laboratory Assessments
COVID-19 infection markers: Serum levels of troponin, procaltonin (PCT), CRP (C-Reaktif Protein), D-dimer, fibrinojen and ferritin were evaluated as infection markers in all patients with COVID-19 infection. When compared between the groups, the CRP level of group-1 was found to be lower than the other groups (p<0.05) at the last dose of MSCs transplant. When serum ferritin, fibrinogen and PCT levels were compared between groups, there was no statistically significant difference (p>0.05) (Table-2). D-dimer levels were compared between groups, the group-1 values were found to be statistically lower (p<0.05) than group 2 and group 3. According to the groups, the 1st, 2nd and 3rd time lymphocyte and white basal cells count measurements of the patients did not show a statistically significant difference during this period (p>0.05).
|
Group 1 |
Group 2 |
Group 3 |
aP |
|||
|
CRP (mg/L) |
||||||
|
Day 1 |
122±115,2 |
129±80,3 |
146±85,4 |
NS |
||
|
82 (6,2-390) |
110 (3-358) |
138 (6-327) |
||||
|
Day 4 |
Mean±Sd |
78±89,2 |
86±99,7 |
87±84,6 |
NS |
|
|
(Median) Min-Max |
59 (1-223) |
68 (1,5-324,9) |
73 (1,4-275) |
|||
|
Day 7 |
58±85,7 |
94±113,5 |
70±72,3 |
a<0,05 |
||
|
35 (1,6-172,5) |
39 (2-352) |
51 (2-221) |
||||
|
PCT (ng/mL) |
||||||
|
Day 1 |
0,6±1,2 |
2,4±7,9 |
0,5±0,5 |
NS |
||
|
0,2 (0,04-4,4) |
0,3 (0,04-4,7) |
0,2 (0,06-2,4) |
||||
|
Day 4 |
Mean±Sd |
4,5±18,1 |
4,9±22,2 |
3,3±13,7 |
NS |
|
|
(Median) Min-Max |
0,16 (0,02-100) |
0,2 (0,04-124) |
0,2 (0,03-59) |
|||
|
Day 7 |
3,8±13,4 |
3,2±7,22 |
3,7±10,7 |
NS |
||
|
0,3 (0,02-60) |
0,2 (0,02-32) |
0,4 (0,01-41) |
||||
|
D-Dimer (μg/ml) |
||||||
|
Day 1 |
1,9±2,8 |
2,1±1,7 |
2,5±2,6 |
a<0,05 |
||
|
0,7 (0,1-10,4) |
1,4 (0,1-7,2) |
1,5 (0,3-13,3) |
||||
|
Day 4 |
Mean±Sd |
1,7±1,6 |
2,6±2 |
2,1±1,2 |
a<0,05 |
|
|
(Median) Min-Max |
0,9 (0,4-6) |
2 (0,2-9) |
1,6 (0,1-6) |
|||
|
Day 7 |
1,7±1,71 |
2,2±1,96 |
2±1,13 |
a<0,05 |
||
|
1,4 (0,4-5,3) |
1,5 (0,4-7,9) |
1,7 (0,2-3,9) |
||||
|
Fibrinojen (mg/dL) |
||||||
|
Day 1 |
575±224 |
596±159 |
613±193 |
NS |
||
|
517 (128-1198) |
572 (181-968) |
635 (247-1113) |
||||
|
Day 4 |
Mean±Sd |
504±203 |
479±246 |
502±171 |
NS |
|
|
(Median) Min-Max |
475 (217-969) |
416 (158-1200) |
497 (72-797) |
|||
|
Day 7 |
507±244 |
488±212 |
538±116 |
NS |
||
|
490 (209-1140) |
421 (239-1040) |
505 (401-745) |
||||
|
Ferritin ( μg/L) |
||||||
|
Day 1 |
824±783 |
1813±1838 |
1170±895 |
a<0,05 |
||
|
627 (71-3761) |
1654 (188-9515) |
724 (209-3237) |
||||
|
Day 4 |
Mean±Sd |
1096±2635 |
999±1044 |
1019±1008 |
NS |
|
|
(Median) Min-Max |
509 (133-14665) |
780(53-5341) |
548 (67-3851) |
|||
|
Day 7 |
1355±2236 |
1315±2985 |
2037±4331 |
NS |
||
|
534 (205-15316) |
620(112-14431) |
514 (63-16188) |
||||
aKruskal Wallis Test.
Table 2: Level of inflammation markers in venous blood samples, p values following comparison between groups. All values are given as minimum, maximum, median and mean, NS: not significant.
Patients’ situation and mortality
We used the Apache-2 score to determine patients' current physiological status and disease severity related to COVID-19. In addition, the lung functions of the patients were evaluated with the Horowitz index. When the Apache-2 and Horowitz Index of the patients followed in the intensive care unit were compared at the beginning of the study, there was no statistically significant difference (p<0,05). In our study, 54 (52%) of 104 patients died and 50 patients (48%) were discharged with clinical and laboratory (PCR-) recovery. When we evaluated the mortality rates between the groups, 10 patients (31%) in group-1, 20 patients (56%) in group-2 and 24 patients (67%) in group-3 died, respectively (Table-3, Figure-2). When groups were compared, the mortality rate in group-1 was found to be statistically lower (p<0.05) than the other groups (Table-3). When the causes of death of the patients are evaluated, secondary infections due to bacteria, and related sepsis come first, followed by myocardial infarction, thromboembolism, and multi-organ failure. When the average hospital stay of the patients was compared, they were 27 days in group-1, 28 days in group 2, and 26 days in group-3, respectively, and no statistical difference was found (p>0,05) (Table 1).
|
Group-1 (n:32) |
p |
||||||
|
Initial day of MSCs transplantation |
1st day |
2nd day |
3rd day |
4th day |
Total Death Number (%) |
||
|
Mortality number |
0 |
2 |
4 |
4 |
10 (%31) |
|
|
|
Group-2 (n:36) |
|||||||
|
Initial day of MSCs transplantation |
5th day |
6th day |
7th day |
8th day |
|||
|
Mortality number |
4 |
5 |
6 |
5 |
20 (%56) |
||
|
Group-3 (n:36) |
|||||||
|
Initial day of MSCs transplantation |
9th day |
10th day |
11th day |
12th day |
|||
|
Mortality number |
6 |
5 |
5 |
8 |
24 (%67) |
||
|
Total death number |
54 (%52) |
P<0.05 |
|||||
Table 3: The number of mortality according to the initial day of MSCs administration, while no death was observed in patients who received MSCs transplant on day-1, the highest mortality rate was observed in patients who were given on the last day [12].
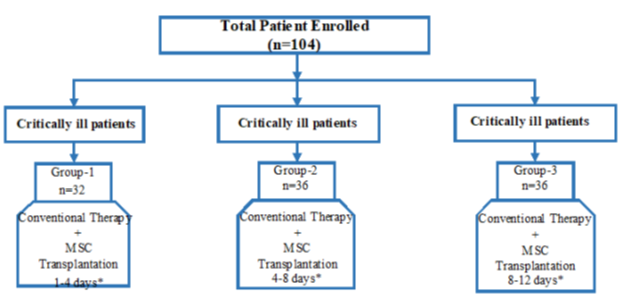
Figure 1: The study design consisting of three groups. *The initial time interval for the first stem cell transplant, after the patients were admitted to the intensive care unit and intubated.
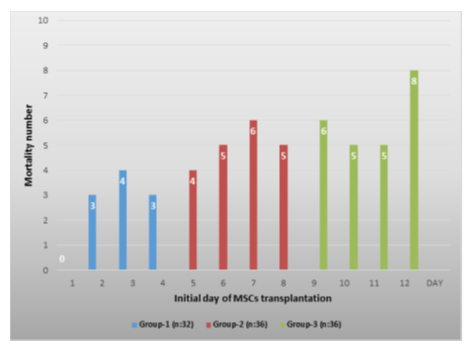
Figure 2: The number of deaths according to the first day of MSCs administration among the groups, the lowest mortality was found in group-1, while the highest mortality was in group-3 (p<0.05).
Discussion
Mesenchymal stem cells could play a pivotal role in combating COVID-19 because of its immunomodulation and regenerative potential as depicted. The immunomodulatory characteristic of MSCs indicate that MSCs can be used as a supportive treatment option for better recovery of critically ill COVID-19 patients (11,12). As of today, there are no detailed and precise data regarding the dose of MSCs and the route of administration in critically ill patients with COVID-19 infection. Furthermore, there is no consensus on which time is the optimal time to begin MSC administration in critically ill patients with COVID-19 infection. The aim of this study was to find the answer to when MSCs should be given to critically ill patients with COVID-19 infection. For this purpose, MSCs were applied to critically ill patients with COVID-19 infection in 3 different time periods, and it was investigated which of these time periods had a more positive effect on recovery. We used the Apache-2 score to determine the patients current physiological state and overall disease severity. In addition, the lung function of the patients in the groups was evaluated with the Horowitz index. This index is useful for assessing the extent of damage to the lungs. The Horowitz index plays a major role in the diagnosis of acute respiratory distress syndrome (ARDS) (8). When the Apache-2 and Horowitz Index of the patients followed in the ICU were compared at the beginning of the MSCs treatment, it was found that there was no statistically significant difference. This result shows that the distribution of patients between the groups is homogeneous. The dose of injected cells ranges generally from 0,5 to 2x106 cells/kg. Apart from these studies, there have been publications using higher MSCs. The number of injections varies between a single dose and up to 4 doses. In general, the interval between repeat injections ranges from 24h to 96 h, which is common [13,14]. In our study, 3x106/kg MSCs were administered as 3 same doses with 3 day intervals [15]. Our clinical practice for the treatment of MSCs is to give to critically ill COVID-19 patients admitted to the ICU. Although it has been given in some centers in the literature, especially in comorbid patients, we do not apply MSCs treatment to mild or moderate patients with good general condition. In general, we prefer to start MSCs treatment within the first 48 hours in intubated patients with COVID-19 infection [15]. One of the hallmarks of COVID-19 is high serum concentrations of CRP (16). In our study, when all groups were evaluated as COVID-19 infection markers, the CRP level of group-1 was found to be lower than the other groups. This level of decrease was statistically significant in favor of group-1 in the last MSCs (3rd dose) administration (p<0.05) (Table-2). An elevated D-dimer, the most common coagulation abnormality in COVID-19 is an independent risk factor for death [17]. When D-dimer levels were compared between the groups, group-1 values were found to be statistically lower than the other groups (p<0.05) (Table-2). These results show the positive effect of MSCs applied in the early period on both COVID-19 infection markers. MSCs are now being used as a potential therapy for treating COVID-19 patients in order to reduce mortality. Generally critically ill patients, and those patients who are at high risk of infection due to other comorbidities, are potential candidates for stem cell-based therapies [18,19]. However, no data is currently available regarding the time of the start of MSC therapy for maximum benefits [19]. In our study, considering the mortality numbers and rates, the highest mortality rate was 24 patients (%67) in group-3. The lowest mortality rate was in group-1 with 10 patients (%31). When the groups were compared statistically, the mortality rate in group-1 was found to be statistically (p<0,05) low. This result shows that MSCs transplantation at an earlier time period reduces mortality in patients with critical COVID-19 infection. In the late period, especially after the first week, the positive effect of MSC transplantation on mortality decreases considerably. This can be explained by the fact that the effect of MSCs on organ regeneration has decreased considerably. These patients especially had multiple organ failure. When the MSCs application initial time is determined, it is seen that MSCs treatment was given to the patients on 12 separate days. When this situation was evaluated, it was observed that the number of mortality increased especially in the late period in group-3. While there was no mortality in the patients who started MSCs on the 1st day of administration, 8 deaths occurred in the patients who were administered on the 12th day (Table-3, Figure-1). This result is that the application of MSCs has no positive effect on improvement in patients with COVID-19 in the late period. The lowest mortality rate (31%) in group-1 is close to the result (%30) of our previously published prospective double control study on this subject [15]. Both results support early initiation of MSCs therapy in critically ill patients with COVID-19 infection. Leng et al. investigated the use of MSCs in hospitalized patients with COVID 19 who were not improving despite standard therapy. Intravenous MSC at a dose of 1x106 cells per kg was administered to seven patients in the early period. MSC infusion was well tolerated all the patients [20]. Sanchez-Guijo et al administered MSCs to thirteen adult Covid-19 patients undergoing invasive mechanical ventilation. Ten of the thirteen patients received two doses (3 days after the first dose). The remaining two patients received a single dose, while the third patient received 3 doses of MSC. While 70% of the patients recovered clinically and were discharged from the intensive care unit, four patients remained intubated and two patients died [21]. As a matter of discussion in this study, it may be asked why treatment for MSCs is not started early and in the same time period to all patients. Our hospital is accepted as a reference center since it is a hospital that provides cellular treatments in Istanbul. For this reason, critical patients with COVID-19 infections are transferred from surrounding hospitals and nearby provinces. In this study, our main goal is to reduce the mortality rate with treatment of MSCs in critically ill COVID-19 patients. These patients were treated with cellular based therapy as one of the last options, since conventional treatments related to COVID-19 had been performed before. Therefore, MSCs treatment initiation time differs in patients. This is one of the limitations of our study. When the average hospital stay of the patients was compared, they were 27 days in group-1, 28 days in group 2, and 26 days in group-3, respectively, and no statistical difference was found (p>0,05). It is seen that the shortest mean duration of stay was in group-3 with 26 days. The reason for this is that the highest death rate is in group-3, which decreases on average. As it is known, the most frequently affected main organ in COVID 19 infection is the lungs. Our opinion is that this is an advantage in favor of systemic MSCs transplantation. Because the vast majority of systemically administered cells are trapped inside the lungs [22,23]. Exogenously given MSCs trapped in the lungs allow stem cell based therapy to be faster and more efficient on COVID-19 infection. This makes it advantageous to apply stem cell-based therapy in COVID-19. The main limitation of our study is that it is a retrospective study. In addition, the transfer of our patients from many different centers to our hospital negatively affects the homogeneity of the study in terms of the start time of the MSC application. The patients transferred to our hospital were generally in poor general condition and were treated with classical treatments for COVID-19 infection. For this reason, our thought in administering MSCs to patients, even in the late period, is to benefit from the regeneration effect of these cells. We also predicted that the positive effect of MSC on regeneration would reduce mortality. MSCs can secrete a variety of growth factors have significant ability to play essential roles in tissue regeneration in lung injuries via diminishing collagen accumulation and fibrosis [24]. Systemic administration of MSCs results in homing to the pulmonary vascular bed where they release soluble factors such as antiinflammatory cytokines, antimicrobial peptides, angiogenic growth factors, and extracellular vesicles and thus could improve the pulmonary microenvironment, protect alveolar epithelial cells, prevent pulmonary fibrosis and improve overall lung function [19,25]. One of our reason for applying MSCs in the late period was to use the reducing effect of these cells on lung fibrosis. However, in our study, we did not find a reducing effect of MSC treatment on mortality, especially in the last time period. As we mentioned in our previously published study, the first 48 hours of MSCs treatment is to start in critically ill patients with COVID-19 who are taken to intensive care and intubated. The results of both studies show that the optimal time to administer MSCs in critically ill patients with COVID-19 is the first 48 hours. Apart from this, we predict the initiation of MSCs treatment in patients who are considered to be transferred to the intensive care unit, where the respiratory parameters and vital functions of the patient begin to deteriorate and the cytokine storm continues.
Conclusion
In conclusion, early initiation of MSC treatment reduces mortality in critically ill patients with COVID-19 who have impaired vital signs and are admitted to the intensive care unit. As treatment initiation time increases, the efficacy of MSCs may decrease or become ineffective.
Abbreviations
MSC: mesenchymal stem cells
ARDS: Acute respiratory distress syndrome
e-CDSS: electronic clinical decision support system
PBW: predicted body weight
PEEP: Positive End Expiratory Pressure
Apache-2 score: Acute Physiology and Chronic Health Evaluation Score
Horowitz Index: PaO2/FiO2
CRP: C-Reaktif Protein
PCT: Procalsitonin
Compliance with ethical standards
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- Kumar A, Ghosh B S. Emerging Treatment Options of Regenerative Medicine in Severe Corona Virus/COVID 19 Infections. International Journal of Stem Cells 31 (2020): 12-23
- Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunpathol 12 (2017): 67-81.
- Irmak KD, Darici H, Karaoz E. Stem cell based therapy option in COVID-19: Is it really promising? Aging and Disease 4 (2020): 1-17.
- Laroye C, Gibot S, Huselstein C, et al. Mesenchymal stromal cells for sepsis and septic shock: Lessons for treatment of COVID-19. Stem Cells Transl Med 9 (2020): 1488-1494.
- Xia S, Zhou C, Kalionis B, et al. Combined antioxidant, anti-inflammaging and mesenchymal stem cell treatment: A possible therapeutic direction in elderly patients with chronic obstructive pulmonary disease. Aging and Disease 11 (2020): 129-140.
- Ahmed MS, Morsi M, Ghoneim IN, et al. Mesenchymal stromal cell therapy for pancreatitis: A systemic review. Oxidative Medicine and Cellular Longevity 13 (2018): 1-14.
- Behnke J, Kremer S, Shahzad T, et al. MSC based therapies-new perspectives for the injured lung. J of Clin Med 13 (2020): 32-45.
- RanieriVM, Rubenfeld GD, et al. ARDS definition Task Force, Acute respiratory distress syndrome: Berlin Definition. JAMA 307 (2012): 2526-2533.
- Dai A, Baspinar O, Yesilyurt A, et al. Efficacy of stem cell therapy in ambulatory and nonambulatory children with Duchenne muscular dystrophy–Phase I–II. Degenerative Neurological and Neuromuscular Disease 8 (2018): 63-77.
- Kabatas S¸ Civelek E, Inci Ç, et al. Wharton’s Jelly-Derived Mesenchymal Stem Cell Transplantation in a Patient with Hypoxic-Ischemic Encephalopathy: A Pilot Study.. Cell Transplantation 27 (2018): 1425-1433.
- Pooja Y, Ravina V, Afsareen B, et al. Mesenchymal stem cell immunomodulation and regeneration therapeutics as an ameliorative approach for COVID-19 pandemics. Life Sci 263 (2020): 118588.
- Liang B, Chen J, Li T, et al. Clinicalremission of a critically ill COVID-19 patient treated by humanumbilical cord mesenchymal stem cells: A case report. Medicine 99 (2020): e21429
- Khoury M, Ikonomou L, Dominici M, et al. The Coronavirus Pandemic: A Pitfall or a Fast Track for Validating Cell Therapy Products? Stem Cells and Development 30 (2019): 887-899
- Shahani P, Datta I. Mesenchymal stromal cell therapy for coronavirus disease 2019: which? when? and how much? Cytotherapy 23 (2021): 861-873.
- Adas G, Z Cukurova, K Kart et al. The Systematic Effect of Mesenchymal Stem Cell Therapy in Critical COVID-19 Patients: A Prospective Double Controlled TrialCell Transplant 30 (2021): 456-469.
- Zhand S, Jazi MS, Mohammadi S, et al. COVID-19: The immune responses and Clinical Therapy Candidates. Int J Mol Sci 34 (2020): 21-15.
- Meaghan EC, Yogendra K. COVID-19 associated coagulopathy: An exploration of mechanisms Vascular Medicine 25 (2020): 471-478.
- Zumla A, Wang SF, Ippolito G, et al. Reducing mortality and morbidity in patients with severe COVID-19 disease by advancing ongoing trials of Mesenchymal Stromal (stem) Cell (MSC) therapy - Achieving global consensus and visibility for cellular host-directed therapies Int J Infect Dis. 96 (2020): 431-439.
- Choudlery SM, Harris TD. Stem cell therapy for COVID-19: possibilities and challenges. Cell Biol Int 44 (2020): 2182-2191.
- Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging and Disease 11 (2020): 216.
- Mallis P, Michalopoulos E, Chatzistamatiou T, et al. Mesenchymal stromal cells as potential immunomodulatory players in severe acute respiratory distress syndrome induced by SARSCoV-2 infection. World J Stem Cells 12 (2020): 731-751.
- Schrepfer S, Deuse T, Reichenspurner H, et al. Pelletier Stem Cell Transplantation: The Lung Barrier. Transplantation Proceedings 39 (2007): 573-576.
- Fischer MU, Harting TM, Jimenez F, et al. Pulmonary Passage is a Major Obstacle for Intravenous Stem Cell Delivery: The Pulmonary First-Pass Effect. Stem Cells and Development 18 (2009): 681-693.
- Susan M, Atieh P, Parisa Z, et al. Mesenchymal stromal/stem cells (MSCs) and MSC-derived extracellular vesicles in COVID-19-induced ARDS: Mechanisms of action, research progress, challenges, and opportunities International Immunopharmacology 97 (2021): 107694
- Lanzoni G, Linetsky E, Correa D, et al. Umbilical Cord-derived Mesenchymal Stem Cells for COVID-19 Patients with Acute Respiratory Distress Syndrome (ARDS). CellR4 Repair Replace Regen Reprogram. 8 (2020): 459-471.
